 Found 49 hits with Last Name = 'berg' and Initial = 'ce'
Found 49 hits with Last Name = 'berg' and Initial = 'ce' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131550
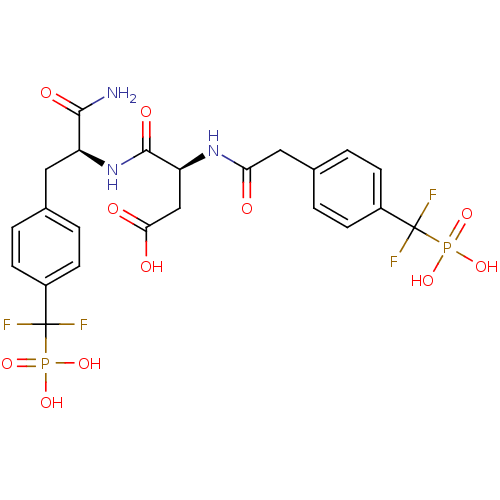
((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM13976

(Aminobenzoic acid analog 5 | CHEMBL116605)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50131550

((3S)-3-{[(1S)-1-carbamoyl-2-{4-[difluoro(phosphono...)Show SMILES NC(=O)[C@H](Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)[C@H](CC(O)=O)NC(=O)Cc1ccc(cc1)C(F)(F)P(O)(O)=O |r| Show InChI InChI=1S/C23H25F4N3O11P2/c24-22(25,42(36,37)38)14-5-1-12(2-6-14)9-16(20(28)34)30-21(35)17(11-19(32)33)29-18(31)10-13-3-7-15(8-4-13)23(26,27)43(39,40)41/h1-8,16-17H,9-11H2,(H2,28,34)(H,29,31)(H,30,35)(H,32,33)(H2,36,37,38)(H2,39,40,41)/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131547

(2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cc(Cl)cc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H36ClN3O11/c1-4-21-15-20(11-12-25(21)38(31(42)33(45)46)26-10-6-5-9-23(26)32(43)44)16-24(37-19(2)39)30(41)36-13-7-8-14-49-28-18-22(35)17-27(40)29(28)34(47)48-3/h5-6,9-12,15,17-18,24,40H,4,7-8,13-14,16H2,1-3H3,(H,36,41)(H,37,39)(H,43,44)(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131555

(2-(4-(2-acetamido-3-(4-(1-carboxy-N-(2-carboxyphen...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C33H35N3O11/c1-3-21-17-20(13-14-24(21)36(30(40)33(45)46)25-10-5-4-9-22(25)31(41)42)18-23(35-19(2)37)29(39)34-15-6-7-16-47-27-12-8-11-26(38)28(27)32(43)44/h4-5,8-14,17,23,38H,3,6-7,15-16,18H2,1-2H3,(H,34,39)(H,35,37)(H,41,42)(H,43,44)(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM13976

(Aminobenzoic acid analog 5 | CHEMBL116605)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O11/c1-4-22-18-21(14-15-25(22)37(31(41)33(44)45)26-11-6-5-10-23(26)32(42)43)19-24(36-20(2)38)30(40)35-16-7-8-17-48-28-13-9-12-27(39)29(28)34(46)47-3/h5-6,9-15,18,24,39H,4,7-8,16-17,19H2,1-3H3,(H,35,40)(H,36,38)(H,42,43)(H,44,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50131555

(2-(4-(2-acetamido-3-(4-(1-carboxy-N-(2-carboxyphen...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C33H35N3O11/c1-3-21-17-20(13-14-24(21)36(30(40)33(45)46)25-10-5-4-9-22(25)31(41)42)18-23(35-19(2)37)29(39)34-15-6-7-16-47-27-12-8-11-26(38)28(27)32(43)44/h4-5,8-14,17,23,38H,3,6-7,15-16,18H2,1-2H3,(H,34,39)(H,35,37)(H,41,42)(H,43,44)(H,45,46) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 73 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM15819

(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)N[C@@H](CCSC)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C32H40N4O10S/c1-4-21-17-20(12-13-25(21)36(29(40)32(45)46)26-10-6-5-9-22(26)30(41)42)18-24(34-19(2)37)28(39)33-15-8-7-11-27(38)35-23(31(43)44)14-16-47-3/h5-6,9-10,12-13,17,23-24H,4,7-8,11,14-16,18H2,1-3H3,(H,33,39)(H,34,37)(H,35,38)(H,41,42)(H,43,44)(H,45,46)/t23-,24?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131545
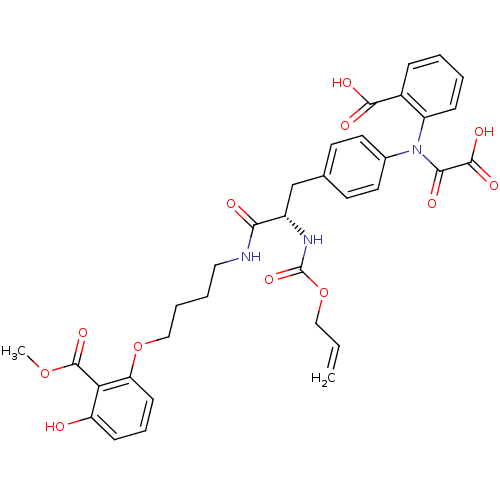
((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(cc1)N(C(=O)C(O)=O)c1ccccc1C(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C34H35N3O12/c1-3-18-49-34(46)36-24(29(39)35-17-6-7-19-48-27-12-8-11-26(38)28(27)33(45)47-2)20-21-13-15-22(16-14-21)37(30(40)32(43)44)25-10-5-4-9-23(25)31(41)42/h3-5,8-16,24,38H,1,6-7,17-20H2,2H3,(H,35,39)(H,36,46)(H,41,42)(H,43,44)/t24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50131553

