

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50261816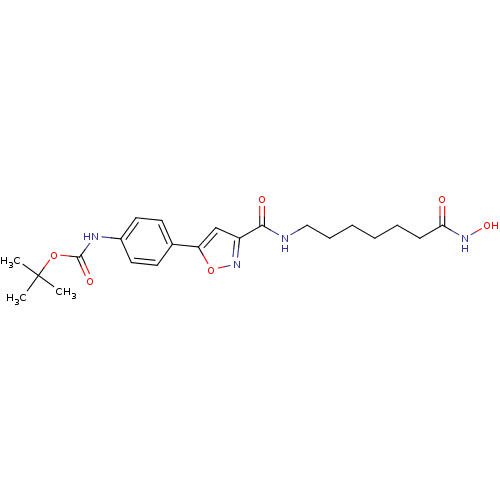 (CHEMBL511749 | tert-butyl 4-(3-((7-(hydroxyamino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 17 hrs | J Med Chem 51: 4370-3 (2008) Article DOI: 10.1021/jm8002894 BindingDB Entry DOI: 10.7270/Q27W6C11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50267760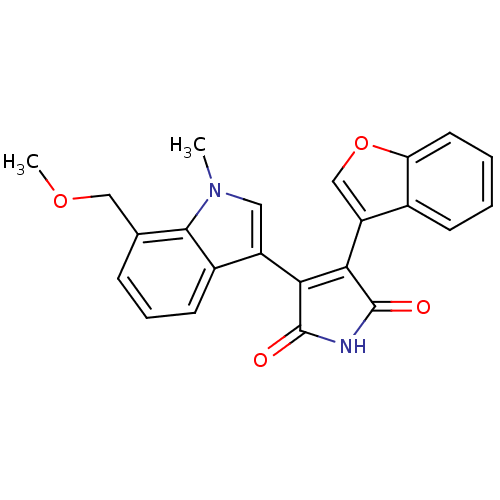 (3-Benzofuran-3-yl-4-(7-methoxymethyl-1-methyl-1H-i...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human GSK3-beta by scintillation counting | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50267461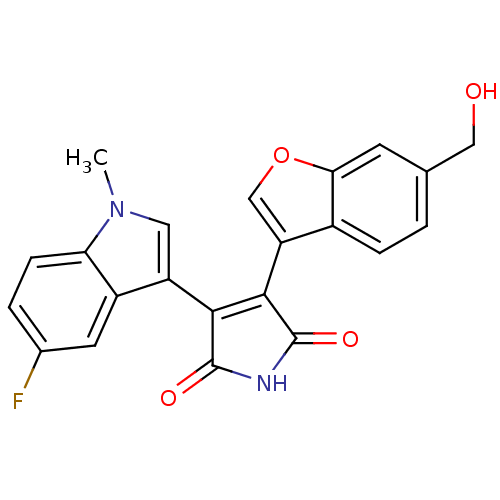 (3-(5-Fluoro-1-methyl-1H-indol-3-yl)-4-(6-hydroxyme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human GSK3-beta by scintillation counting | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50261816 (CHEMBL511749 | tert-butyl 4-(3-((7-(hydroxyamino)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) after 17 hrs | J Med Chem 51: 4370-3 (2008) Article DOI: 10.1021/jm8002894 BindingDB Entry DOI: 10.7270/Q27W6C11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase Chk1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of CHK1 | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50267607 (3-(5-Bromo-1-methyl-1H-indol-3-yl)-4-(6-hydroxymet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human GSK3-beta by scintillation counting | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RAC-gamma serine/threonine-protein kinase (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of AKT3 | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50267800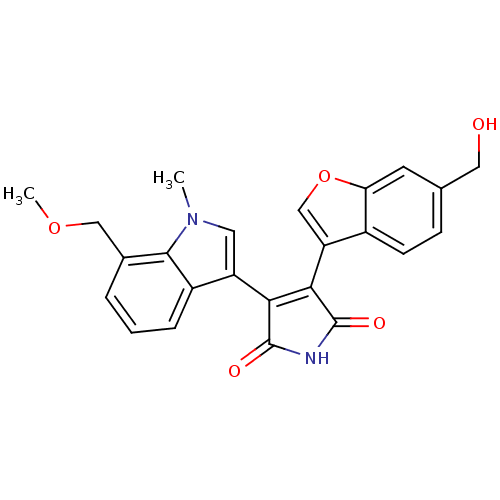 (3-(6-Hydroxymethylbenzofuran-3-yl)-4-(7-methoxymet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human GSK3-beta by scintillation counting | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50267523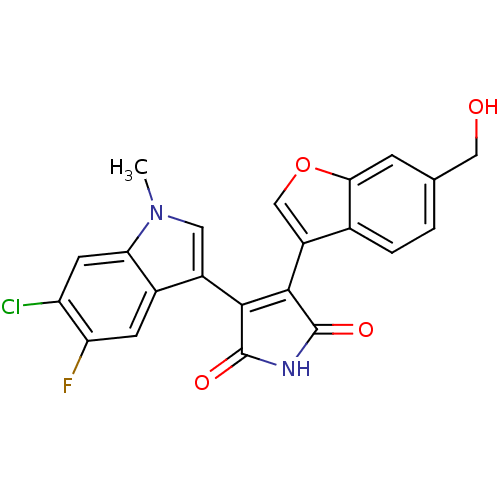 (3-(6-Chloro-5-fluoro-1-methyl-1H-indol-3-yl)-4-(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human GSK3-beta by scintillation counting | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC6 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 17 hrs | J Med Chem 51: 4370-3 (2008) Article DOI: 10.1021/jm8002894 BindingDB Entry DOI: 10.7270/Q27W6C11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50312620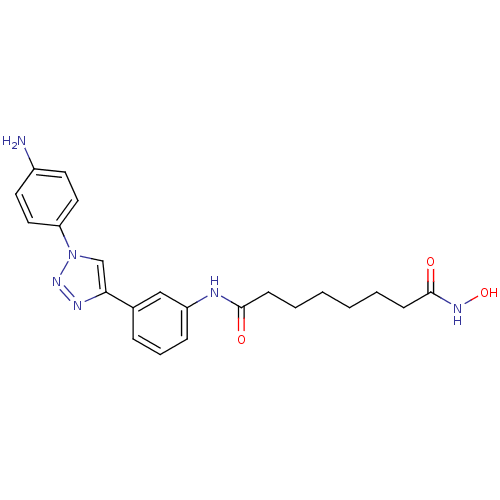 (CHEMBL1094708 | Octanedioic Acid-{3-[1-(3-Aminophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.01 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC1 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50267802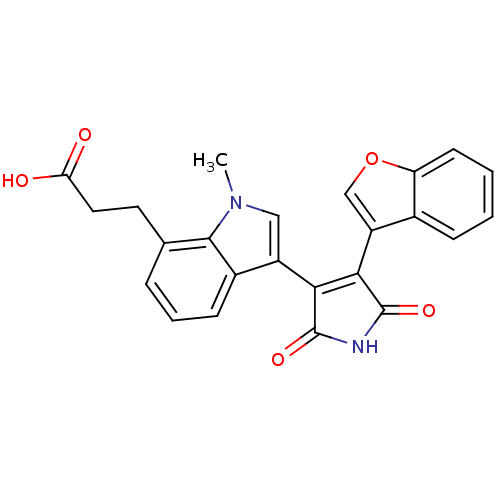 (3-[3-(4-Benzofuran-3-yl-2,5-dioxo-2,5-dihydro-1H-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human GSK3-beta by scintillation counting | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24347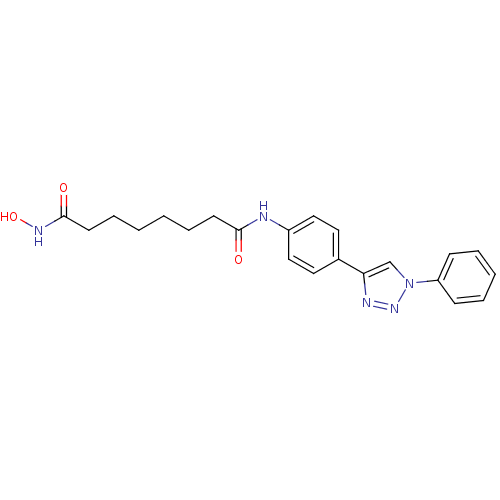 (N-hydroxy-N'-[4-(1-phenyl-1H-1,2,3-triazol-4-yl)ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 8 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.38 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC8 (unknown origin) after 17 hrs | J Med Chem 51: 4370-3 (2008) Article DOI: 10.1021/jm8002894 BindingDB Entry DOI: 10.7270/Q27W6C11 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50312621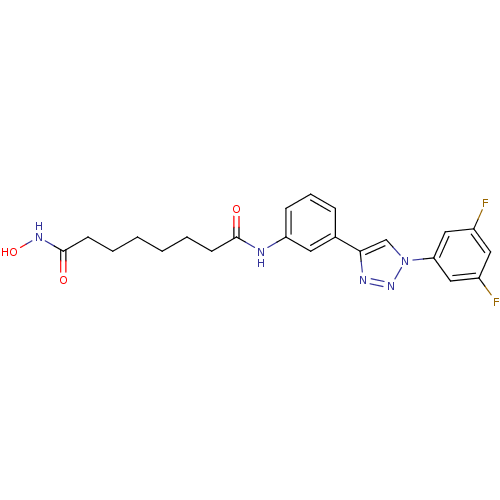 (CHEMBL1091815 | Octanedioic Acid-{3-[1-(3,5-Difluo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC3 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of CDK2/Cyclin A | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50312620 (CHEMBL1094708 | Octanedioic Acid-{3-[1-(3-Aminophe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC3 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50312622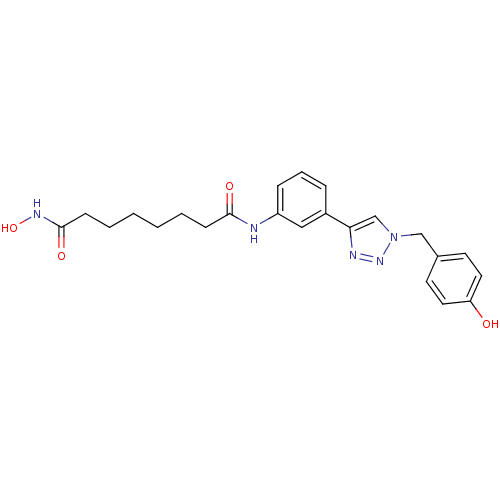 (CHEMBL1088735 | Octanedioic Acid Hydroxyamide-{3-[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC3 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50312621 (CHEMBL1091815 | Octanedioic Acid-{3-[1-(3,5-Difluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC6 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24346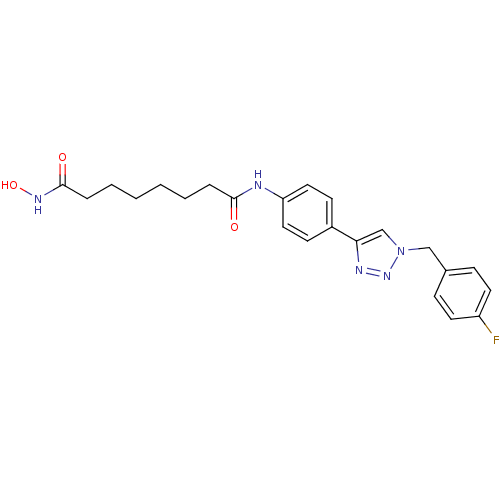 (N-(4-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24352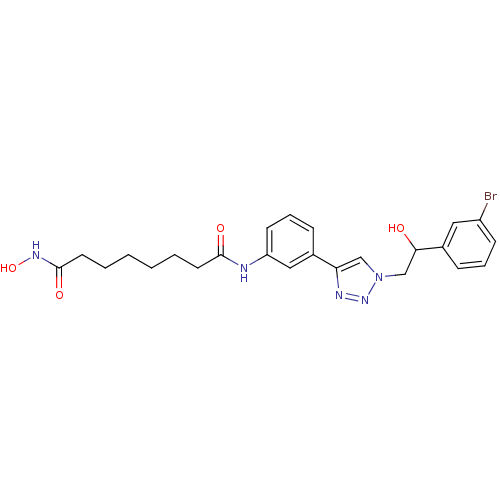 (N-(3-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50312629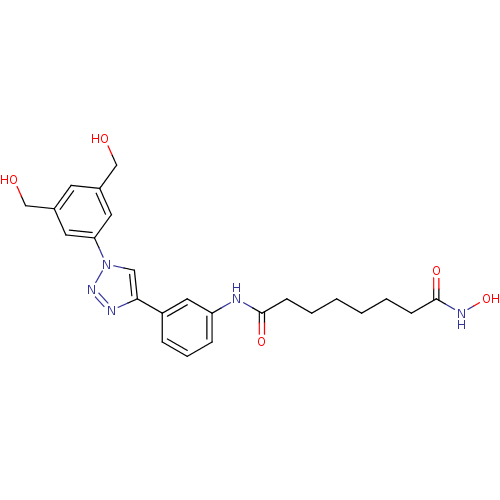 (CHEMBL1088736 | Octanedioic Acid-{3-[1-(3,5-Bis(hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC6 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC3 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC3 (unknown origin) after 17 hrs | J Med Chem 51: 4370-3 (2008) Article DOI: 10.1021/jm8002894 BindingDB Entry DOI: 10.7270/Q27W6C11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM19130 ((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50312624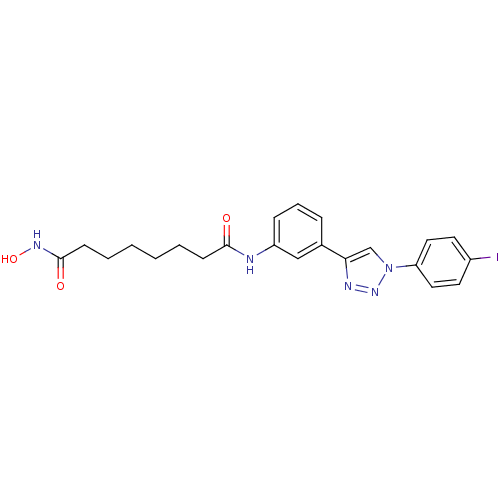 (CHEMBL1091475 | Octanedioic Acid Hydroxyamide-{3-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC6 