

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-4 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Binding affinity against nicotinic acetylcholine receptor using [3H]epibatidine as radioligand in rat brain tissue | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor; alpha2/beta4 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.0910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Agonistic potency against nicotinic acetylcholine receptor alpha3-beta4 | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50049757 (()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Binding affinity of the compound was determined against Nicotinic acetylcholine receptor using [3H]-(-)-nicotine radioligand | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50137787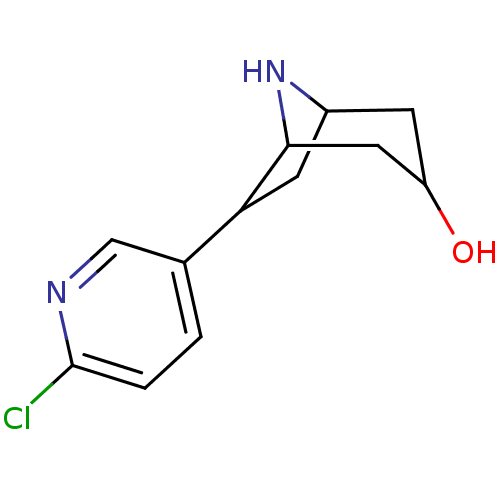 (6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Binding affinity against nicotinic acetylcholine receptor using [3H]epibatidine as radioligand in rat brain tissue | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor; alpha2/beta4 (Homo sapiens (Human)) | BDBM50137787 (6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description In vitro ability to displace [3H]-(-)-cytisine binding to whole rat brain membranes at Nicotinic acetylcholine receptor alpha4-beta2 | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-4 (Homo sapiens (Human)) | BDBM50137787 (6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Binding affinity against nicotinic acetylcholine receptor alpha2-beta4 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM50137787 (6-(6-Chloro-pyridin-3-yl)-8-aza-bicyclo[3.2.1]octa...