

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50452590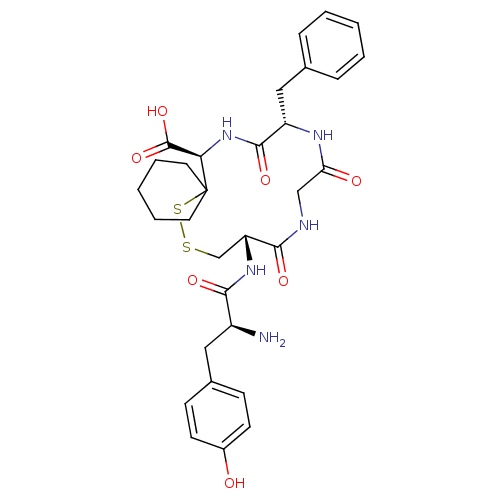 (CHEMBL3084309) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]DADLE | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001465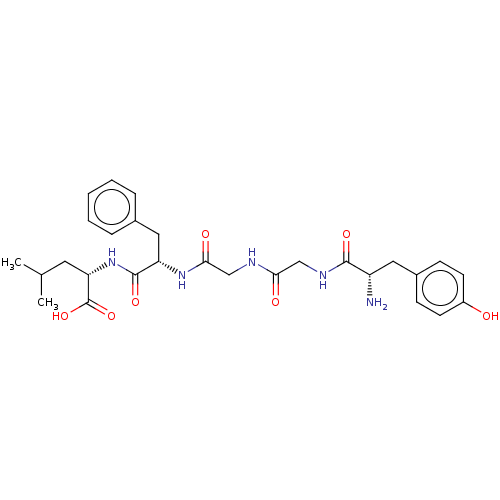 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]DADLE | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016752 (CHEMBL339943 | N*1*-[1-(1-Carbamoyl-4-guanidino-bu...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase (Vasopressin V2 receptor) of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016829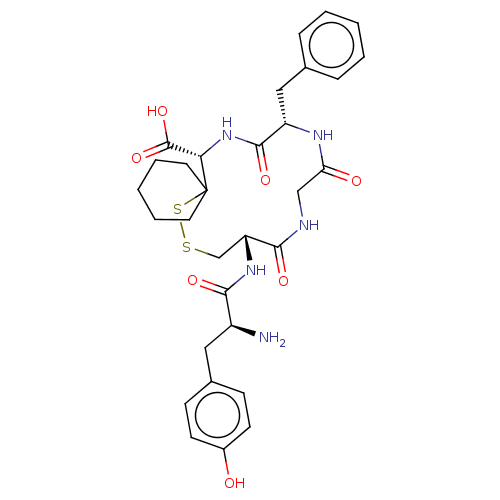 (10-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace delta-receptor specific radioligand [3H]DSLET | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016829 (10-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace opioid receptor delta specific radioligand [3H]-DSLET | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace delta-receptor specific radioligand [3H]DSLET | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016752 (CHEMBL339943 | N*1*-[1-(1-Carbamoyl-4-guanidino-bu...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney (Vasopressin V2 receptor) | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016760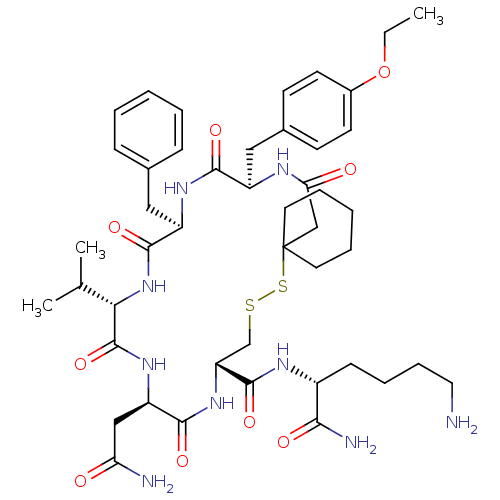 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50452590 (CHEMBL3084309) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace delta-receptor