

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036257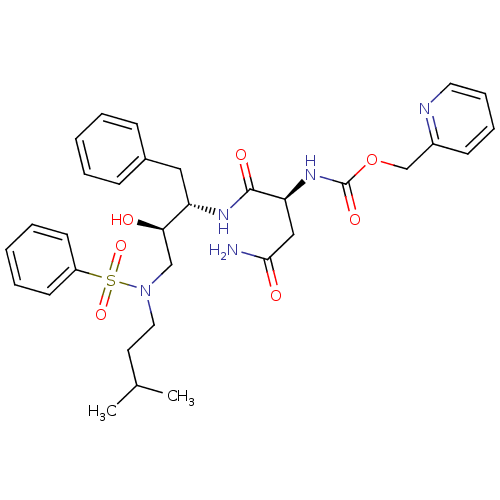 (((S)-1-{(1S,2R)-3-[Benzenesulfonyl-(3-methyl-butyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036261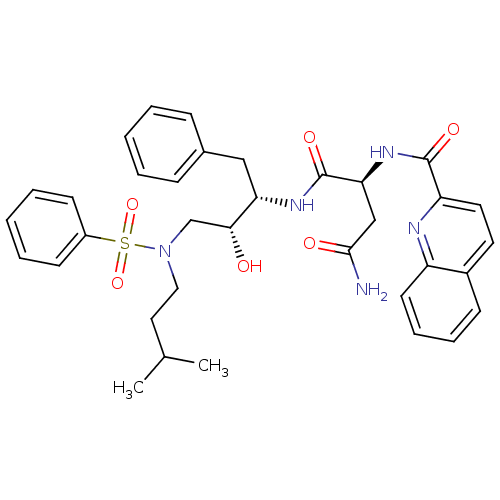 ((S)-N*1*-{(1S,2R)-3-[Benzenesulfonyl-(3-methyl-but...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined in vitro | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036251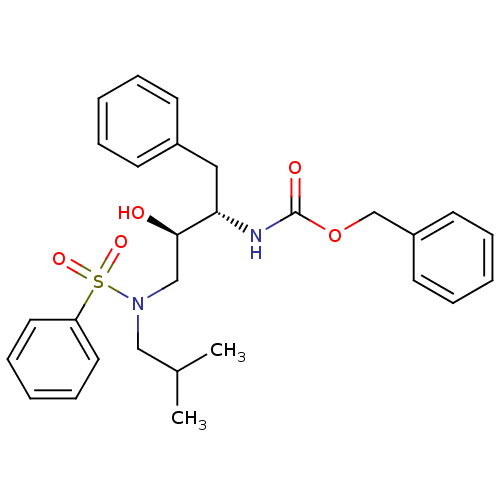 (CHEMBL141265 | [(1S,2R)-3-(Benzenesulfonyl-isobuty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined in vitro | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036252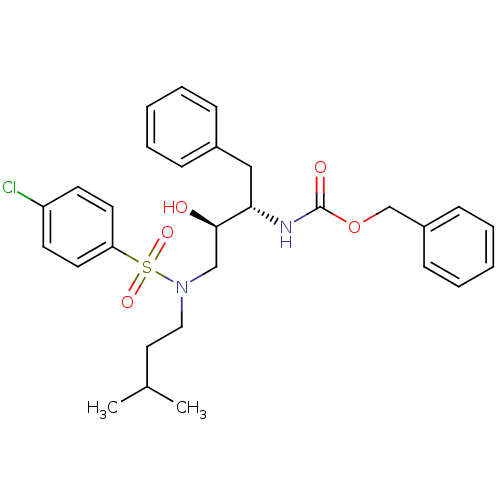 (CHEMBL347671 | {(1S,2R)-1-Benzyl-3-[(4-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036262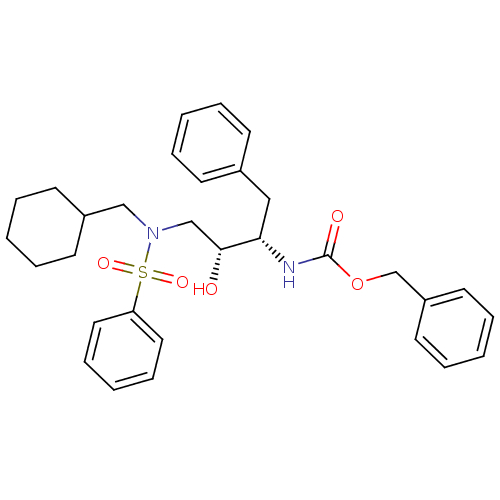 (CHEMBL348282 | [(1S,2R)-3-(Benzenesulfonyl-cyclohe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036253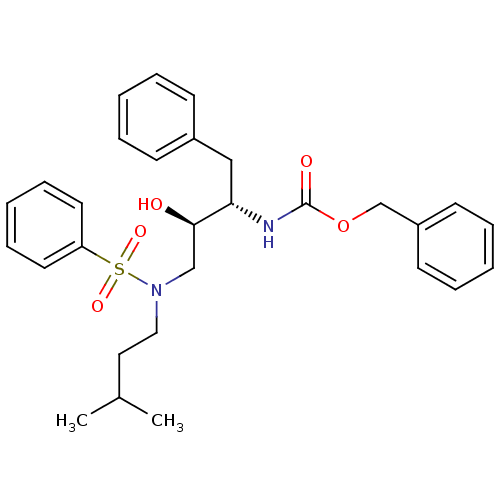 (CHEMBL347431 | {(1S,2R)-3-[Benzenesulfonyl-(3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined in vitro. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036259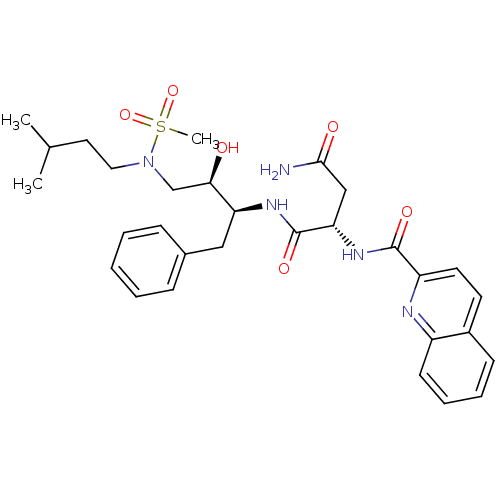 ((S)-N*1*-{(1S,2R)-1-Benzyl-2-hydroxy-3-[methanesul...