(2-[(4-{2-Acetylamino-2-[4-(3-hydroxy-2-nitro-pheno...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2[N+]([O-])=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C32H34N4O11/c1-3-21-17-20(13-14-24(21)35(30(40)32(43)44)25-10-5-4-9-22(25)31(41)42)18-23(34-19(2)37)29(39)33-15-6-7-16-47-27-12-8-11-26(38)28(27)36(45)46/h4-5,8-14,17,23,38H,3,6-7,15-16,18H2,1-2H3,(H,33,39)(H,34,37)(H,41,42)(H,43,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131553

(2-[(4-{2-Acetylamino-2-[4-(3-hydroxy-2-nitro-pheno...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2[N+]([O-])=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C32H34N4O11/c1-3-21-17-20(13-14-24(21)35(30(40)32(43)44)25-10-5-4-9-22(25)31(41)42)18-23(34-19(2)37)29(39)33-15-6-7-16-47-27-12-8-11-26(38)28(27)36(45)46/h4-5,8-14,17,23,38H,3,6-7,15-16,18H2,1-2H3,(H,33,39)(H,34,37)(H,41,42)(H,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50131547

(2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cc(Cl)cc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H36ClN3O11/c1-4-21-15-20(11-12-25(21)38(31(42)33(45)46)26-10-6-5-9-23(26)32(43)44)16-24(37-19(2)39)30(41)36-13-7-8-14-49-28-18-22(35)17-27(40)29(28)34(47)48-3/h5-6,9-12,15,17-18,24,40H,4,7-8,13-14,16H2,1-3H3,(H,36,41)(H,37,39)(H,43,44)(H,45,46) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131546

(2-[(4-{2-Acetylamino-2-[4-(3-hydroxy-2-methylcarba...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)NC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H38N4O10/c1-4-22-18-21(14-15-25(22)38(32(43)34(46)47)26-11-6-5-10-23(26)33(44)45)19-24(37-20(2)39)30(41)36-16-7-8-17-48-28-13-9-12-27(40)29(28)31(42)35-3/h5-6,9-15,18,24,40H,4,7-8,16-17,19H2,1-3H3,(H,35,42)(H,36,41)(H,37,39)(H,44,45)(H,46,47) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM15819

(1:1 mixture of diastereomers | 2-[(4-{2-[(4-{[(1S)...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCC(=O)N[C@@H](CCSC)C(O)=O)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O |r| Show InChI InChI=1S/C32H40N4O10S/c1-4-21-17-20(12-13-25(21)36(29(40)32(45)46)26-10-6-5-9-22(26)30(41)42)18-24(34-19(2)37)28(39)33-15-8-7-11-27(38)35-23(31(43)44)14-16-47-3/h5-6,9-10,12-13,17,23-24H,4,7-8,11,14-16,18H2,1-3H3,(H,33,39)(H,34,37)(H,35,38)(H,41,42)(H,43,44)(H,45,46)/t23-,24?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131544
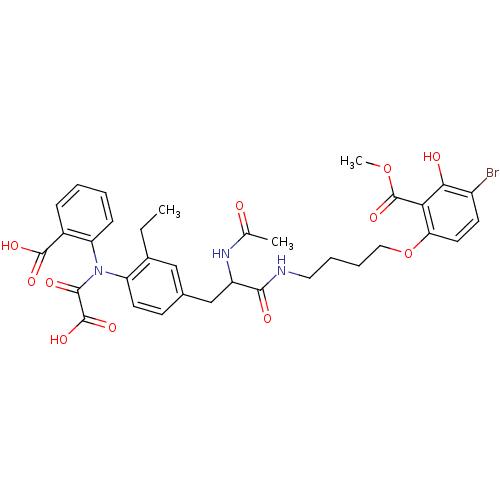
(6-[4-(2-Acetylamino-3-{4-[(2-carboxy-phenyl)-oxaly...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2ccc(Br)c(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H36BrN3O11/c1-4-21-17-20(11-13-25(21)38(31(42)33(45)46)26-10-6-5-9-22(26)32(43)44)18-24(37-19(2)39)30(41)36-15-7-8-16-49-27-14-12-23(35)29(40)28(27)34(47)48-3/h5-6,9-14,17,24,40H,4,7-8,15-16,18H2,1-3H3,(H,36,41)(H,37,39)(H,43,44)(H,45,46) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50131545