specific radioligand [3H]DSLET | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016830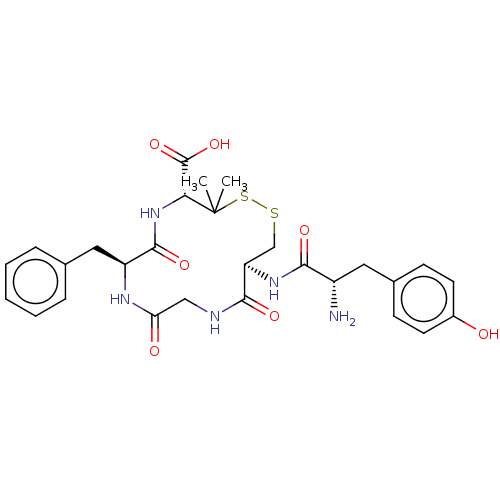 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]DADLE | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016756 (CHEMBL265591 | c(Pmp-D-Tyr(Et)-Phe-Val-Asn-Cys)-Pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016753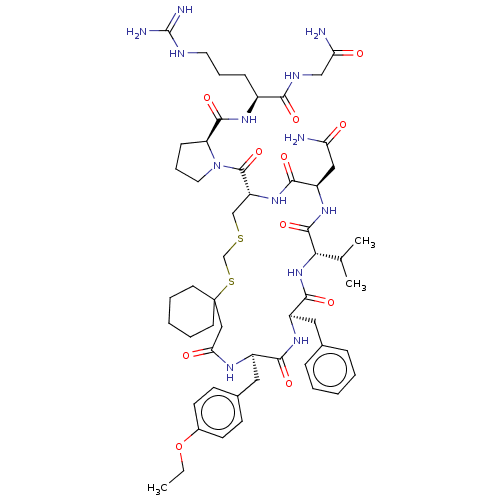 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016753 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016757 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016757 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020655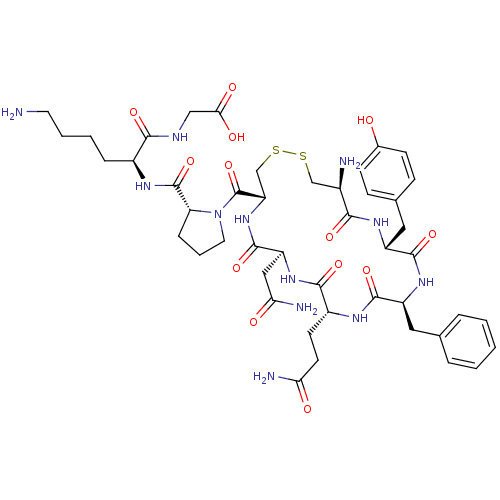 (CHEMBL408474 | [6-Amino-2-({1-[19-amino-13-benzyl-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of hog kidney renin | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020655 (CHEMBL408474 | [6-Amino-2-({1-[19-amino-13-benzyl-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016747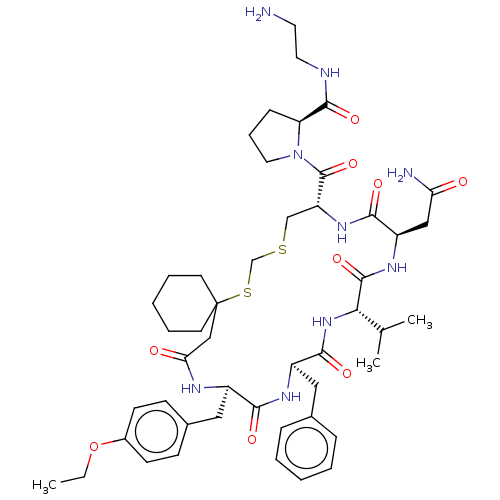 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016747 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016833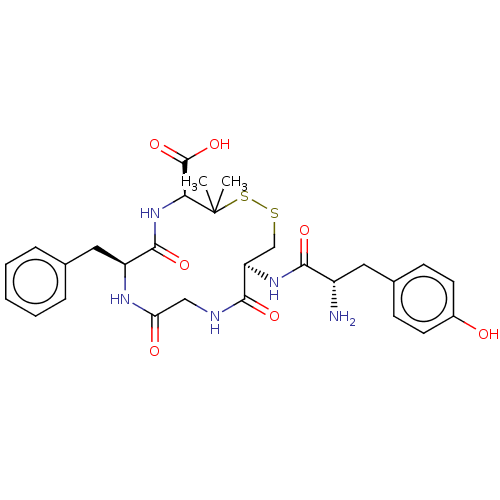 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]DADLE | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016833 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]DADLE | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016760 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016760 