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined in vitro | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036258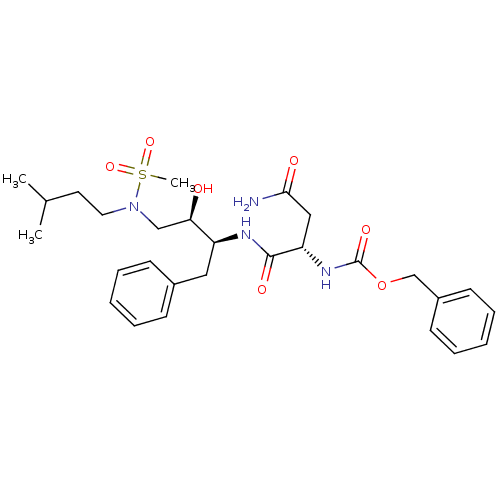 (((S)-1-{(1S,2R)-1-Benzyl-2-hydroxy-3-[methanesulfo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036260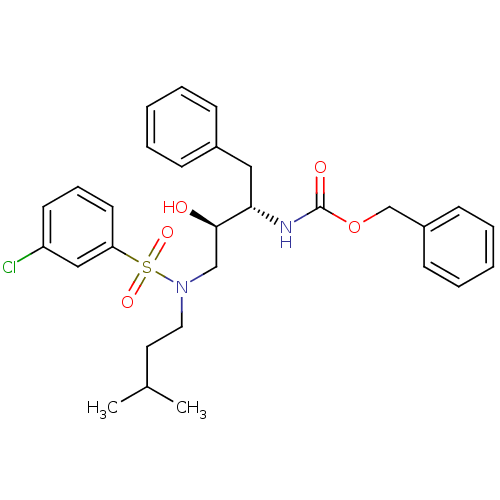 (CHEMBL156424 | {(1S,2R)-1-Benzyl-3-[(3-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036255 (CHEMBL154197 | [(1S,2R)-3-(Benzenesulfonyl-benzyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036254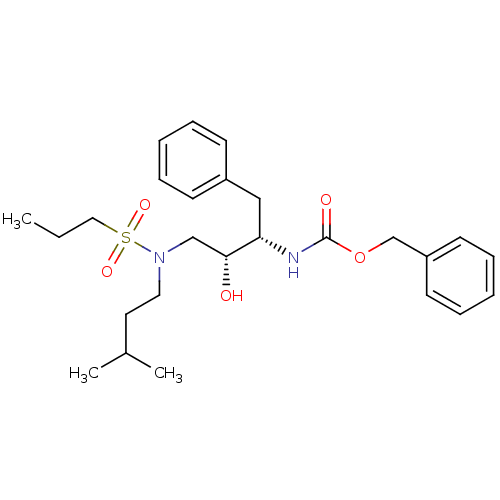 (CHEMBL155051 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[(3-m...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036264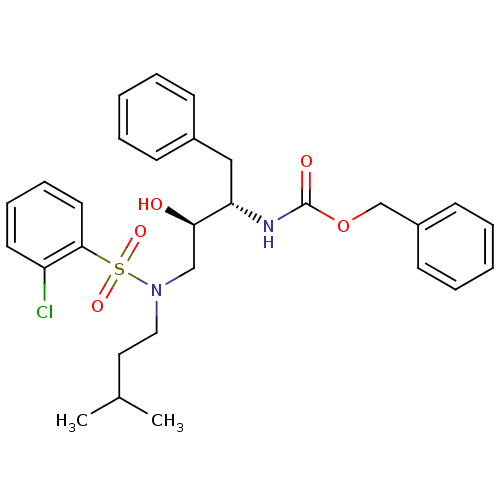 (CHEMBL154600 | {(1S,2R)-1-Benzyl-3-[(2-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036263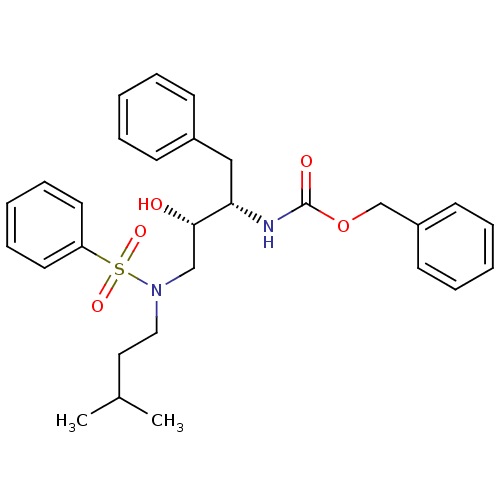 (CHEMBL154638 | {(1S,2S)-3-[Benzenesulfonyl-(3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036256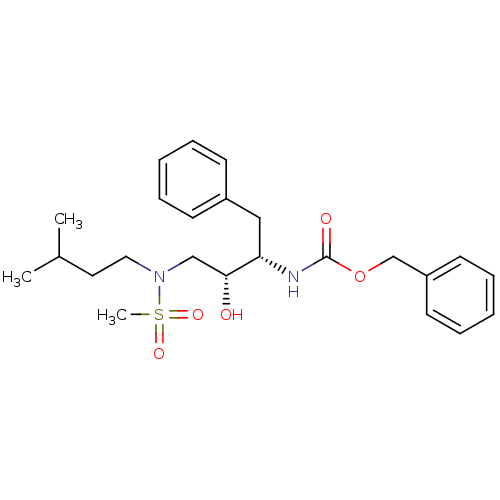 (CHEMBL154798 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Binding affinity of the compound for HIV-1 protease was determined | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036261 ((S)-N*1*-{(1S,2R)-3-[Benzenesulfonyl-(3-methyl-but...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit HIV-1 protease was determined using spectrofluorometric assay | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036257 (((S)-1-{(1S,2R)-3-[Benzenesulfonyl-(3-methyl-butyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit HIV-1 protease was determined using spectrofluorometric assay | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288807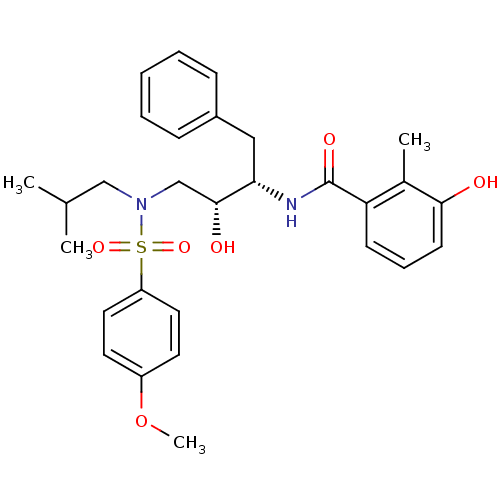 (CHEMBL263540 | N-{(1S,2R)-1-Benzyl-2-hydroxy-3-[is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against recombinant HIV-1 protease using spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM486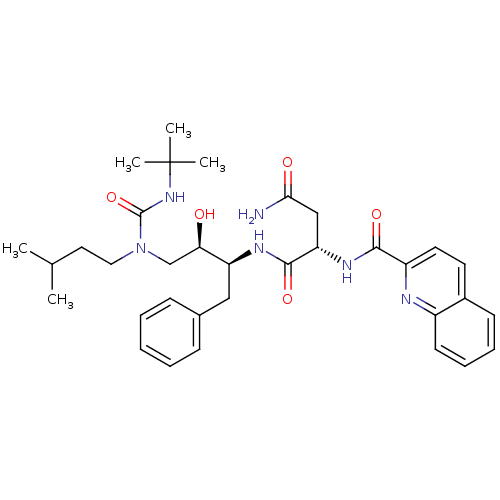 ((2S)-N-[(2S,3R)-4-[(tert-butylcarbamoyl)(3-methylb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM490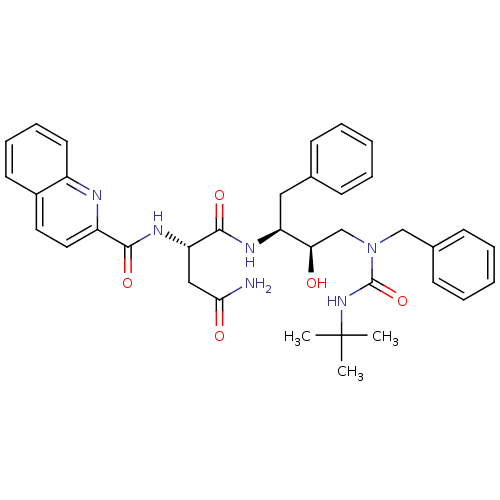 ((2S)-N-[(2S,3R)-4-[benzyl(tert-butylcarbamoyl)amin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288806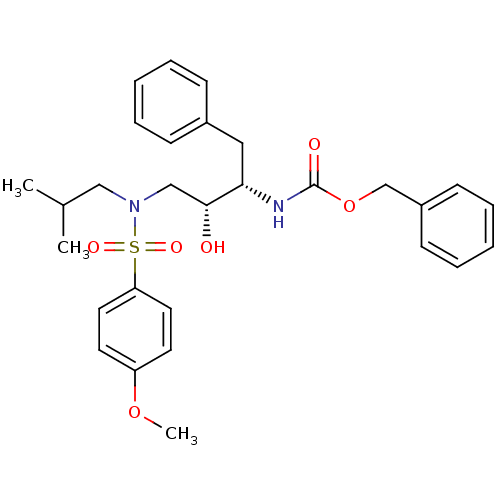 (CHEMBL336684 | {(1S,2R)-1-Benzyl-2-hydroxy-3-[isob...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease in spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288804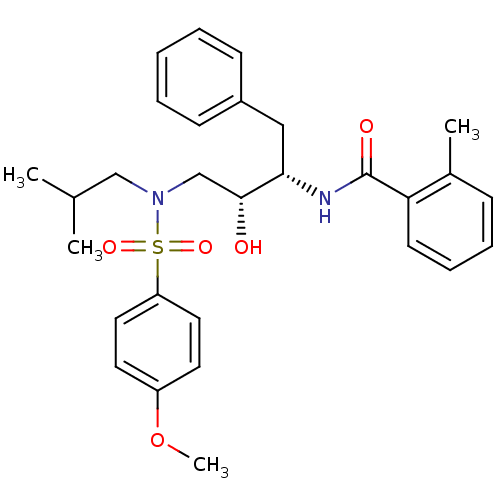 (CHEMBL139538 | N-{(1S,2R)-1-Benzyl-2-hydroxy-3-[is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against recombinant HIV-1 protease using spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM488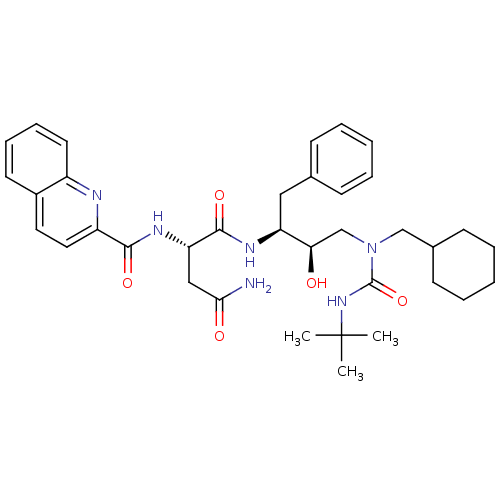 ((2S)-N-[(2S,3R)-4-[(tert-butylcarbamoyl)(cyclohexy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288817 (CHEMBL139444 | N-{(1S,2R)-1-Benzyl-2-hydroxy-3-[is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against recombinant HIV-1 protease using spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288808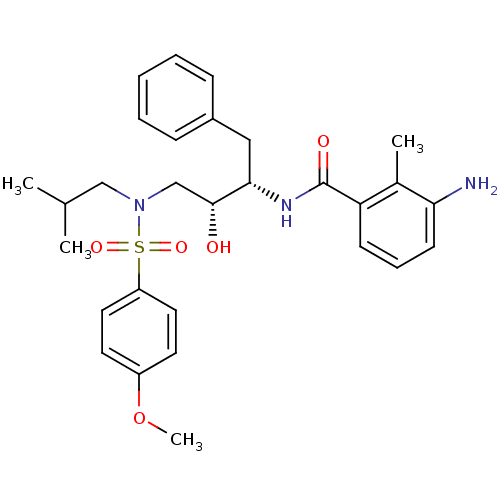 (3-Amino-N-{(1S,2R)-1-benzyl-2-hydroxy-3-[isobutyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against recombinant HIV-1 protease using spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288815 (CHEMBL344233 | N-{(1S,2R)-1-Benzyl-2-hydroxy-3-[is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease in spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM483 ((2S)-N-[(2S,3R)-4-[(tert-butylcarbamoyl)(2-methylp...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036251 (CHEMBL141265 | [(1S,2R)-3-(Benzenesulfonyl-isobuty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease in spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036251 (CHEMBL141265 | [(1S,2R)-3-(Benzenesulfonyl-isobuty...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit HIV-1 protease was determined using spectrofluorometric assay | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288811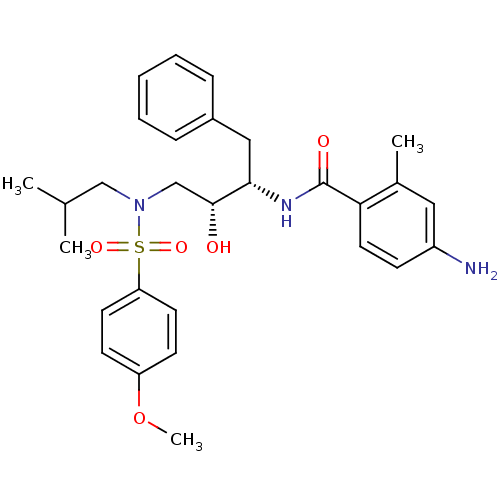 (4-Amino-N-{(1S,2R)-1-benzyl-2-hydroxy-3-[isobutyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against recombinant HIV-1 protease using spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036252 (CHEMBL347671 | {(1S,2R)-1-Benzyl-3-[(4-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit HIV-1 protease was determined using spectrofluorometric assay. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288805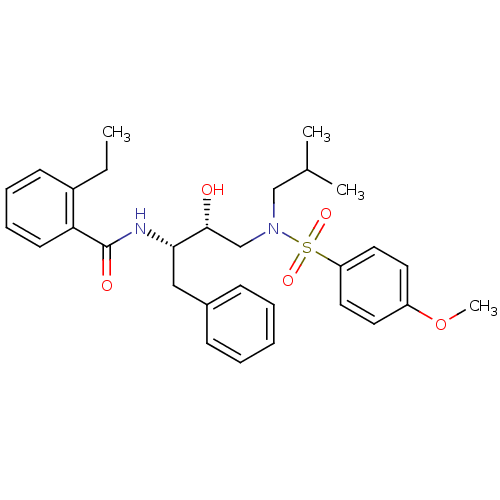 (CHEMBL343108 | N-{(1S,2R)-1-Benzyl-2-hydroxy-3-[is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease in spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288812 (5-Amino-N-{(1S,2R)-1-benzyl-2-hydroxy-3-[isobutyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against recombinant HIV-1 protease using spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM485 ((Hydroxyethyl)urea Isostere deriv. 13 | benzyl N-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036262 (CHEMBL348282 | [(1S,2R)-3-(Benzenesulfonyl-cyclohe...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit HIV-1 protease was determined using spectrofluorometric assay | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288803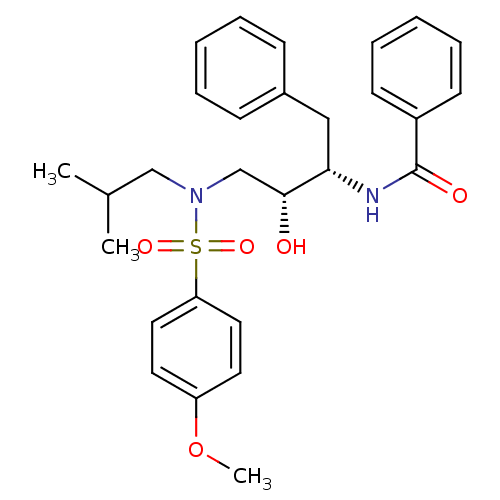 (CHEMBL141046 | N-{(1S,2R)-1-Benzyl-2-hydroxy-3-[is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease in spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288802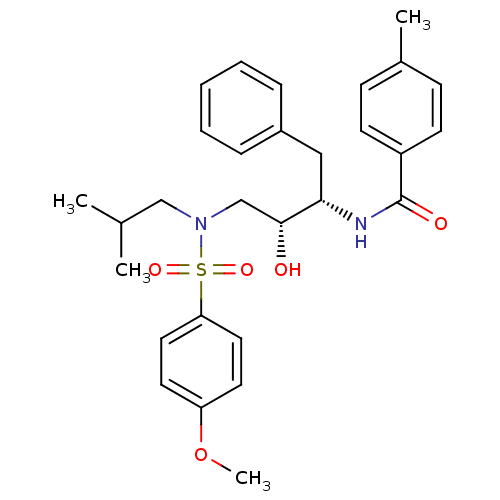 (CHEMBL423189 | N-{(1S,2R)-1-Benzyl-2-hydroxy-3-[is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease in spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036253 (CHEMBL347431 | {(1S,2R)-3-[Benzenesulfonyl-(3-meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit HIV-1 protease was determined using spectrofluorometric assay. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM494 ((2S)-N-[(2S,3R)-4-[(tert-butylcarbamoyl)(pyridin-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM489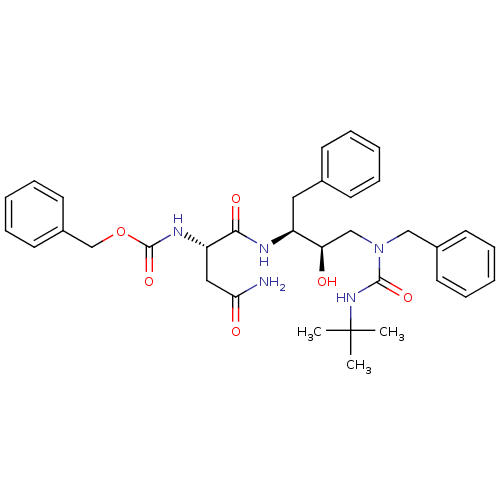 ((Hydroxyethyl)urea Isostere deriv. 