((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(cc1)N(C(=O)C(O)=O)c1ccccc1C(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C34H35N3O12/c1-3-18-49-34(46)36-24(29(39)35-17-6-7-19-48-27-12-8-11-26(38)28(27)33(45)47-2)20-21-13-15-22(16-14-21)37(30(40)32(43)44)25-10-5-4-9-23(25)31(41)42/h3-5,8-16,24,38H,1,6-7,17-20H2,2H3,(H,35,39)(H,36,46)(H,41,42)(H,43,44)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131548
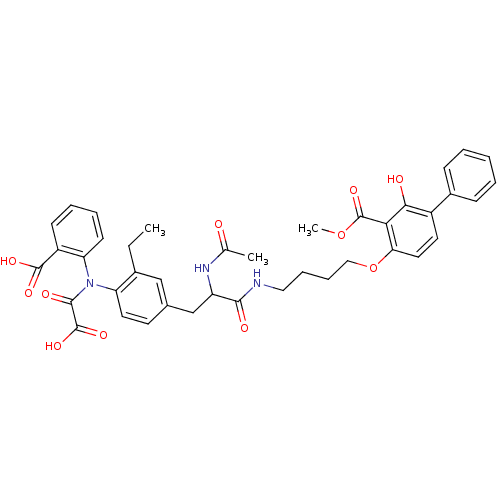
(4-[4-(2-Acetylamino-3-{4-[(2-carboxy-phenyl)-oxaly...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2ccc(c(O)c2C(=O)OC)-c2ccccc2)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C40H41N3O11/c1-4-26-22-25(16-18-31(26)43(37(47)39(50)51)32-15-9-8-14-29(32)38(48)49)23-30(42-24(2)44)36(46)41-20-10-11-21-54-33-19-17-28(27-12-6-5-7-13-27)35(45)34(33)40(52)53-3/h5-9,12-19,22,30,45H,4,10-11,20-21,23H2,1-3H3,(H,41,46)(H,42,44)(H,48,49)(H,50,51) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50131546

(2-[(4-{2-Acetylamino-2-[4-(3-hydroxy-2-methylcarba...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cccc(O)c2C(=O)NC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H38N4O10/c1-4-22-18-21(14-15-25(22)38(32(43)34(46)47)26-11-6-5-10-23(26)33(44)45)19-24(37-20(2)39)30(41)36-16-7-8-17-48-28-13-9-12-27(40)29(28)31(42)35-3/h5-6,9-15,18,24,40H,4,7-8,16-17,19H2,1-3H3,(H,35,42)(H,36,41)(H,37,39)(H,44,45)(H,46,47) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50131548

(4-[4-(2-Acetylamino-3-{4-[(2-carboxy-phenyl)-oxaly...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2ccc(c(O)c2C(=O)OC)-c2ccccc2)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C40H41N3O11/c1-4-26-22-25(16-18-31(26)43(37(47)39(50)51)32-15-9-8-14-29(32)38(48)49)23-30(42-24(2)44)36(46)41-20-10-11-21-54-33-19-17-28(27-12-6-5-7-13-27)35(45)34(33)40(52)53-3/h5-9,12-19,22,30,45H,4,10-11,20-21,23H2,1-3H3,(H,41,46)(H,42,44)(H,48,49)(H,50,51) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50131551

(2-carboxy(2-ethyl-4-{2-methylcarboxamido-2-[4-(2-m...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2ccccc2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O10/c1-4-23-19-22(15-16-27(23)37(31(40)33(43)44)28-13-7-5-11-24(28)32(41)42)20-26(36-21(2)38)30(39)35-17-9-10-18-47-29-14-8-6-12-25(29)34(45)46-3/h5-8,11-16,19,26H,4,9-10,17-18,20H2,1-3H3,(H,35,39)(H,36,38)(H,41,42)(H,43,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131551

(2-carboxy(2-ethyl-4-{2-methylcarboxamido-2-[4-(2-m...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2ccccc2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H37N3O10/c1-4-23-19-22(15-16-27(23)37(31(40)33(43)44)28-13-7-5-11-24(28)32(41)42)20-26(36-21(2)38)30(39)35-17-9-10-18-47-29-14-8-6-12-25(29)34(45)46-3/h5-8,11-16,19,26H,4,9-10,17-18,20H2,1-3H3,(H,35,39)(H,36,38)(H,41,42)(H,43,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Protein-tyrosine phosphatase 1B (PTP 1B) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Receptor-type tyrosine-protein phosphatase alpha
(Homo sapiens (Human)) | BDBM50131544