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016756 (CHEMBL265591 | c(Pmp-D-Tyr(Et)-Phe-Val-Asn-Cys)-Pr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020653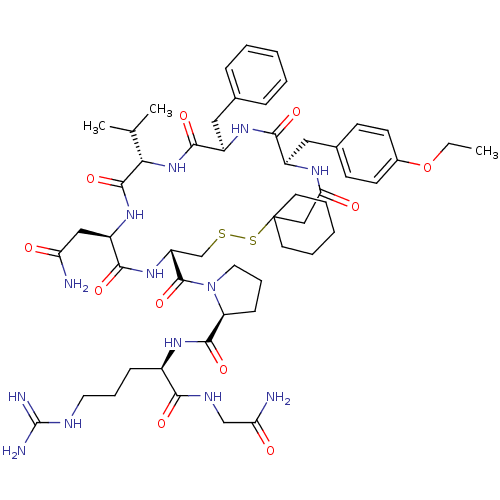 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016755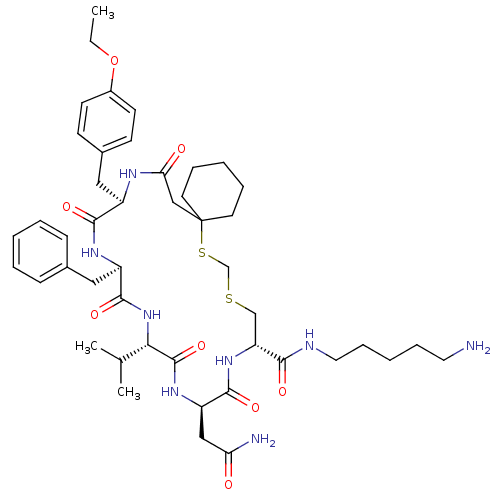 (20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase (Vasopressin V2 receptor) of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016755 (20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney (Vasopressin V2 receptor) | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016763 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50016763 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of human kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016754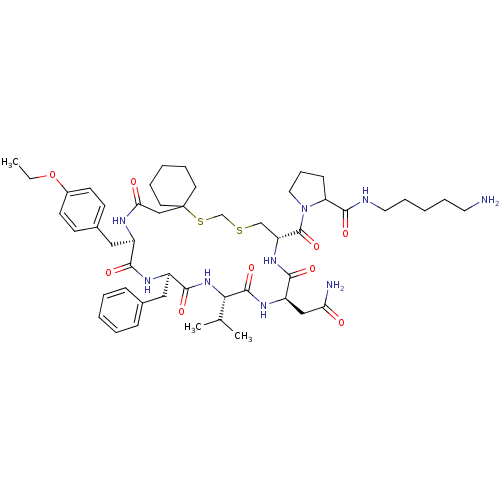 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020656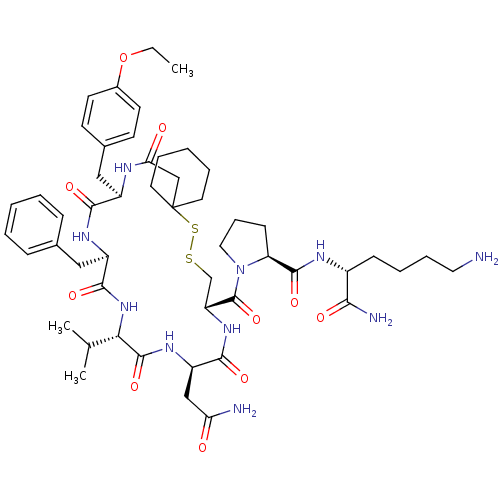 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020656 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016761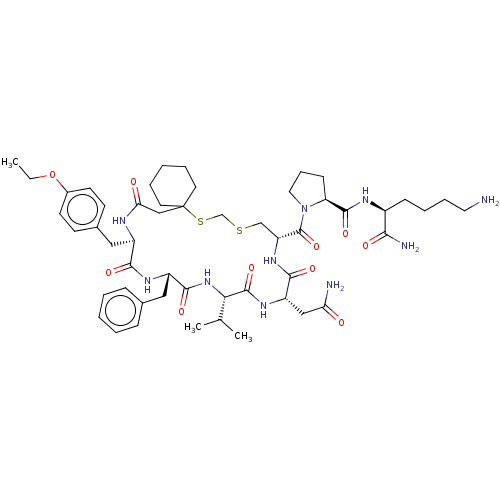 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace Opioid receptor mu 1 specific radioligand [3H]DAGO | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020701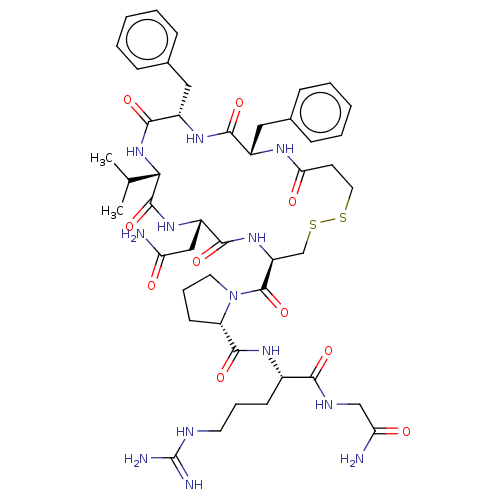 (1-(13,16-Dibenzyl-7-carbamoylmethyl-10-isopropyl-6...