17 | benzyl N-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288814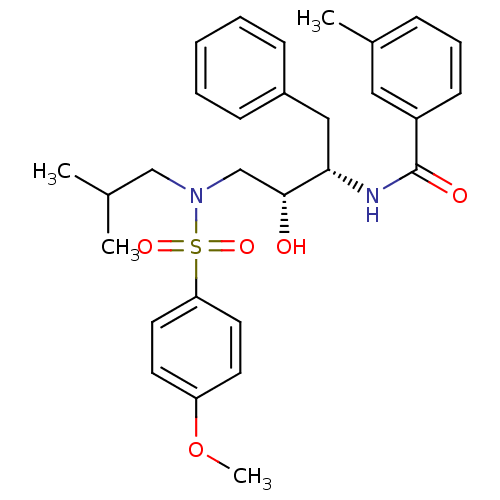 (CHEMBL341697 | N-{(1S,2R)-1-Benzyl-2-hydroxy-3-[is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease in spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036259 ((S)-N*1*-{(1S,2R)-1-Benzyl-2-hydroxy-3-[methanesul...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit HIV-1 protease was determined using spectrofluorometric assay | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM487 ((Hydroxyethyl)urea Isostere deriv. 15 | benzyl N-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM481 ((Hydroxyethyl)urea Isostere deriv. 9 | benzyl N-[(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288816 (CHEMBL140653 | N-{(1S,2R)-1-Benzyl-2-hydroxy-3-[is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease in spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288813 (CHEMBL141002 | N-{(1S,2R)-1-Benzyl-2-hydroxy-3-[is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity of the compound was evaluated against recombinant HIV-1 protease using spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50288810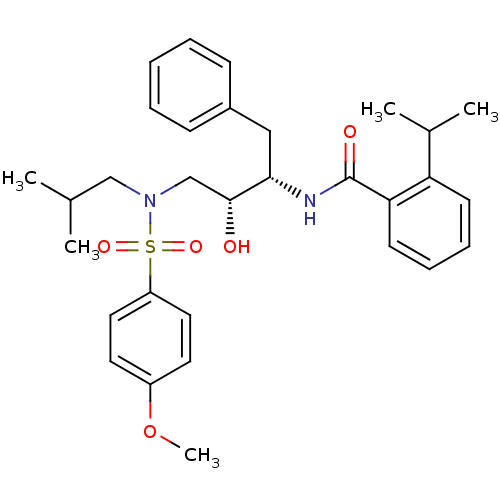 (CHEMBL139309 | N-{(1S,2R)-1-Benzyl-2-hydroxy-3-[is...