(6-[4-(2-Acetylamino-3-{4-[(2-carboxy-phenyl)-oxaly...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2ccc(Br)c(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H36BrN3O11/c1-4-21-17-20(11-13-25(21)38(31(42)33(45)46)26-10-6-5-9-22(26)32(43)44)18-24(37-19(2)39)30(41)36-15-7-8-16-49-27-14-12-23(35)29(40)28(27)34(47)48-3/h5-6,9-14,17,24,40H,4,7-8,15-16,18H2,1-3H3,(H,36,41)(H,37,39)(H,43,44)(H,45,46) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory constant against T cell protein tyrosine phosphatase |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F
(Homo sapiens (Human)) | BDBM50131547

(2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cc(Cl)cc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H36ClN3O11/c1-4-21-15-20(11-12-25(21)38(31(42)33(45)46)26-10-6-5-9-23(26)32(43)44)16-24(37-19(2)39)30(41)36-13-7-8-14-49-28-18-22(35)17-27(40)29(28)34(47)48-3/h5-6,9-12,15,17-18,24,40H,4,7-8,13-14,16H2,1-3H3,(H,36,41)(H,37,39)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against SH-domain containing phosphotyrosine phosphatase-2 (Tyrosine phosphatase SHP2) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50131547

(2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cc(Cl)cc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H36ClN3O11/c1-4-21-15-20(11-12-25(21)38(31(42)33(45)46)26-10-6-5-9-23(26)32(43)44)16-24(37-19(2)39)30(41)36-13-7-8-14-49-28-18-22(35)17-27(40)29(28)34(47)48-3/h5-6,9-12,15,17-18,24,40H,4,7-8,13-14,16H2,1-3H3,(H,36,41)(H,37,39)(H,43,44)(H,45,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 tyrosine phosphatase was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50131547

(2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cc(Cl)cc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H36ClN3O11/c1-4-21-15-20(11-12-25(21)38(31(42)33(45)46)26-10-6-5-9-23(26)32(43)44)16-24(37-19(2)39)30(41)36-13-7-8-14-49-28-18-22(35)17-27(40)29(28)34(47)48-3/h5-6,9-12,15,17-18,24,40H,4,7-8,13-14,16H2,1-3H3,(H,36,41)(H,37,39)(H,43,44)(H,45,46) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against cell division cycle 25 degree C (Cdc25 C) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50131547

(2-(N-(4-(2-acetamido-3-(4-(5-chloro-3-hydroxy-2-(m...)Show SMILES CCc1cc(CC(NC(C)=O)C(=O)NCCCCOc2cc(Cl)cc(O)c2C(=O)OC)ccc1N(C(=O)C(O)=O)c1ccccc1C(O)=O Show InChI InChI=1S/C34H36ClN3O11/c1-4-21-15-20(11-12-25(21)38(31(42)33(45)46)26-10-6-5-9-23(26)32(43)44)16-24(37-19(2)39)30(41)36-13-7-8-14-49-28-18-22(35)17-27(40)29(28)34(47)48-3/h5-6,9-12,15,17-18,24,40H,4,7-8,13-14,16H2,1-3H3,(H,36,41)(H,37,39)(H,43,44)(H,45,46) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against (calcineurin) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase F
(Homo sapiens (Human)) | BDBM50131545

((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(cc1)N(C(=O)C(O)=O)c1ccccc1C(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C34H35N3O12/c1-3-18-49-34(46)36-24(29(39)35-17-6-7-19-48-27-12-8-11-26(38)28(27)33(45)47-2)20-21-13-15-22(16-14-21)37(30(40)32(43)44)25-10-5-4-9-23(25)31(41)42/h3-5,8-16,24,38H,1,6-7,17-20H2,2H3,(H,35,39)(H,36,46)(H,41,42)(H,43,44)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against SH-domain containing phosphotyrosine phosphatase-2 (Tyrosine phosphatase SHP2) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50131545

((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(cc1)N(C(=O)C(O)=O)c1ccccc1C(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C34H35N3O12/c1-3-18-49-34(46)36-24(29(39)35-17-6-7-19-48-27-12-8-11-26(38)28(27)33(45)47-2)20-21-13-15-22(16-14-21)37(30(40)32(43)44)25-10-5-4-9-23(25)31(41)42/h3-5,8-16,24,38H,1,6-7,17-20H2,2H3,(H,35,39)(H,36,46)(H,41,42)(H,43,44)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.78E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cell division cycle 25 degree C was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein phosphatase C
(Homo sapiens (Human)) | BDBM50131545

((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(cc1)N(C(=O)C(O)=O)c1ccccc1C(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C34H35N3O12/c1-3-18-49-34(46)36-24(29(39)35-17-6-7-19-48-27-12-8-11-26(38)28(27)33(45)47-2)20-21-13-15-22(16-14-21)37(30(40)32(43)44)25-10-5-4-9-23(25)31(41)42/h3-5,8-16,24,38H,1,6-7,17-20H2,2H3,(H,35,39)(H,36,46)(H,41,42)(H,43,44)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against CD45 tyrosine phosphatase was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Protein phosphatase 3 catalytic subunit alpha
(Homo sapiens (Human)) | BDBM50131545

