

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029464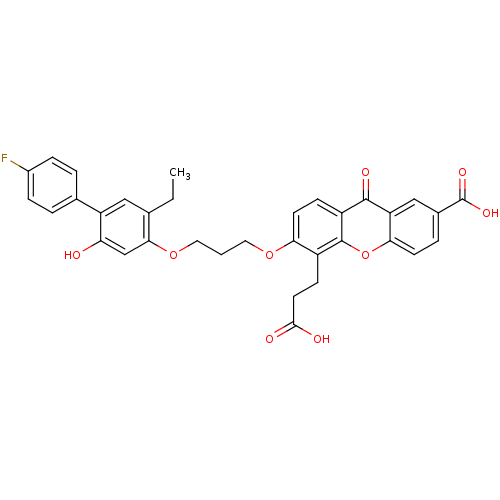 (5-(2-Carboxy-ethyl)-6-[3-(5-ethyl-4'-fluoro-2-hydr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50052027 ((E)-3-[6-(2,6-Dichloro-phenylsulfanylmethyl)-3-phe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for binding affinity against human neutrophil LTB4 (leukotriene) receptor | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50052025 (6-{2-(2-Carboxy-ethyl)-3-[6-(4-oxo-8-propyl-chroma...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416285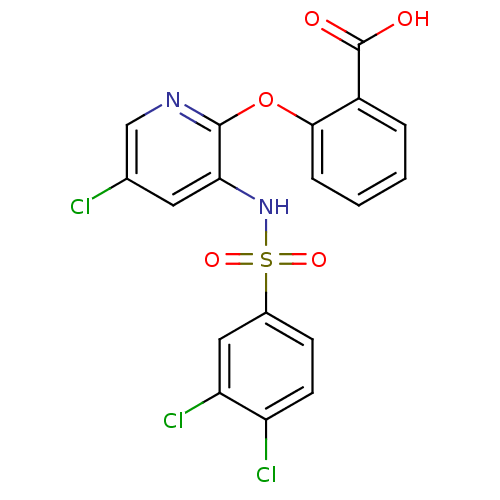 (CHEMBL1171008) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416284 (CHEMBL1170828) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416308 (CHEMBL1170827) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (RAT) | BDBM50384444 (CHEMBL2035510) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50384444 (CHEMBL2035510) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416283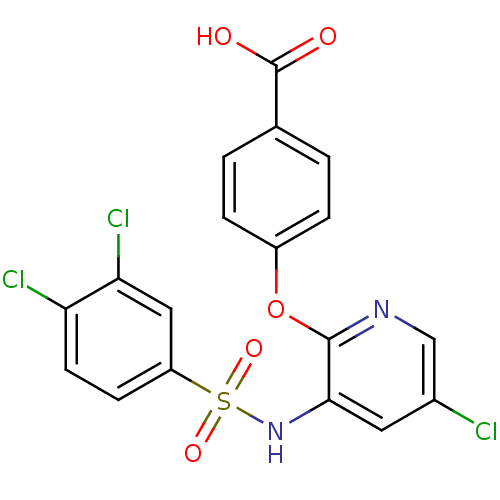 (CHEMBL1170829) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416304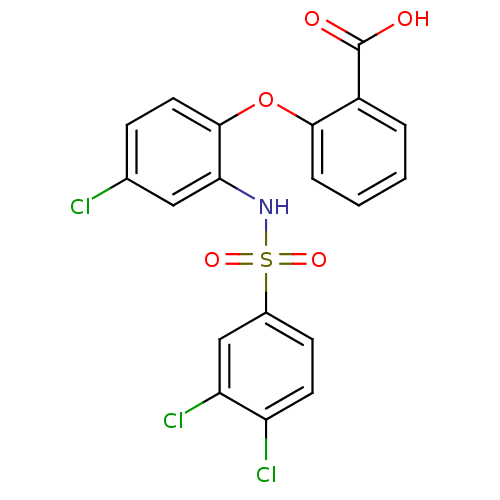 (CHEMBL1171786) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037382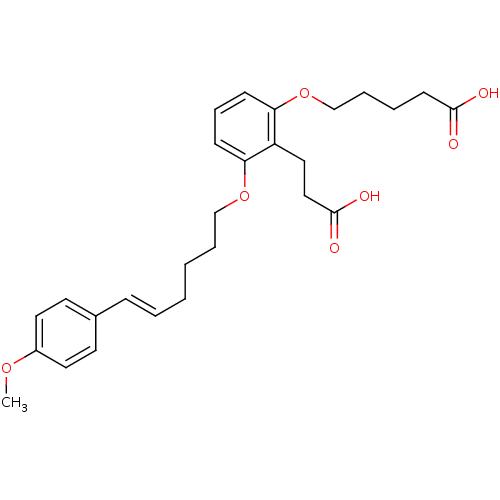 (5-{2-(2-Carboxy-ethyl)-3-[(E)-6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416289 (CHEMBL1171787) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50384443 (CHEMBL1770317) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding affinity to human EP3 | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483809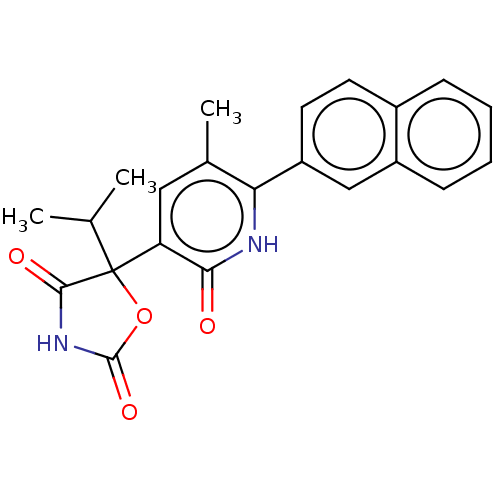 (CHEMBL1770337) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50384445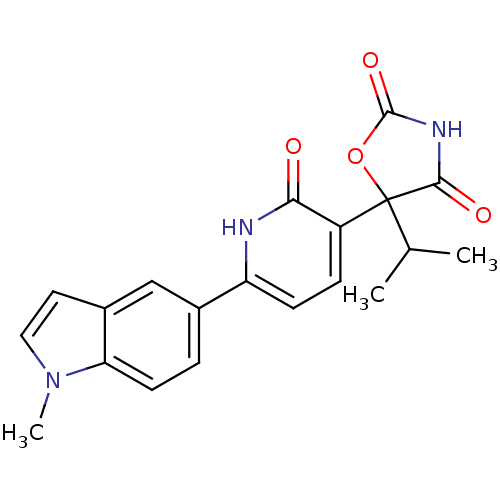 (CHEMBL2035508) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50037385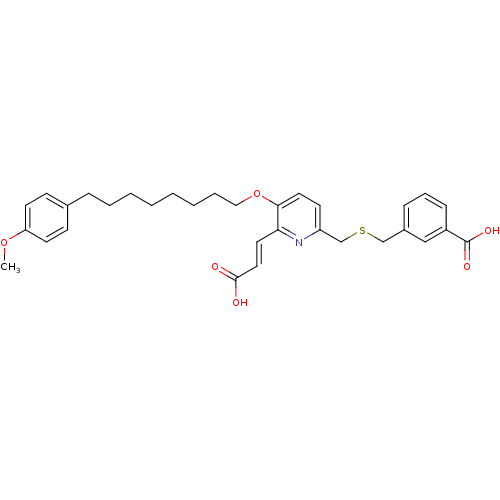 (3-{6-((E)-2-Carboxy-vinyl)-5-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for binding affinity against Leukotriene B4 receptor | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483823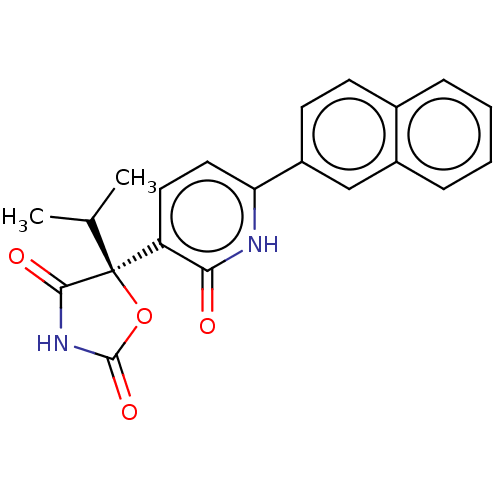 (CHEMBL1770341) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483821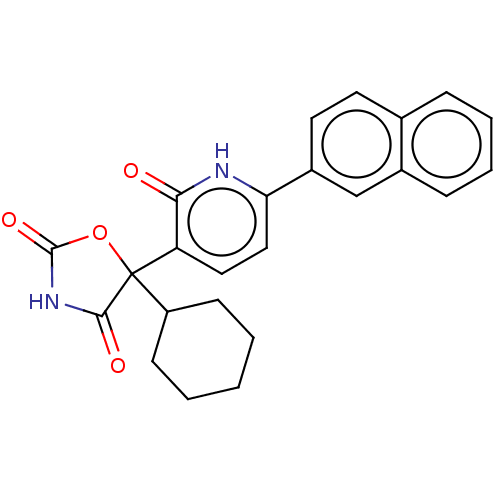 (CHEMBL1770320) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483814 (CHEMBL1770319) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (RAT) | BDBM50384445 (CHEMBL2035508) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416290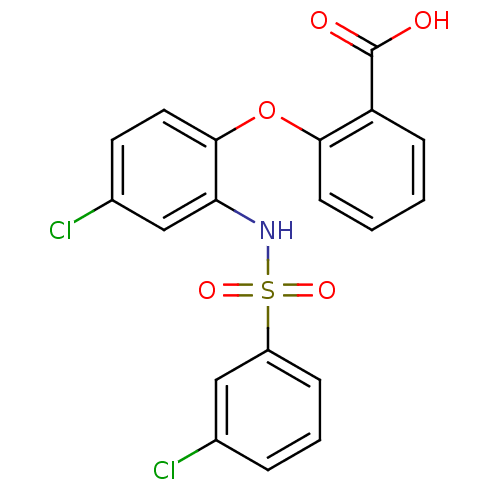 (CHEMBL1171614) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50384443 (CHEMBL1770317) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50359023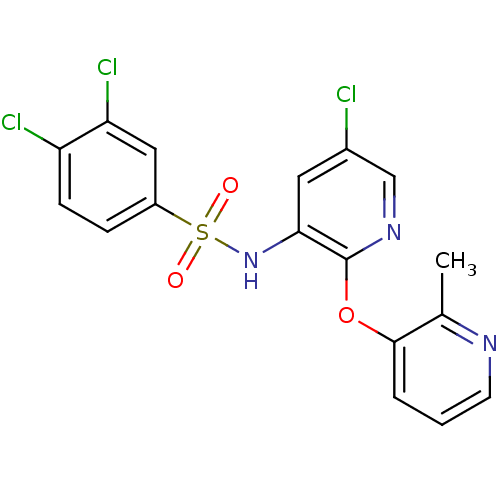 (CHEMBL1170725) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416307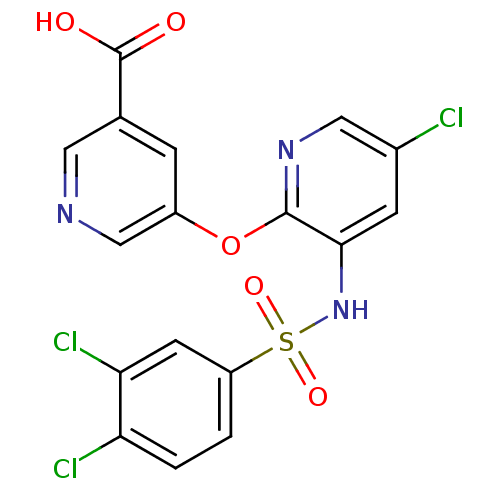 (CHEMBL1171011) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401549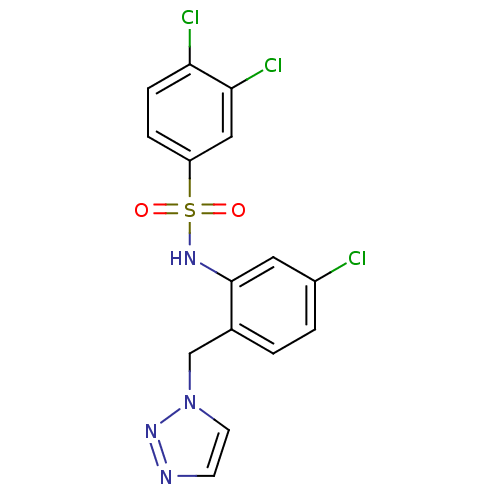 (CHEMBL2207081) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to human CCR2 | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401547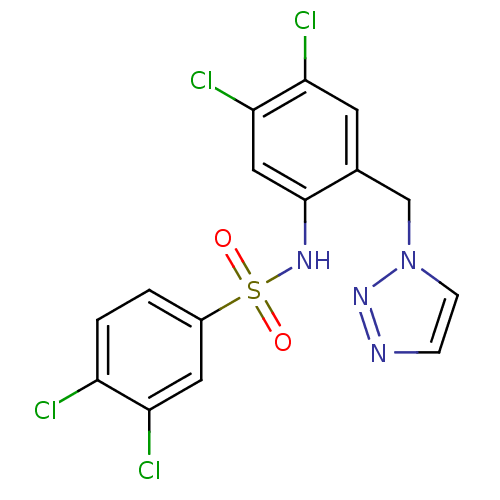 (CHEMBL2207098) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to CCR2 by 35S-gamma-GTP membrane assay | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401550 (CHEMBL2207084) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to CCR2 by 35S-gamma-GTP membrane assay | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401561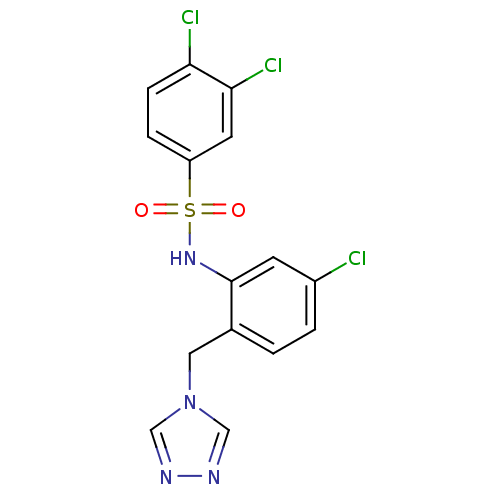 (CHEMBL2207082) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to CCR2 by 35S-gamma-GTP membrane assay | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401549 (CHEMBL2207081) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to CCR2 by 35S-gamma-GTP membrane assay | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (RAT) | BDBM50384443 (CHEMBL1770317) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50384443 (CHEMBL1770317) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401546 (CHEMBL2207100) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to CCR2 by 35S-gamma-GTP membrane assay | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401555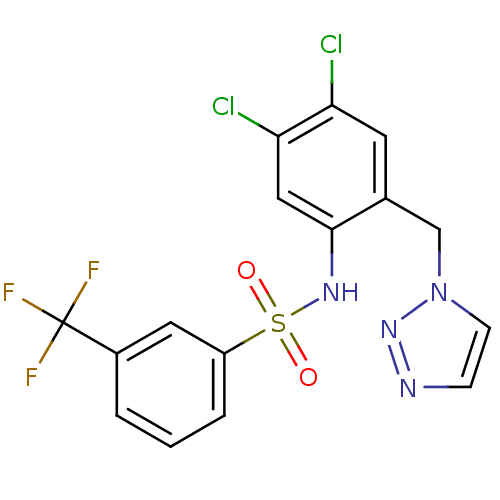 (CHEMBL2207101) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to CCR2 by 35S-gamma-GTP membrane assay | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (RAT) | BDBM50384446 (CHEMBL2035509) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at rat EP3 receptor expressed in human U2OS cells co-expressing Gqi5 assessed as inhibition of PGE2-induced response after 24 hrs... | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416281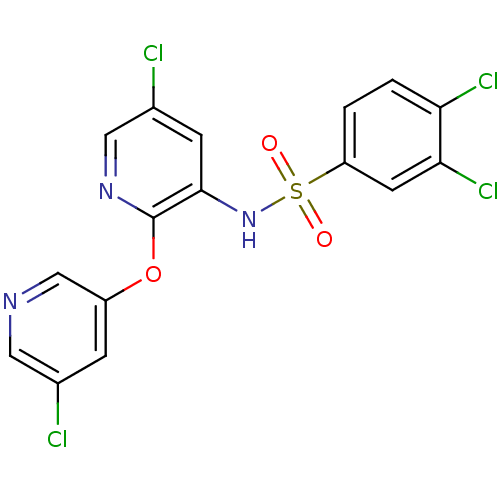 (CHEMBL1169994) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401553 (CHEMBL2207085) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to CCR2 by 35S-gamma-GTP membrane assay | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416306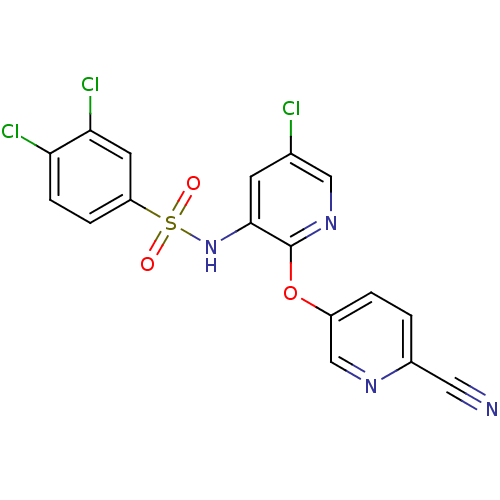 (CHEMBL1169995) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483827 (CHEMBL1770322) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50384446 (CHEMBL2035509) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Antagonist activity at human EP3c receptor expressed in human U2OS cells assessed as inhibition of PGE2-induced calcium mobilization after 24 hrs by ... | ACS Med Chem Lett 1: 316-320 (2010) Article DOI: 10.1021/ml100077x BindingDB Entry DOI: 10.7270/Q2BG2Q1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416294 (CHEMBL1171760) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416282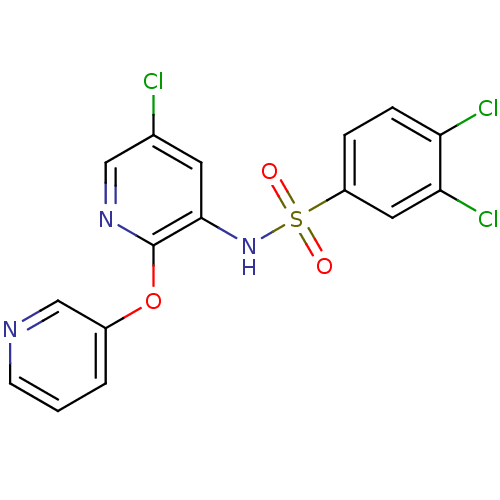 (CHEMBL1170722) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483811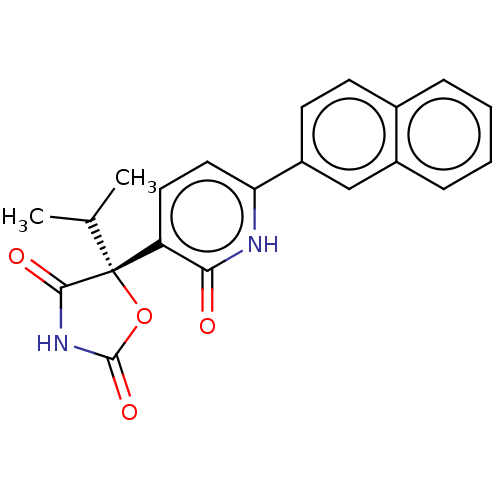 (CHEMBL1770340) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50483812 (CHEMBL1770316) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Antagonist activity at human EP3 receptor expressed in CHO cells assessed as inhibition of PGE2-induced increase in intracellular calcium concentrati... | Bioorg Med Chem Lett 21: 2806-11 (2011) Article DOI: 10.1016/j.bmcl.2011.03.107 BindingDB Entry DOI: 10.7270/Q2NZ8BGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50029450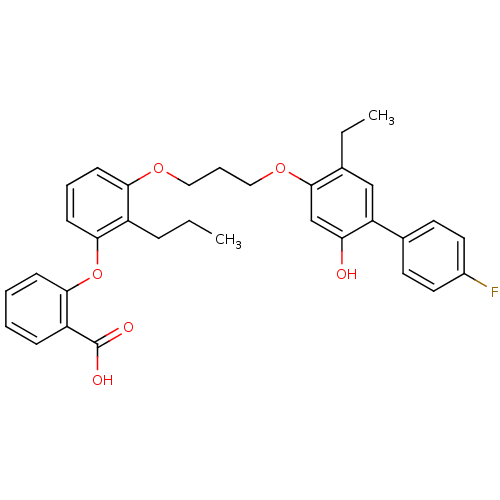 (2-{3-[3-(5-Ethyl-4'-fluoro-2-hydroxy-biphenyl-4-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against human neutrophil LTB4 receptor binding | J Med Chem 39: 2629-54 (1996) Article DOI: 10.1021/jm960088k BindingDB Entry DOI: 10.7270/Q21J9BFT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401548 (CHEMBL2207094) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to CCR2 by 35S-gamma-GTP membrane assay | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416305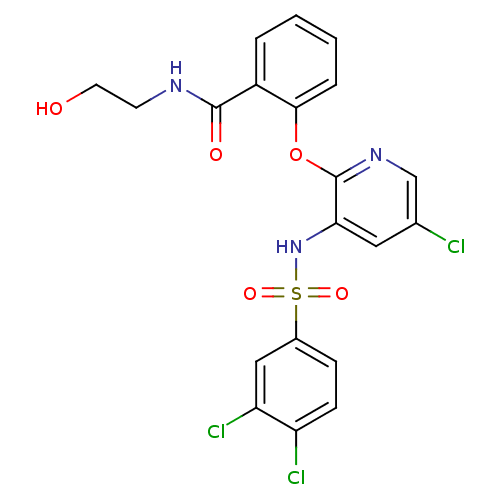 (CHEMBL1169660) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401557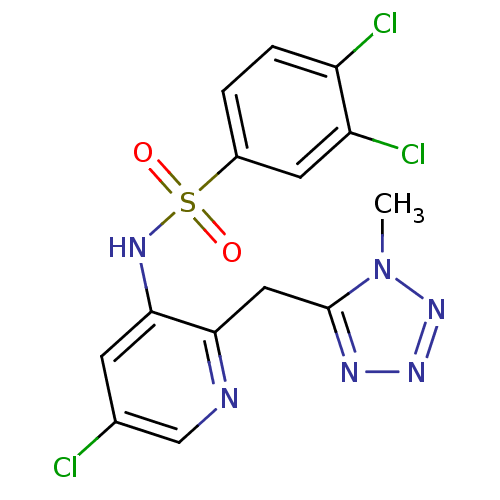 (CHEMBL2207099) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to CCR2 by 35S-gamma-GTP