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 protease in spectrofluorometric assay | Bioorg Med Chem Lett 6: 445-450 (1996) Article DOI: 10.1016/0960-894X(96)00035-2 BindingDB Entry DOI: 10.7270/Q2R78F6Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036260 (CHEMBL156424 | {(1S,2R)-1-Benzyl-3-[(3-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit HIV-1 protease was determined using spectrofluorometric assay. | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50036258 (((S)-1-{(1S,2R)-1-Benzyl-2-hydroxy-3-[methanesulfo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Searle Discovery Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit HIV-1 protease was determined using spectrofluorometric assay | J Med Chem 38: 581-4 (1995) BindingDB Entry DOI: 10.7270/Q2SF2V61 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM493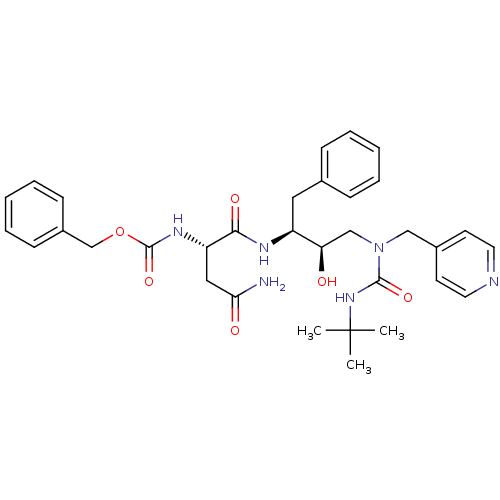 ((Hydroxyethyl)urea Isostere deriv. 21 | benzyl N-[...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM477 ((2S)-N-[(2S,3R)-4-[(butylcarbamoyl)(2-methylpropyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | 6.4 | 25 |
Monsanto Corporate Research | Assay Description IC50 values for inhibition of recombinant HIV protease were determined using the spectrofluorometric assay, which utilized an intramolecularly quench... | J Med Chem 36: 288-91 (1993) Article DOI: 10.1021/jm00054a014 BindingDB Entry DOI: 10.7270/Q2WW7FTF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 73 total ) | Next | Last >> |