((S)-2-(N-(4-(2-(allyloxycarbonylamino)-3-(4-(3-hyd...)Show SMILES COC(=O)c1c(O)cccc1OCCCCNC(=O)[C@H](Cc1ccc(cc1)N(C(=O)C(O)=O)c1ccccc1C(O)=O)NC(=O)OCC=C Show InChI InChI=1S/C34H35N3O12/c1-3-18-49-34(46)36-24(29(39)35-17-6-7-19-48-27-12-8-11-26(38)28(27)33(45)47-2)20-21-13-15-22(16-14-21)37(30(40)32(43)44)25-10-5-4-9-23(25)31(41)42/h3-5,8-16,24,38H,1,6-7,17-20H2,2H3,(H,35,39)(H,36,46)(H,41,42)(H,43,44)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against (calcineurin) was determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50022087
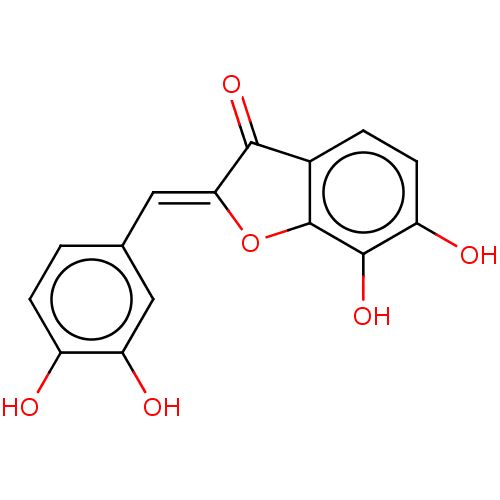
(CHEBI:6694 | CHEMBL3298066)Show InChI InChI=1S/C15H10O6/c16-9-3-1-7(5-11(9)18)6-12-13(19)8-2-4-10(17)14(20)15(8)21-12/h1-6,16-18,20H/b12-6- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Manufacturing Flagship (Biomedical)
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta1-42 (unknown origin) aggregation assessed as amyloid fibril formation tested after 17 hrs by thioflavin T fluorescence met... |
Bioorg Med Chem Lett 24: 3108-12 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.008
BindingDB Entry DOI: 10.7270/Q2S1842P |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50070942

((-)-Epigallocatechin gallate | (-)-Epigallocatechi...)Show SMILES Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c1cc(O)c(O)c(O)c1 |r| Show InChI InChI=1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Manufacturing Flagship (Biomedical)
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta1-42 (unknown origin) aggregation assessed as amyloid fibril formation tested after 17 hrs by thioflavin T fluorescence met... |
Bioorg Med Chem Lett 24: 3108-12 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.008
BindingDB Entry DOI: 10.7270/Q2S1842P |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM50022088
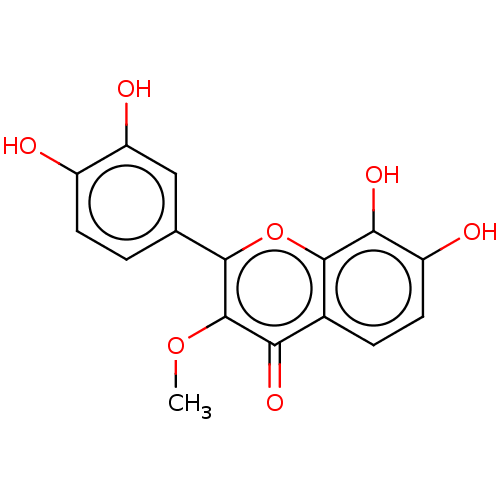
(CHEMBL3297746)Show InChI InChI=1S/C16H12O7/c1-22-16-12(20)8-3-5-10(18)13(21)15(8)23-14(16)7-2-4-9(17)11(19)6-7/h2-6,17-19,21H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Manufacturing Flagship (Biomedical)
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta1-42 (unknown origin) aggregation assessed as amyloid fibril formation tested after 17 hrs by thioflavin T fluorescence met... |
Bioorg Med Chem Lett 24: 3108-12 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.008
BindingDB Entry DOI: 10.7270/Q2S1842P |
More data for this
Ligand-Target Pair | |
Amyloid-beta precursor protein
(Homo sapiens (Human)) | BDBM7459

(2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIRO Manufacturing Flagship (Biomedical)
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta1-42 (unknown origin) aggregation assessed as amyloid fibril formation tested after 17 hrs by thioflavin T fluorescence met... |
Bioorg Med Chem Lett 24: 3108-12 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.008
BindingDB Entry DOI: 10.7270/Q2S1842P |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304180

(2-(8-Aminooctanamido)-5-(3-aminopropoxy)-N-(4-phen...)Show SMILES NCCCCCCCC(=O)Nc1ccc(OCCCN)cc1C(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C30H38N4O4/c31-19-8-3-1-2-7-12-29(35)34-28-18-17-26(37-21-9-20-32)22-27(28)30(36)33-23-13-15-25(16-14-23)38-24-10-5-4-6-11-24/h4-6,10-11,13-18,22H,1-3,7-9,12,19-21,31-32H2,(H,33,36)(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.20E+4 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304181