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Binding affinity against nicotinic acetylcholine receptor alpha4-beta2 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor; alpha2/beta4 (Homo sapiens (Human)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PubMed | 103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Binding affinity against nicotinic acetylcholine receptor alpha2-beta4 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-4 (Homo sapiens (Human)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Binding affinity against nicotinic acetylcholine receptor alpha4-beta4 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-3/beta-4 (Homo sapiens (Human)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Wollongong Curated by ChEMBL | Assay Description Binding affinity against nicotinic acetylcholine receptor alpha3-beta4 using [3H]epibatidine as radioligand expressed in HEK293 cells or tsA cells | Bioorg Med Chem Lett 14: 271-3 (2003) BindingDB Entry DOI: 10.7270/Q2MC8ZD0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-lactamase TEM (Escherichia coli) | BDBM50505403 (CHEMBL4533632) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant bacterial class A serine beta-lactamase TEM-1 expressed in Escherichia coli assessed as reduction in breakdown of cephalosp... | J Med Chem 62: 8544-8556 (2019) Article DOI: 10.1021/acs.jmedchem.9b00911 BindingDB Entry DOI: 10.7270/Q2P272DC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271950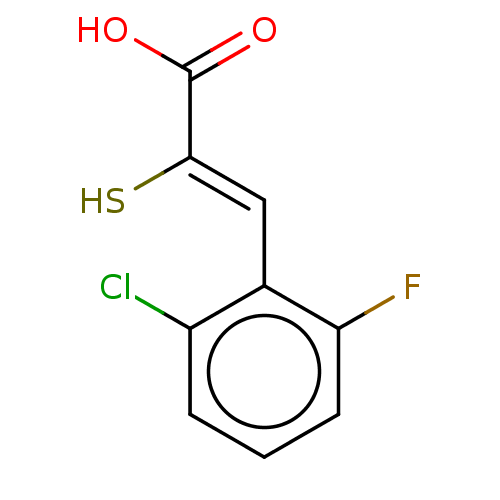 (CHEMBL4127821) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271847 (CHEMBL4127736) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271833 (CHEMBL3792857) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271878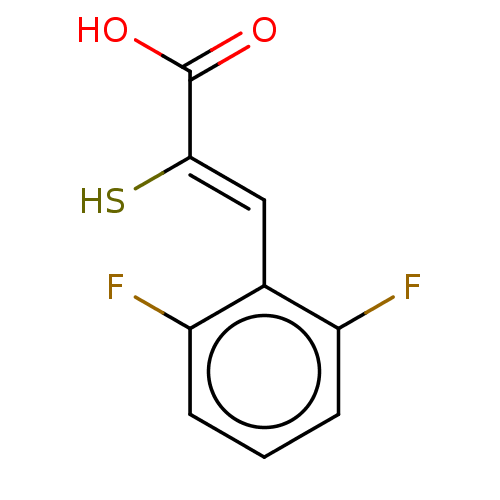 (CHEMBL4125829) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271834 (CHEMBL3234727) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidoglycan D,D-transpeptidase FtsI (Pseudomonas aeruginosa) | BDBM50240426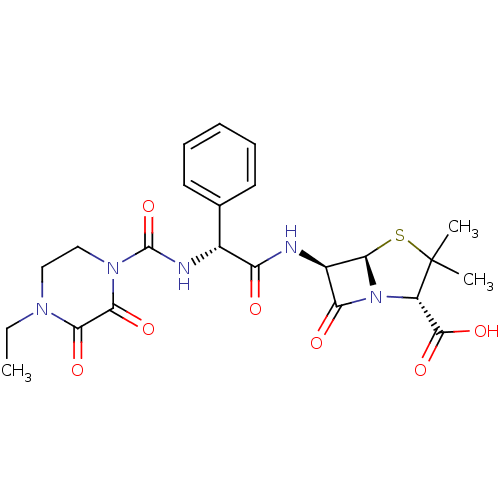 ((2S,5R,6R)-6-{[(2R)-2-{[(4-ethyl-2,3-dioxopiperazi...) | PDB MMDB KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 166 | n/a | n/a | n/a | n/a | 7.2 | 25 |
University of Oxford | Assay Description An appropriate amount of enzyme (4uM) was pre-incubated with the (5R)- or 5(S)-penicilloic acid in the assay buffer (50mM HEPES-NaOH buffer (pH 7.2) ... | ACS Chem Biol 8: 2112-6 (2013) Article DOI: 10.1021/cb400200h BindingDB Entry DOI: 10.7270/Q2VT1QRK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271963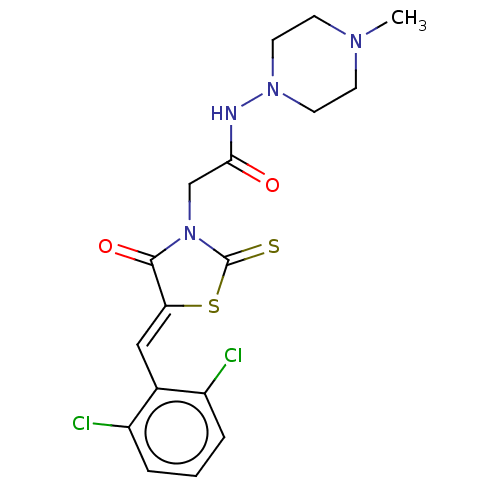 (CHEMBL1559342) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271893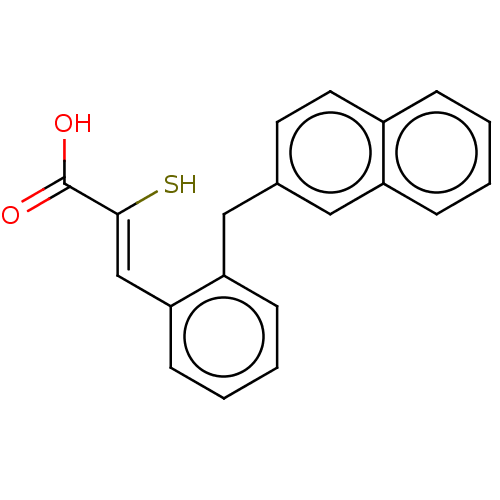 (CHEMBL4128221) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271889 (CHEMBL4129411) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Aeromonas hydrophila) | BDBM50271833 (CHEMBL3792857) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Aeromonas hydrophila CphA using fluorogenic cephalosporin as substrate | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50247639 (CHEMBL4068716) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271952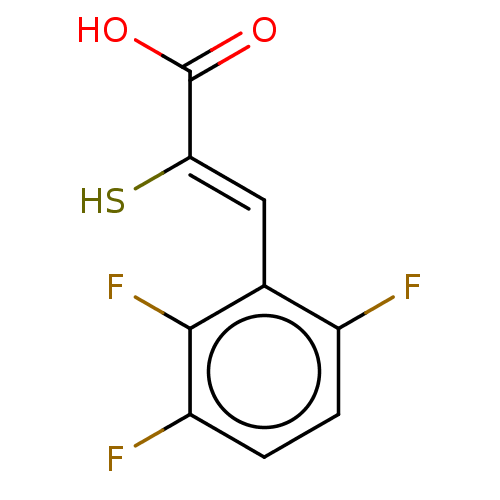 (CHEMBL4126465) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271891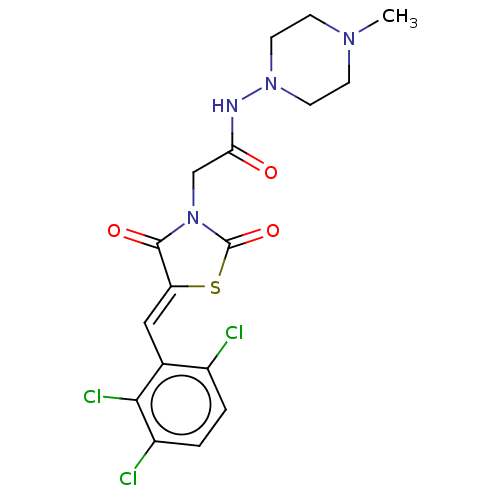 (CHEMBL4129450) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 690 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 1 min