

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176411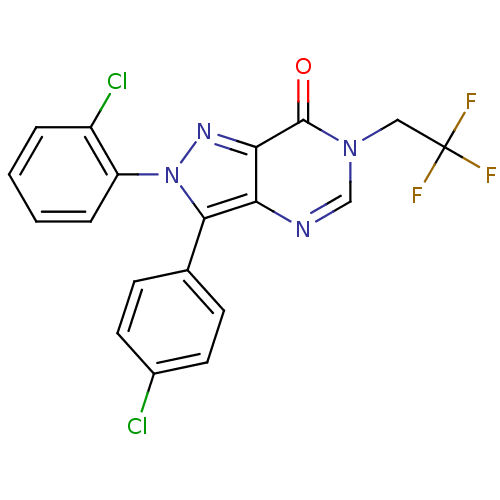 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176407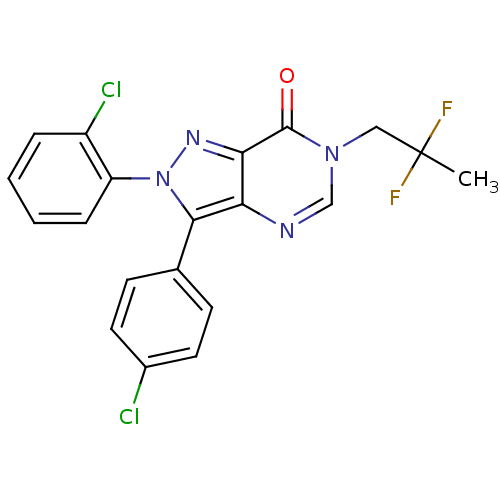 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2-diflu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027094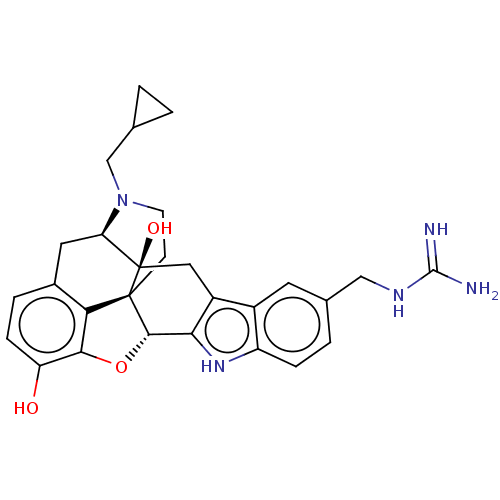 (CHEMBL2112374) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176407 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2-diflu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122859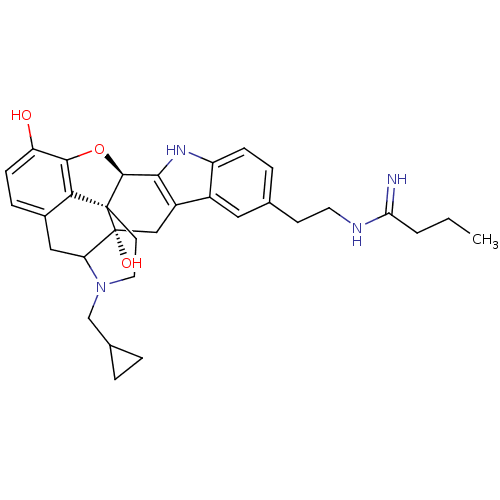 (22-cyclopropylmethyl-7-[2-(1-iminobutylamino)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122869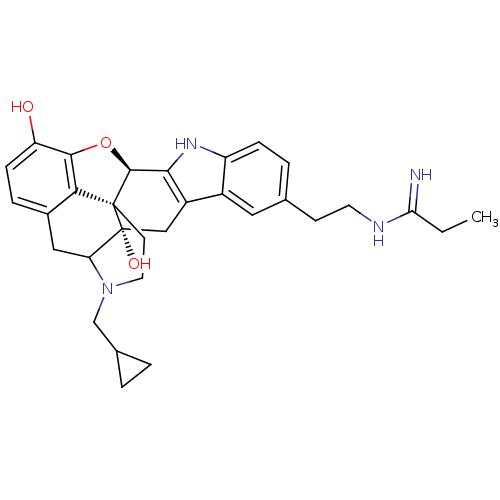 (22-cyclopropylmethyl-7-[2-(1-iminopropylamino)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122862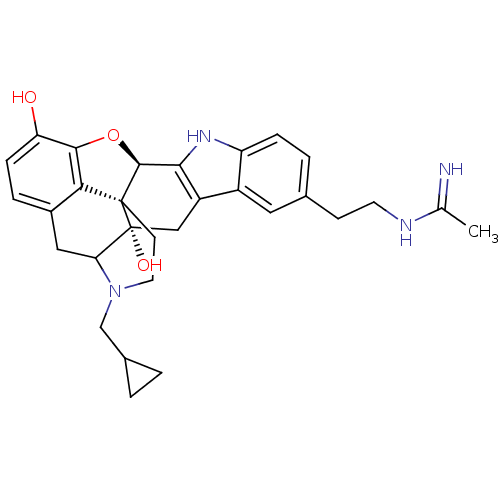 (22-cyclopropylmethyl-7-[2-(1-iminoethylamino)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176411 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122863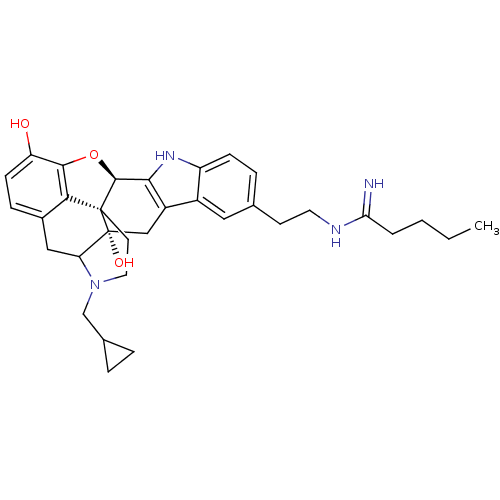 (22-cyclopropylmethyl-7-[2-(1-iminopentylamino)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027093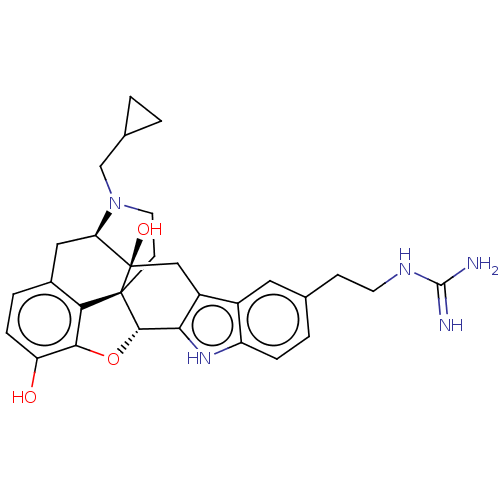 (CHEMBL2112373) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29061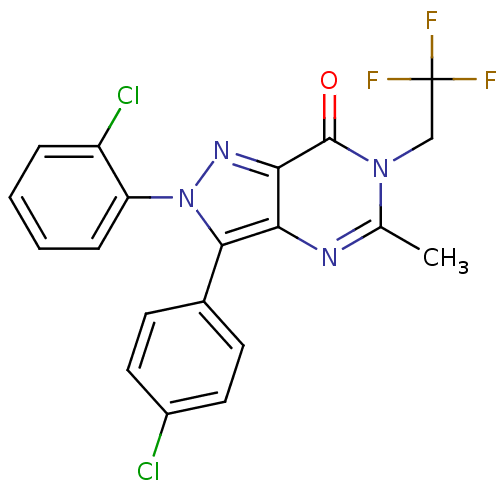 (CHEMBL201602 | pyrazolopyrimidinone-based antagoni...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | -53.5 | n/a | n/a | 1 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29061 (CHEMBL201602 | pyrazolopyrimidinone-based antagoni...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176403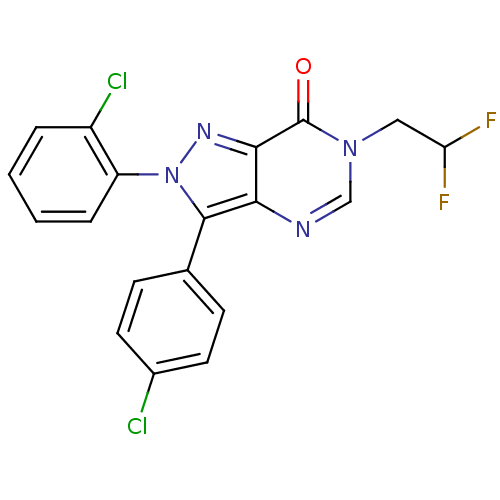 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2-diflu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50136119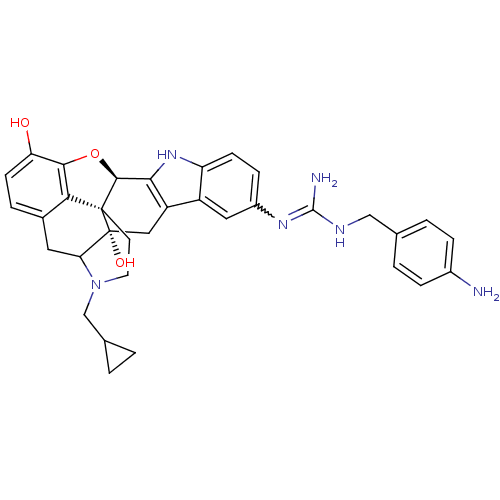 (7-[4-aminobenzylamino(imino)methylamino]-22-cyclop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.650 | -53.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50136114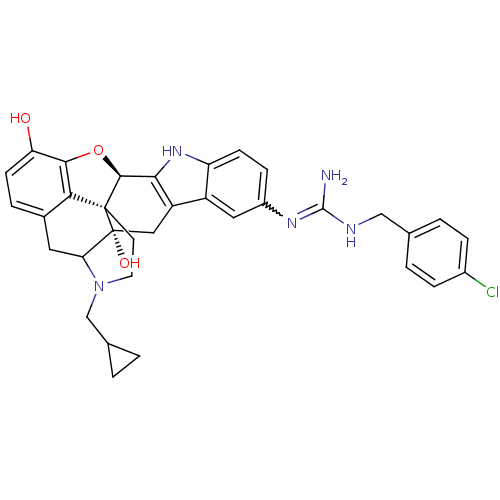 (7-[4-chlorobenzylamino(imino)methylamino]-22-cyclo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122855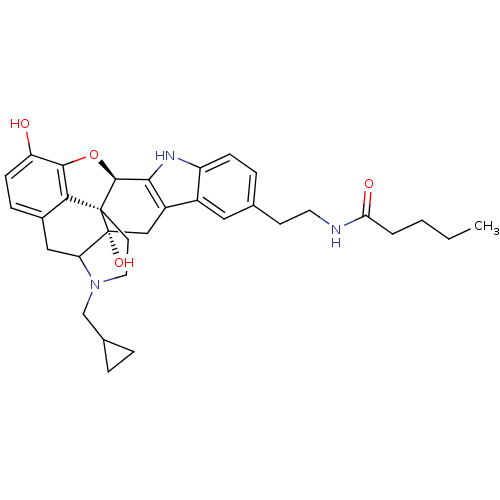 (1N-{2-[22-cyclopropylmethyl-2,16-dihydroxy-14-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29070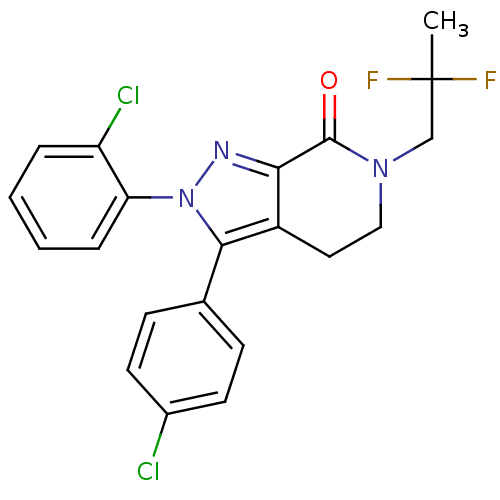 (lactam-based compound, 12i) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.700 | -53.1 | n/a | n/a | 0.800 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM27337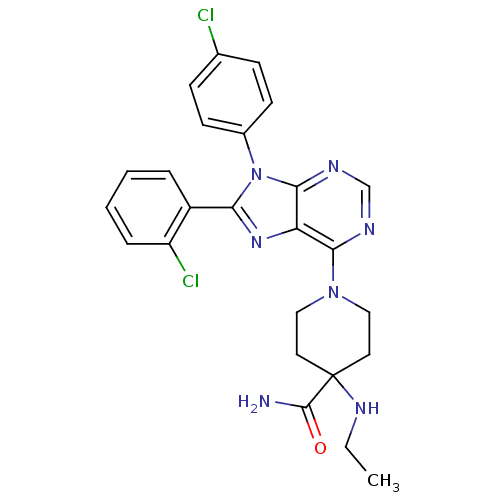 (1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50534464 (CHEMBL4483714) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122852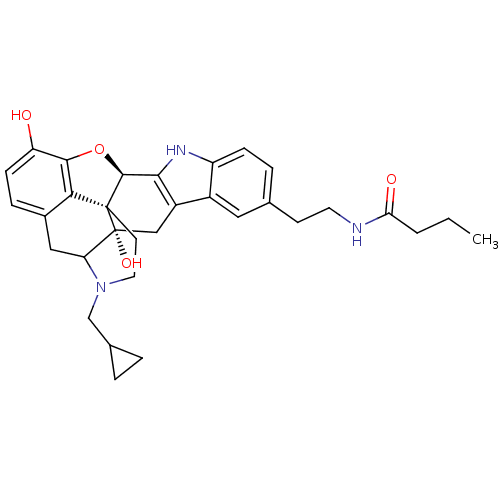 (1N-{2-[22-cyclopropylmethyl-2,16-dihydroxy-14-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50534464 (CHEMBL4483714) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.859 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50136122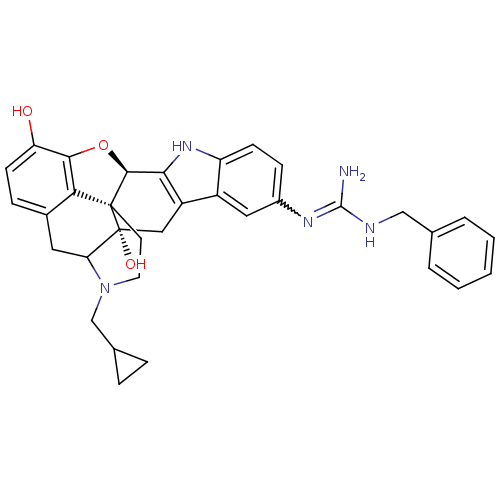 (7-benzylamino(imino)methylamino-22-cyclopropylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.900 | -52.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 234-7 (2009) Article DOI: 10.1021/jm8012932 BindingDB Entry DOI: 10.7270/Q2CN7276 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50136111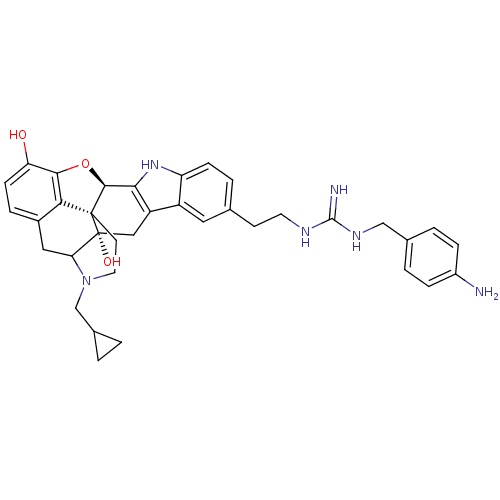 (7-{2-[4-aminobenzylamino(imino)methylamino]ethyl}-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50534476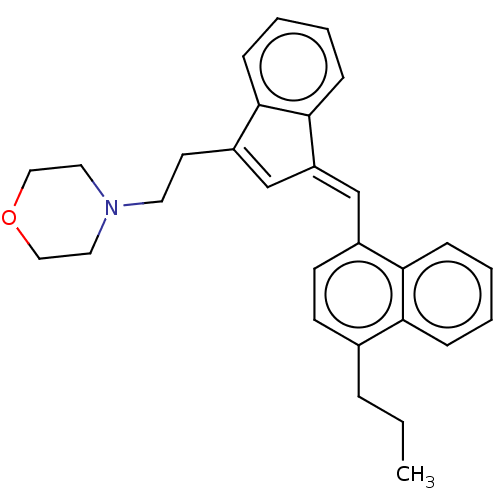 (CHEMBL4443660) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176402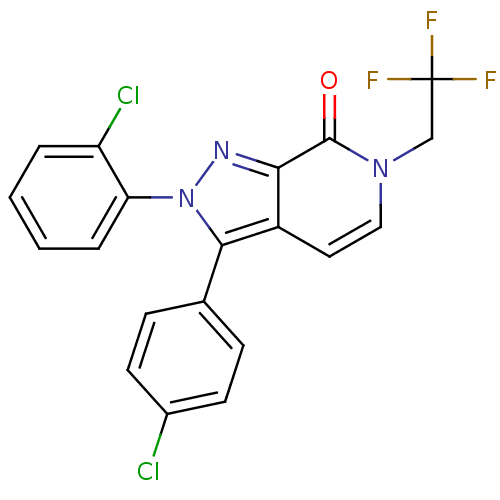 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29075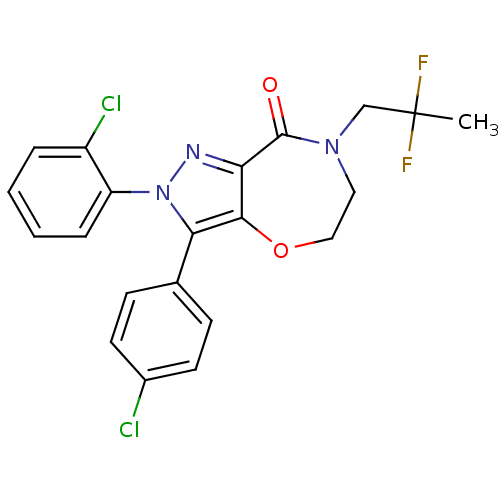 (PF-514273) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | -52.2 | n/a | n/a | 0.820 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176418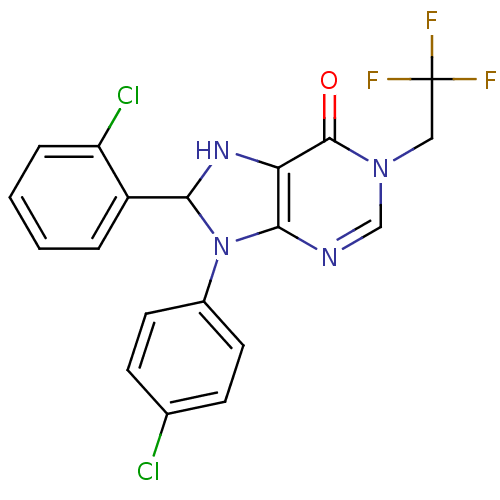 (8-(2-chlorophenyl)-9-(4-chlorophenyl)-1-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29061 (CHEMBL201602 | pyrazolopyrimidinone-based antagoni...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176403 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2-diflu...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176402 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Functional activity at human CB1 receptor transfected in CHOK1 cells by [35SGTP]gammaS assay | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176416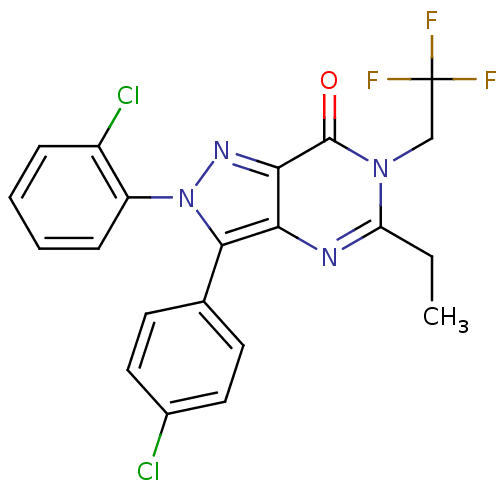 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-5-ethyl-6-(2...