(5-(4-Aminobutoxy)-2-(6-aminohexanamido)-N-(4-pheno...)Show SMILES NCCCCCC(=O)Nc1ccc(OCCCCN)cc1C(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C29H36N4O4/c30-18-6-2-5-11-28(34)33-27-17-16-25(36-20-8-7-19-31)21-26(27)29(35)32-22-12-14-24(15-13-22)37-23-9-3-1-4-10-23/h1,3-4,9-10,12-17,21H,2,5-8,11,18-20,30-31H2,(H,32,35)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.90E+4 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304182

(5-(4-Aminobutoxy)-2-(8-aminooctanamido)-N-(4-pheno...)Show SMILES NCCCCCCCC(=O)Nc1ccc(OCCCCN)cc1C(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C31H40N4O4/c32-20-8-3-1-2-7-13-30(36)35-29-19-18-27(38-22-10-9-21-33)23-28(29)31(37)34-24-14-16-26(17-15-24)39-25-11-5-4-6-12-25/h4-6,11-12,14-19,23H,1-3,7-10,13,20-22,32-33H2,(H,34,37)(H,35,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304183

(2-(5-Guanidinopentanamido)-5-(3-guanidinopropoxy)-...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-c1ccc(-[#8]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])cc1-[#6](=O)-[#7]-c1ccc(-[#8]-c2ccccc2)cc1 Show InChI InChI=1S/C29H36N8O4/c30-28(31)34-16-5-4-9-26(38)37-25-15-14-23(40-18-6-17-35-29(32)33)19-24(25)27(39)36-20-10-12-22(13-11-20)41-21-7-2-1-3-8-21/h1-3,7-8,10-15,19H,4-6,9,16-18H2,(H,36,39)(H,37,38)(H4,30,31,34)(H4,32,33,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.16E+5 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304184
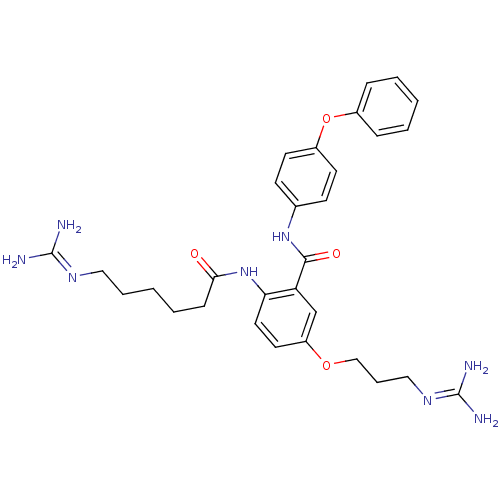
(2-(5-Guanidinohexanamido)-5-(3-guanidinopropoxy)-N...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-c1ccc(-[#8]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])cc1-[#6](=O)-[#7]-c1ccc(-[#8]-c2ccccc2)cc1 Show InChI InChI=1S/C30H38N8O4/c31-29(32)35-17-6-2-5-10-27(39)38-26-16-15-24(41-19-7-18-36-30(33)34)20-25(26)28(40)37-21-11-13-23(14-12-21)42-22-8-3-1-4-9-22/h1,3-4,8-9,11-16,20H,2,5-7,10,17-19H2,(H,37,40)(H,38,39)(H4,31,32,35)(H4,33,34,36) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304185