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description Inhibition of LVP-sensitive adenylate cyclase in a pig renal medullary preparation | J Med Chem 31: 742-4 (1988) BindingDB Entry DOI: 10.7270/Q2VD6XFS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016762 (20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney (Vasopressin V2 receptor) | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016750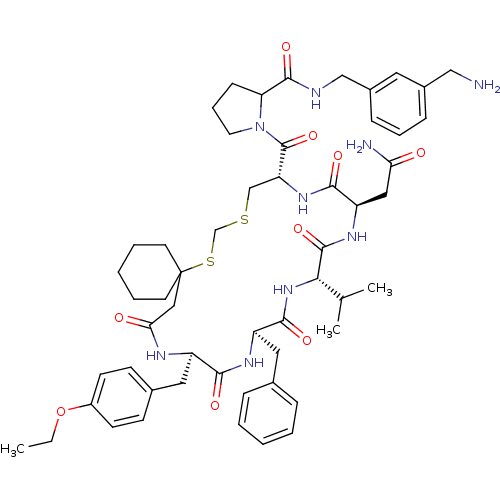 (1-[20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney (Vasopressin V2 receptor) | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020654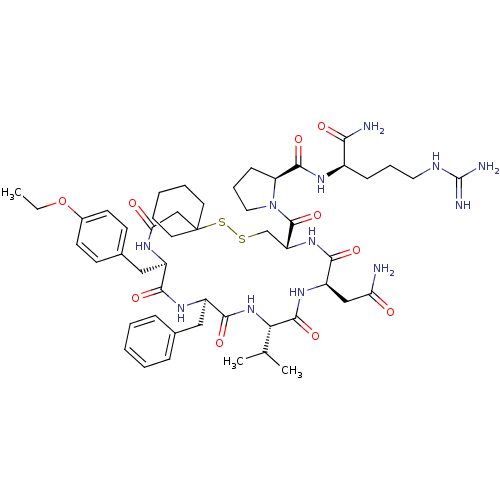 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020653 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020654 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016834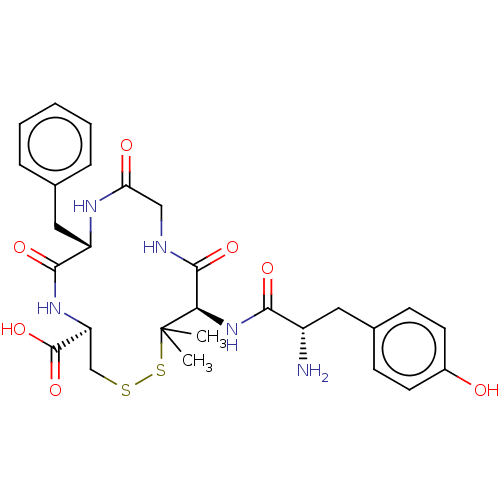 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]naloxone | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50452590 (CHEMBL3084309) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace Opioid receptor mu 1 specific radioligand [3H]DAGO | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016832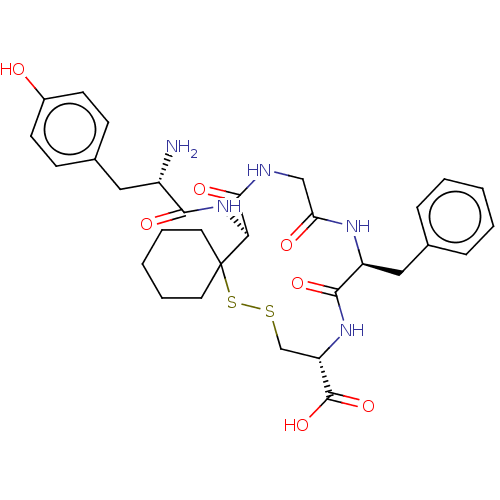 (19-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace receptor specific radioligand [3H]DPDPE | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020651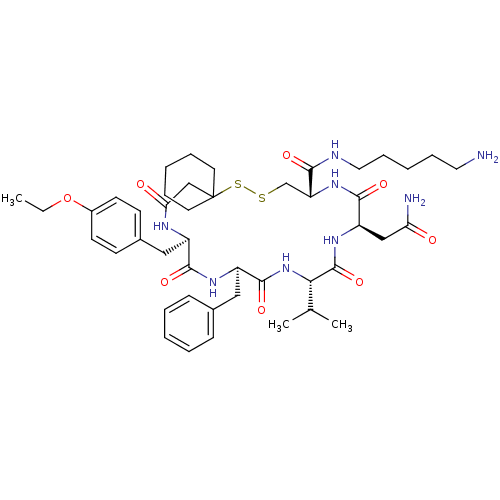 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of [3H]-LVP binding to vasopressin receptor in medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020651 