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50312620 (CHEMBL1094708 | Octanedioic Acid-{3-[1-(3-Aminophe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC6 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50261753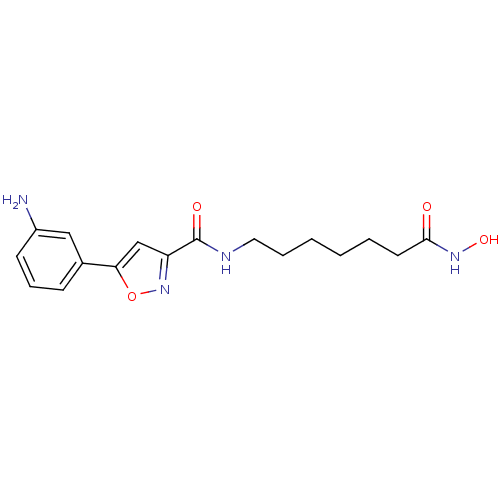 (5-(3-aminophenyl)-N-(7-(hydroxyamino)-7-oxoheptyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.54 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC6 (unknown origin) after 17 hrs | J Med Chem 51: 4370-3 (2008) Article DOI: 10.1021/jm8002894 BindingDB Entry DOI: 10.7270/Q27W6C11 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24350 (N-(3-{1-[(4-fluorophenyl)methyl]-1H-1,2,3-triazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM24352 (N-(3-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50312628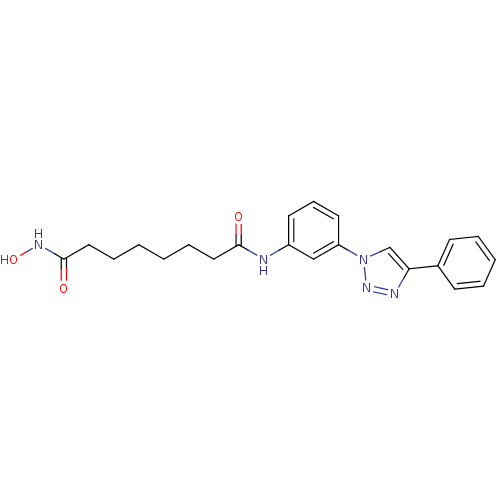 (CHEMBL1094709 | Octanedioic Acid Hydroxyamide-[3-(...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC3 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24360 (N-(3-{1-[2-(3-bromophenyl)-2-hydroxyethyl]-1H-1,2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50312626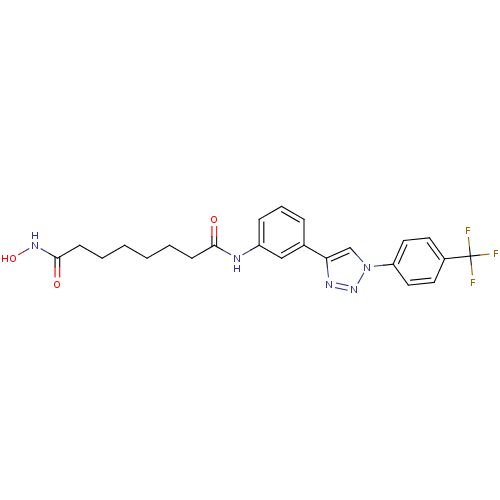 (CHEMBL1088734 | Octanedioic Acid Hydroxyamide-{3-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC6 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50258646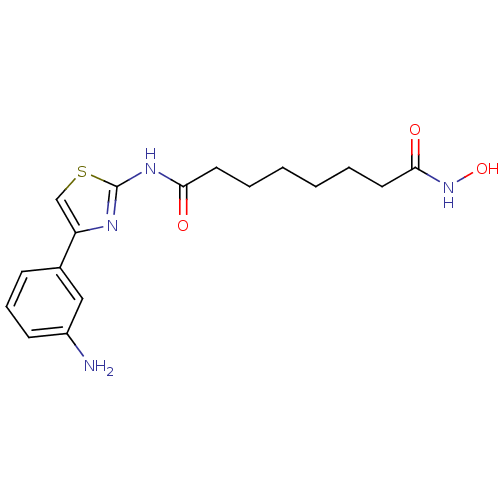 (CHEMBL468935 | N1-(4-(3-aminophenyl)thiazol-2-yl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 | Bioorg Med Chem Lett 19: 3023-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.058 BindingDB Entry DOI: 10.7270/Q2833RXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50258645 (CHEMBL511212 | N1-hydroxy-N8-(4-phenylthiazol-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC1 | Bioorg Med Chem Lett 19: 3023-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.058 BindingDB Entry DOI: 10.7270/Q2833RXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50312624 (CHEMBL1091475 | Octanedioic Acid Hydroxyamide-{3-[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC3 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50258645 (CHEMBL511212 | N1-hydroxy-N8-(4-phenylthiazol-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of human recombinant HDAC6 | Bioorg Med Chem Lett 19: 3023-6 (2009) Article DOI: 10.1016/j.bmcl.2009.04.058 BindingDB Entry DOI: 10.7270/Q2833RXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 1 (Homo sapiens (Human)) | BDBM50312622 (CHEMBL1088735 | Octanedioic Acid Hydroxyamide-{3-[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.04 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC1 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24349 (N-[3-(1-benzyl-1H-1,2,3-triazol-4-yl)phenyl]-N'-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24353 (N-[4-(1-benzyl-1H-1,2,3-triazol-5-yl)phenyl]-N'-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50312628 (CHEMBL1094709 | Octanedioic Acid Hydroxyamide-[3-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC6 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM50312627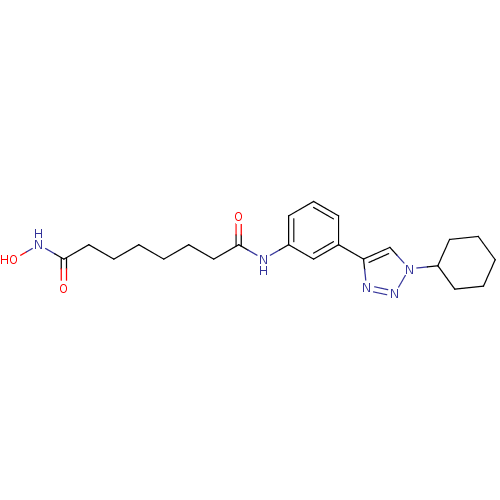 (CHEMBL1094707 | Octanedioic Acid-[3-(1-Cyclohexyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC6 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 9 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of CDK9/cyclin T1 | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 6 (Homo sapiens (Human)) | BDBM24357 (N-[3-(1-benzyl-1H-1,2,3-triazol-5-yl)phenyl]-N'-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago | Assay Description The inhibitory effects of compounds on histone deacetylase (HDAC) activity were determined using a fluorescence-based assay with electrophoretic sepa... | J Med Chem 51: 3437-48 (2008) Article DOI: 10.1021/jm701606b BindingDB Entry DOI: 10.7270/Q2ZC815S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM2579 ((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of CDK1/Cyclin B | J Med Chem 52: 1853-63 (2009) Article DOI: 10.1021/jm801317h BindingDB Entry DOI: 10.7270/Q24M94FZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50312629 (CHEMBL1088736 | Octanedioic Acid-{3-[1-(3,5-Bis(hy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC3 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50312623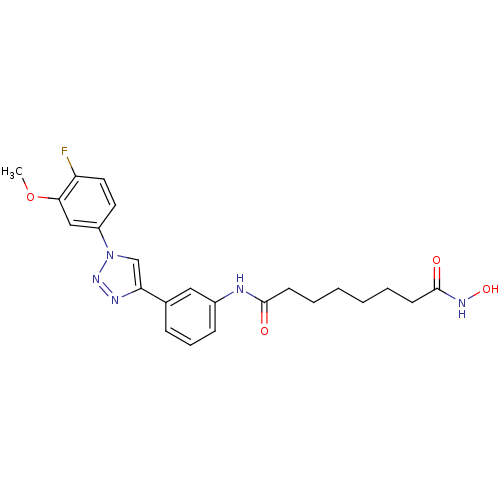 (CHEMBL1076939 | Octanedioic Acid-{3-[1-(3-Fluoro-4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.39 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC3 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 3 (Homo sapiens (Human)) | BDBM50312626 (CHEMBL1088734 | Octanedioic Acid Hydroxyamide-{3-[...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago Curated by ChEMBL | Assay Description Inhibition of HDAC3 assessed as blockade of decorboxylation of carboxyfluorescein labeled acetylated peptide substrate after 17 hrs | J Med Chem 53: 1347-56 (2010) Article DOI: 10.1021/jm901667k BindingDB Entry DOI: 10.7270/Q29S1R52 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 422 total ) | Next | Last >> |