(2-(5-Guanidino-octanamido)-5-(3-guanidinopropoxy)-...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-c1ccc(-[#8]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])cc1-[#6](=O)-[#7]-c1ccc(-[#8]-c2ccccc2)cc1 Show InChI InChI=1S/C32H42N8O4/c33-31(34)37-19-8-3-1-2-7-12-29(41)40-28-18-17-26(43-21-9-20-38-32(35)36)22-27(28)30(42)39-23-13-15-25(16-14-23)44-24-10-5-4-6-11-24/h4-6,10-11,13-18,22H,1-3,7-9,12,19-21H2,(H,39,42)(H,40,41)(H4,33,34,37)(H4,35,36,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304186
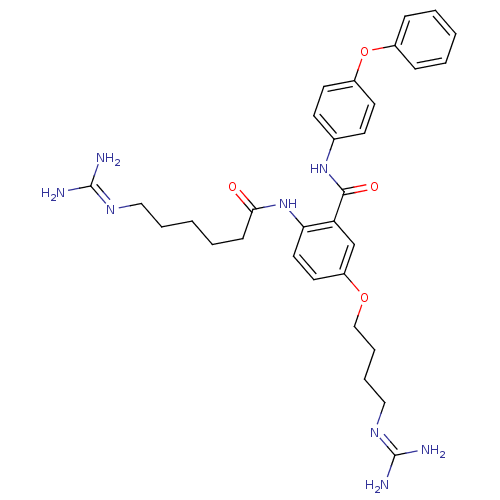
(2-(5-Guanidinohexanamido)-5-(3-guanidinobutoxy)-N-...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-c1ccc(-[#8]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])cc1-[#6](=O)-[#7]-c1ccc(-[#8]-c2ccccc2)cc1 Show InChI InChI=1S/C31H40N8O4/c32-30(33)36-18-6-2-5-11-28(40)39-27-17-16-25(42-20-8-7-19-37-31(34)35)21-26(27)29(41)38-22-12-14-24(15-13-22)43-23-9-3-1-4-10-23/h1,3-4,9-10,12-17,21H,2,5-8,11,18-20H2,(H,38,41)(H,39,40)(H4,32,33,36)(H4,34,35,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304187
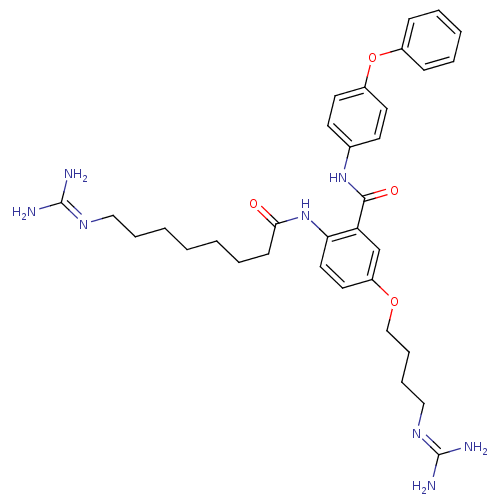
(2-(5-Guanidino-octanamido)-5-(3-guanidinobutoxy)-N...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-c1ccc(-[#8]-[#6]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])cc1-[#6](=O)-[#7]-c1ccc(-[#8]-c2ccccc2)cc1 Show InChI InChI=1S/C33H44N8O4/c34-32(35)38-20-8-3-1-2-7-13-30(42)41-29-19-18-27(44-22-10-9-21-39-33(36)37)23-28(29)31(43)40-24-14-16-26(17-15-24)45-25-11-5-4-6-12-25/h4-6,11-12,14-19,23H,1-3,7-10,13,20-22H2,(H,40,43)(H,41,42)(H4,34,35,38)(H4,36,37,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304188

(2-(8-Guanidinooctanamido)-5-(3-guanidinopropoxy)-N...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-c1ccc(-[#8]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])cc1-[#6](=O)-[#7]-c1ccc(-[#8]-c2ccc(-[#8])cc2)cc1 Show InChI InChI=1S/C32H42N8O5/c33-31(34)37-18-5-3-1-2-4-7-29(42)40-28-17-16-26(44-20-6-19-38-32(35)36)21-27(28)30(43)39-22-8-12-24(13-9-22)45-25-14-10-23(41)11-15-25/h8-17,21,41H,1-7,18-20H2,(H,39,43)(H,40,42)(H4,33,34,37)(H4,35,36,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304189
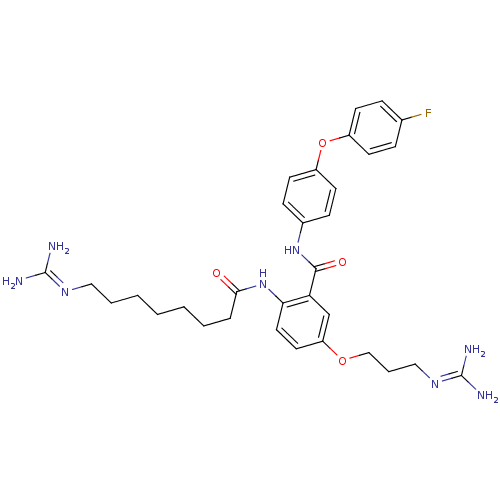
(CHEMBL593380 | N-(4-(4-Fluorophenoxy)phenyl)-2-(8-...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#7]-c1ccc(-[#8]-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])cc1-[#6](=O)-[#7]-c1ccc(-[#8]-c2ccc(F)cc2)cc1 Show InChI InChI=1S/C32H41FN8O4/c33-22-8-12-24(13-9-22)45-25-14-10-23(11-15-25)40-30(43)27-21-26(44-20-6-19-39-32(36)37)16-17-28(27)41-29(42)7-4-2-1-3-5-18-38-31(34)35/h8-17,21H,1-7,18-20H2,(H,40,43)(H,41,42)(H4,34,35,38)(H4,36,37,39) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304190
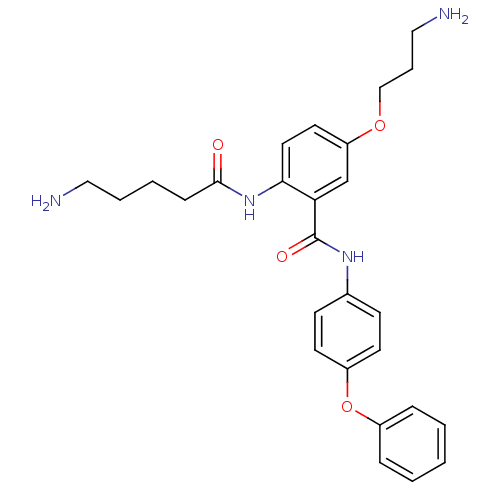
(2-(5-Aminopentanamido)-5-(3-aminopropoxy)-N-(4-phe...)Show SMILES NCCCCC(=O)Nc1ccc(OCCCN)cc1C(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C27H32N4O4/c28-16-5-4-9-26(32)31-25-15-14-23(34-18-6-17-29)19-24(25)27(33)30-20-10-12-22(13-11-20)35-21-7-2-1-3-8-21/h1-3,7-8,10-15,19H,4-6,9,16-18,28-29H2,(H,30,33)(H,31,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Voltage-dependent N-type calcium channel subunit alpha-1B
(Rattus norvegicus (Rat)) | BDBM50304191