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176418 (8-(2-chlorophenyl)-9-(4-chlorophenyl)-1-(2,2,2-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50534476 (CHEMBL4443660) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor | J Med Chem 59: 7525-43 (2016) Article DOI: 10.1021/acs.jmedchem.6b00516 BindingDB Entry DOI: 10.7270/Q2862KX2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29073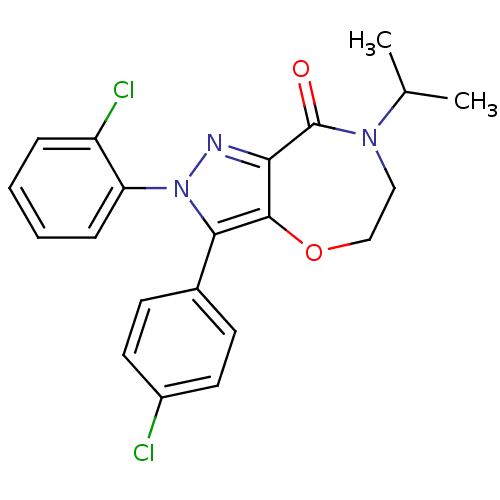 (ether-based lactam, 19b) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | -51.6 | n/a | n/a | 4.80 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122865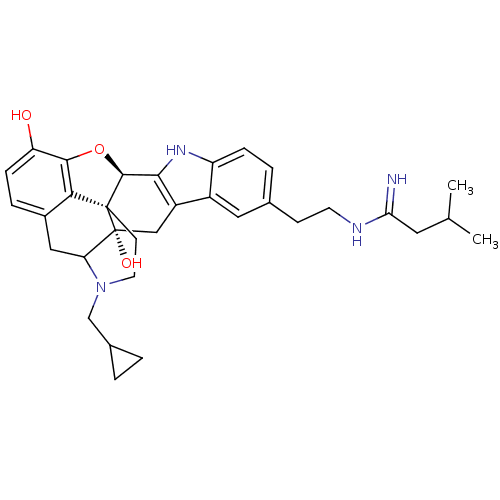 (22-cyclopropylmethyl-7-[2-(1-imino-3-methylbutylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50136116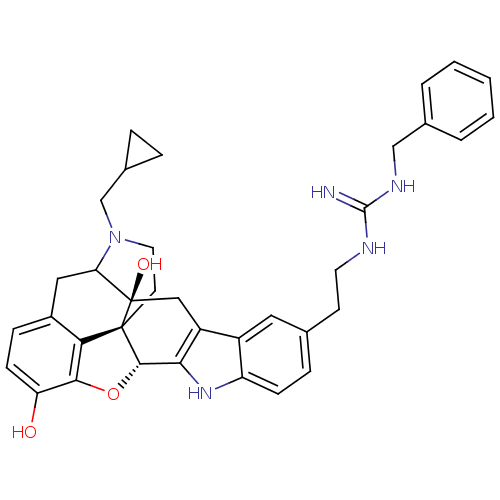 (7-[2-benzylamino(imino)methylaminoethyl]-22-cyclop...