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016829 (10-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace mu-receptor specific radioligand [3H]DAGO | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50016830 (13-[2-Amino-3-(4-hydroxy-phenyl)-propionylamino]-7...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]naloxone | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016760 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for inhibition of vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney. | J Med Chem 28: 1759-60 (1986) BindingDB Entry DOI: 10.7270/Q2CC0ZNX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50452589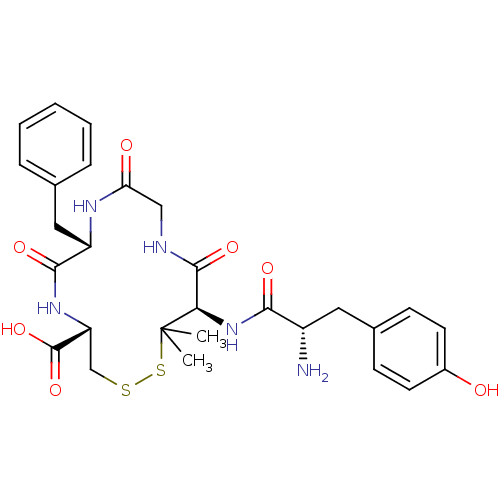 (CHEMBL2370362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline and French Laboratories Curated by ChEMBL | Assay Description The compound was tested for the ability to displace opioid receptor kappa specific radioligand [3H]DADLE | J Med Chem 32: 302-4 (1989) BindingDB Entry DOI: 10.7270/Q2SX6DT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50016749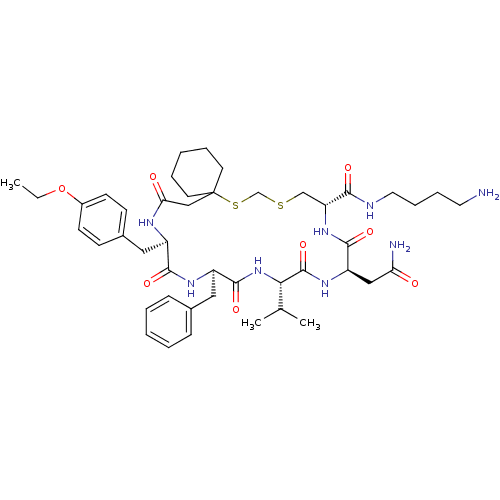 (20-Benzyl-14-carbamoylmethyl-23-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description Inhibition constant for vasopressin-stimulated adenylate cyclase of medullary membranes of pig kidney (Vasopressin V2 receptor) | J Med Chem 32: 391-6 (1989) BindingDB Entry DOI: 10.7270/Q2GM8688 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 96 total ) | Next | Last >> |