(2-(6-Aminohexanamido)-5-(3-aminopropoxy)-N-(4-phen...)Show SMILES NCCCCCC(=O)Nc1ccc(OCCCN)cc1C(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C28H34N4O4/c29-17-6-2-5-10-27(33)32-26-16-15-24(35-19-7-18-30)20-25(26)28(34)31-21-11-13-23(14-12-21)36-22-8-3-1-4-9-22/h1,3-4,8-9,11-16,20H,2,5-7,10,17-19,29-30H2,(H,31,34)(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.07E+5 | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Displacement of [125I]GVIA from Cav2.2 channel in rat brain by scintillation counting |
Bioorg Med Chem 17: 6659-70 (2009)
Article DOI: 10.1016/j.bmc.2009.07.063
BindingDB Entry DOI: 10.7270/Q2K937M3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131554
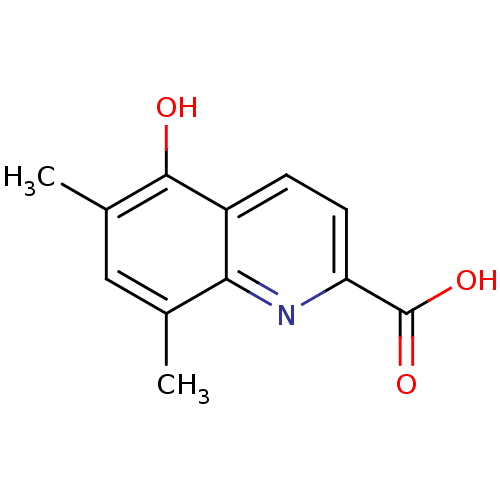
(5-Hydroxy-6,8-dimethyl-quinoline-2-carboxylic acid...)Show InChI InChI=1S/C12H11NO3/c1-6-5-7(2)11(14)8-3-4-9(12(15)16)13-10(6)8/h3-5,14H,1-2H3,(H,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.56E+7 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Protein-tyrosine phosphatase 1B (PTP 1B) site 2 Ligands were identified using [13C]-labeled protein and their Dissociation Constants were determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM48319
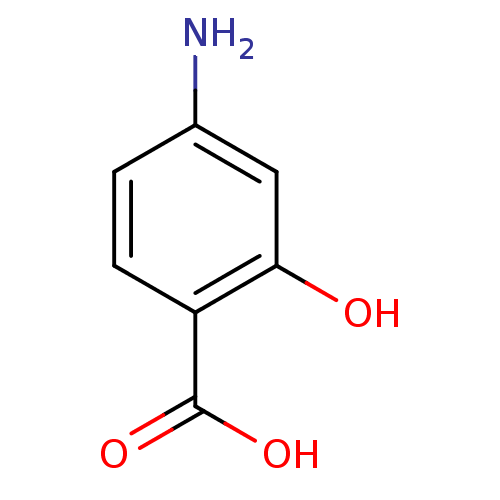
(4-amino-2-hydroxy-benzoic acid | 4-amino-2-hydroxy...)Show InChI InChI=1S/C7H7NO3/c8-4-1-2-5(7(10)11)6(9)3-4/h1-3,9H,8H2,(H,10,11) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.20E+6 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Protein-tyrosine phosphatase 1B (PTP 1B) site 2 Ligands were identified using [13C]-labeled protein and their Dissociation Constants were determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50131549

(4-Amino-3-bromo-5-cyano-2-hydroxy-benzoic acid | C...)Show InChI InChI=1S/C8H5BrN2O3/c9-5-6(11)3(2-10)1-4(7(5)12)8(13)14/h1,12H,11H2,(H,13,14) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 9.10E+5 | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Protein-tyrosine phosphatase 1B (PTP 1B) site 2 Ligands were identified using [13C]-labeled protein and their Dissociation Constants were determined |
J Med Chem 46: 3437-40 (2003)
Article DOI: 10.1021/jm034088d
BindingDB Entry DOI: 10.7270/Q2WW7H1Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data