using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271835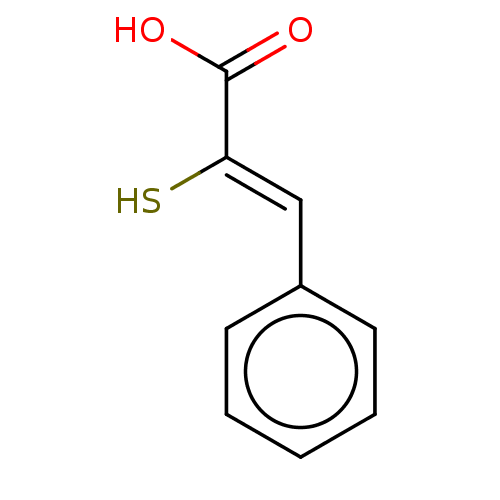 (CHEMBL4095898) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271824 (CHEMBL4129233) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 710 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 10 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 25 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 15 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 20 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271798 (CHEMBL3221923) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271868 (CHEMBL4125695) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271867 (CHEMBL4128976) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271836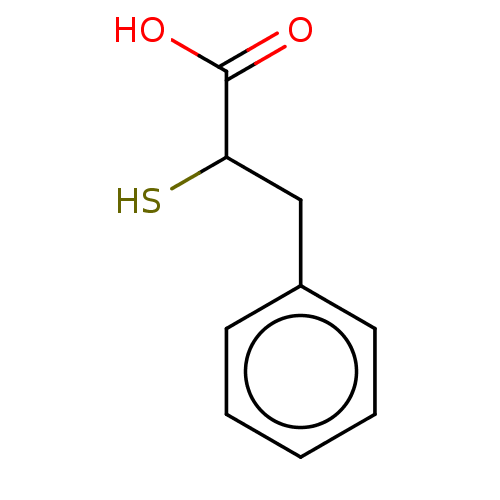 (CHEMBL4126979) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271876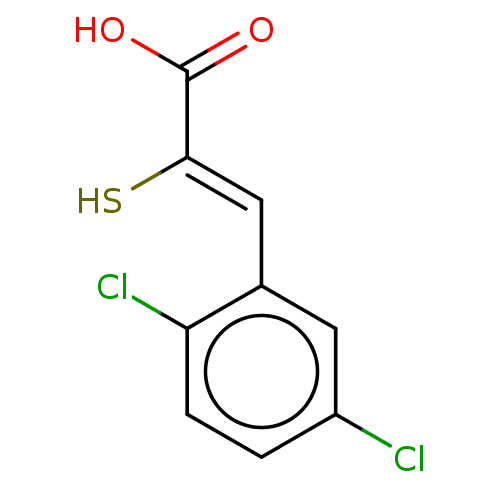 (CHEMBL4125965) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271800 (CHEMBL4129222) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Aeromonas hydrophila) | BDBM50271835 (CHEMBL4095898) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of Aeromonas hydrophila CphA using fluorogenic cephalosporin as substrate | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM50028968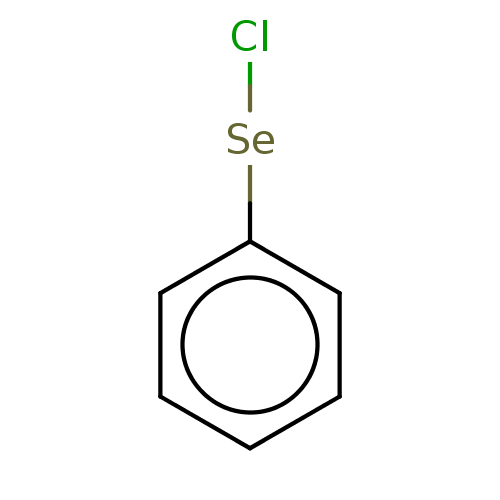 (CHEMBL3335712) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 25 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM50028971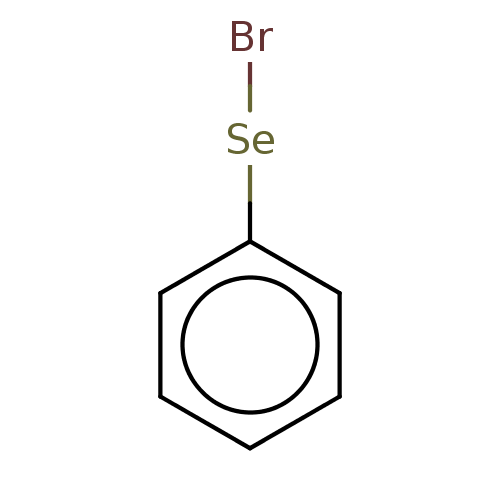 (CHEMBL3335713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 25 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271915 (CHEMBL4129039) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM50028972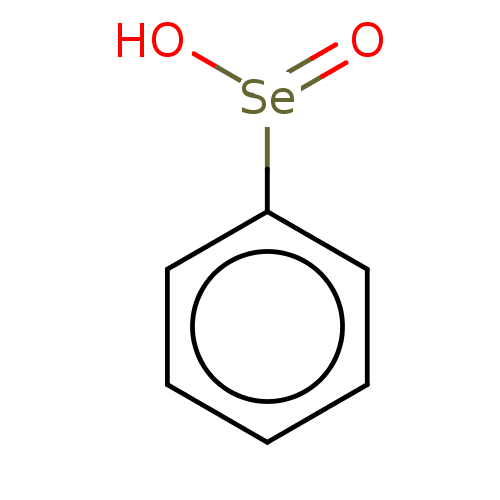 (Benzene Selenoic Acid) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 25 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM50028968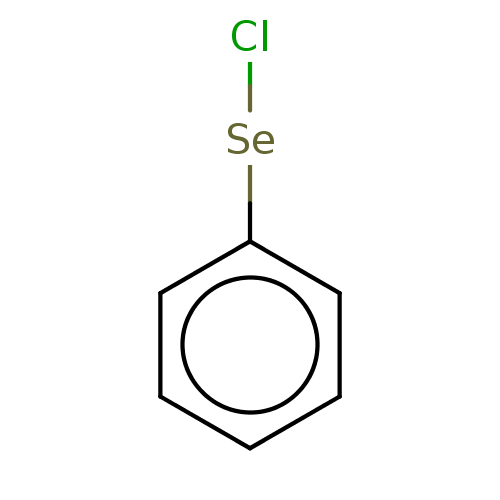 (CHEMBL3335712) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 20 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271825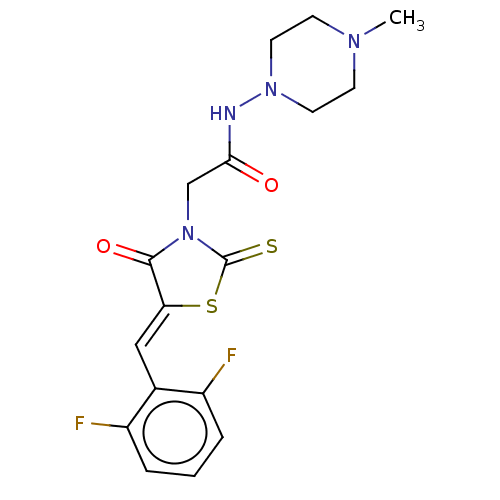 (CHEMBL4127204) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM50028971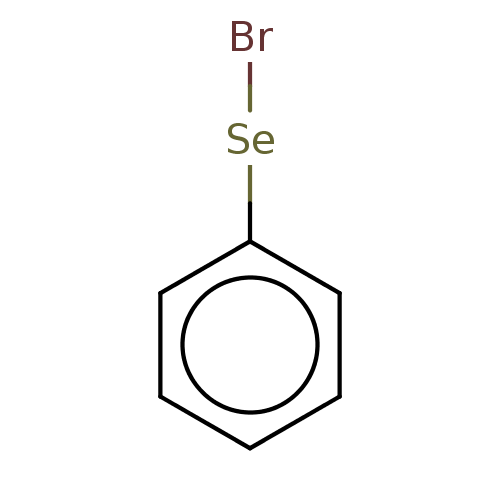 (CHEMBL3335713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 20 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metallo-beta-lactamase type 2 (Bacillus cereus) | BDBM50271802 (CHEMBL4126927) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of recombinant Bacillus cereus BC2 expressed in Escherichia coli BL21 (DE3) cells using FC4-FC5 as substrate by fluorescence-based assay | Bioorg Med Chem 26: 2928-2936 (2018) Article DOI: 10.1016/j.bmc.2018.02.043 BindingDB Entry DOI: 10.7270/Q2M61NRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM50028968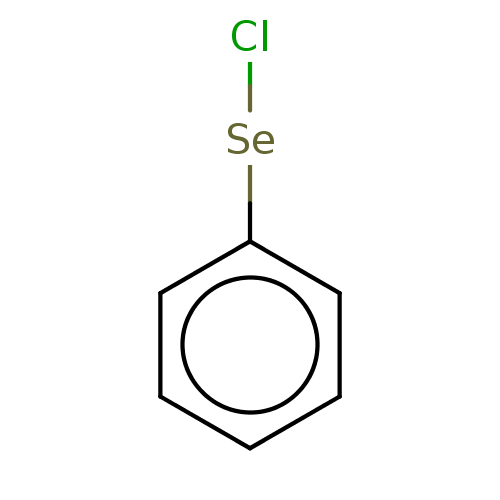 (CHEMBL3335712) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 15 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-butyrobetaine dioxygenase (Homo sapiens (Human)) | BDBM50028971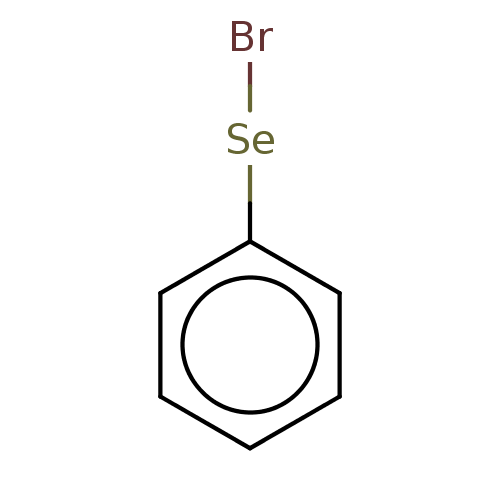 (CHEMBL3335713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of human BBOX pre-incubated for 10 mins using TBS-protected fluorescein probe and Fe (II) (Fe(NH4)2(SO4)2 salt by fluoride release assay | Bioorg Med Chem Lett 24: 4954-7 (2014) Article DOI: 10.1016/j.bmcl.2014.09.035 BindingDB Entry DOI: 10.7270/Q2D2207X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 145 total ) | Next | Last >> |