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29076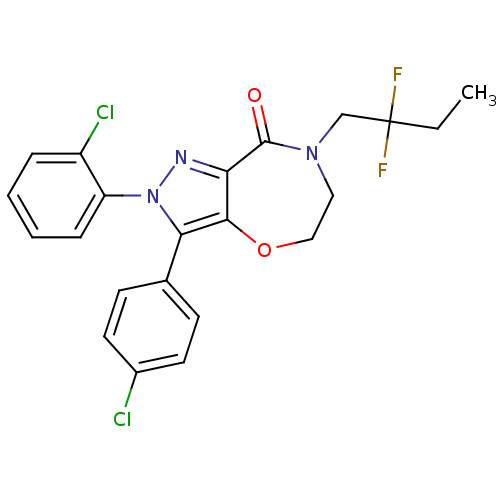 (ether-based lactam, 19e) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | -51.4 | n/a | n/a | 0.700 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122856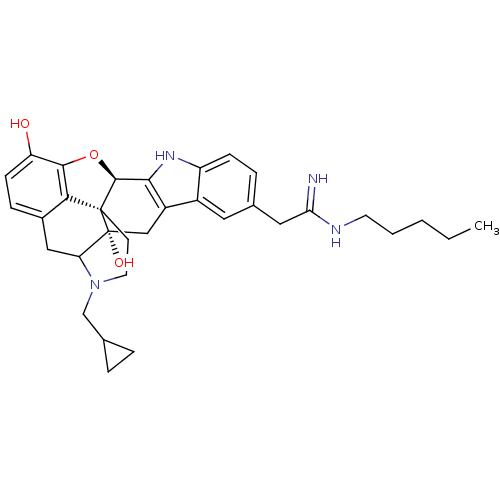 (22-cyclopropylmethyl-7-(2-imino-2-pentylaminoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122864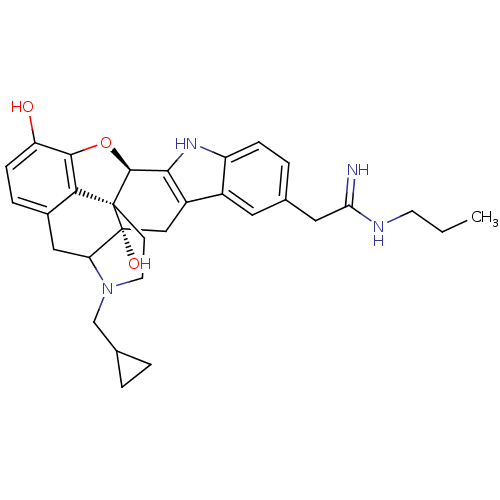 (22-cyclopropylmethyl-7-(2-imino-2-propylaminoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122860 (1N-{2-[22-cyclopropylmethyl-2,16-dihydroxy-14-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50136113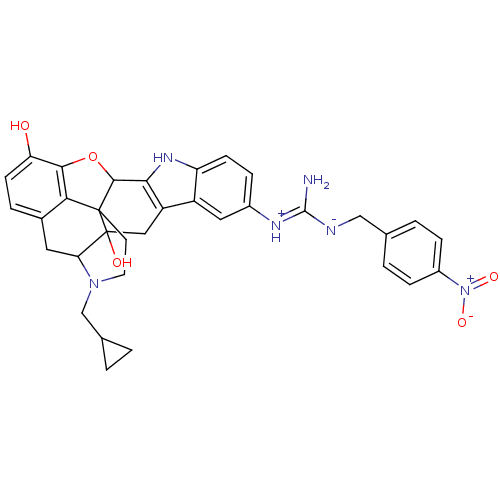 (22-cyclopropylmethyl-7-imino(4-nitrobenzylamino)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Binding affinity against cloned human Opioid receptor kappa 1 transfected into chinese hamster ovary cells using [3H]U-69593 as radioligand | J Med Chem 46: 5505-11 (2003) Article DOI: 10.1021/jm0309203 BindingDB Entry DOI: 10.7270/Q2V69K9X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29069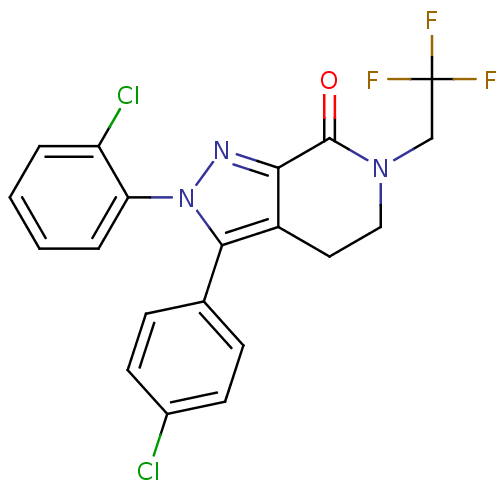 (lactam-based compound, 12h) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.70 | -50.9 | n/a | n/a | 3.10 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.80 | -50.7 | n/a | n/a | 1.60 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50176415 (2-(2-chlorophenyl)-3-(4-chlorophenyl)-6-(3,3,3-tri...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development-Groton Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SR141716 from human CB1 receptor transfected in HEK293 cells | Bioorg Med Chem Lett 16: 731-6 (2005) Article DOI: 10.1016/j.bmcl.2005.10.019 BindingDB Entry DOI: 10.7270/Q2TM79PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM29074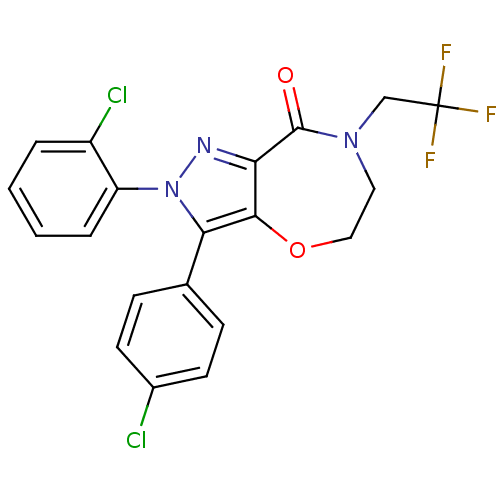 (ether-based lactam, 19c) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.90 | -50.6 | n/a | n/a | 1.5 | n/a | n/a | 7.4 | 30 |
Pfizer | Assay Description IC50 values for test compounds were determined from nonlinear regression analysis of data collected from ligand binding experiments. The inhibition c... | J Med Chem 52: 2652-5 (2009) Article DOI: 10.1021/jm900255t BindingDB Entry DOI: 10.7270/Q2F47MGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 837 total ) | Next | Last >> |