membrane assay | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50416286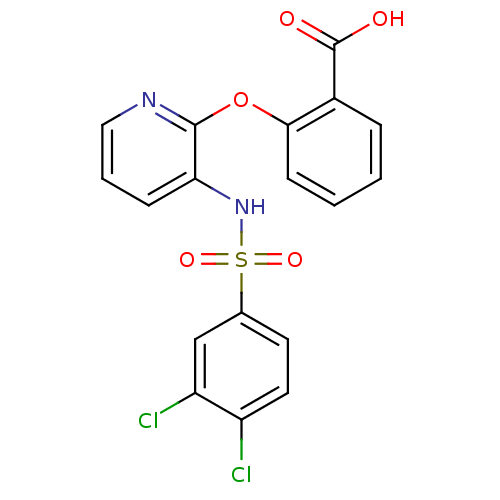 (CHEMBL1170801) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Medicines Research Centre Curated by ChEMBL | Assay Description Antagonist activity at human CCR2 expressed in CHO cells assessed as inhibition of MCP1-induced [35S]-GTPgammaS binding after 3 hrs | Bioorg Med Chem Lett 20: 3961-4 (2010) Article DOI: 10.1016/j.bmcl.2010.04.142 BindingDB Entry DOI: 10.7270/Q2B56KZ9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401558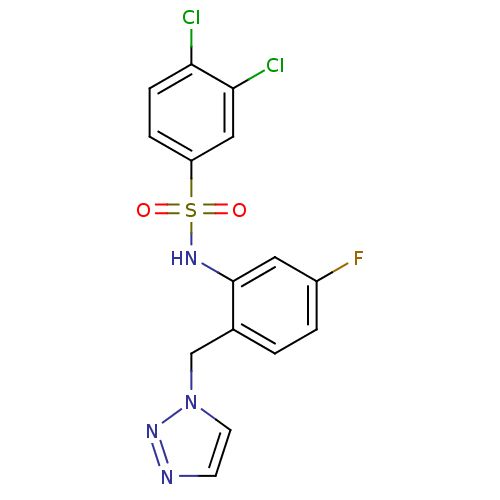 (CHEMBL2207097) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to CCR2 by 35S-gamma-GTP membrane assay | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 2 (Homo sapiens (Human)) | BDBM50401552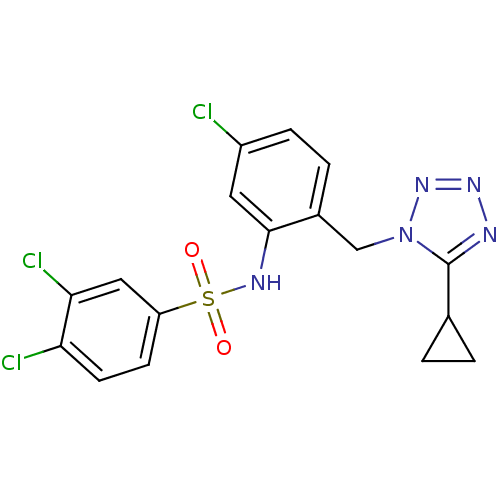 (CHEMBL2207080) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity to CCR2 by 35S-gamma-GTP membrane assay | Bioorg Med Chem Lett 22: 7252-5 (2012) Article DOI: 10.1016/j.bmcl.2012.09.020 BindingDB Entry DOI: 10.7270/Q21J9BZJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1109 total ) | Next | Last >> |