

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM160666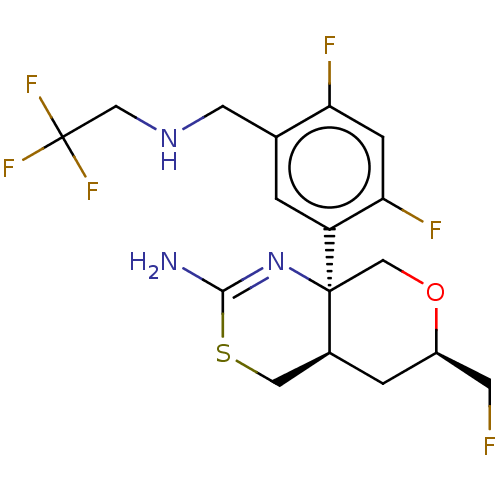 (US9045498, 8) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223332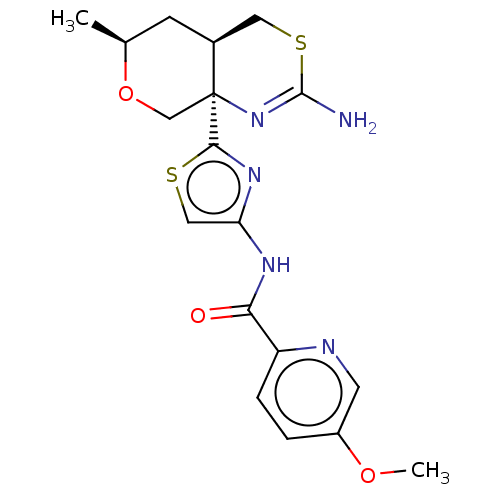 (US9315520, 19 | US9605007, Example 19 | US9744173,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259962 (CHEMBL4088234) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452875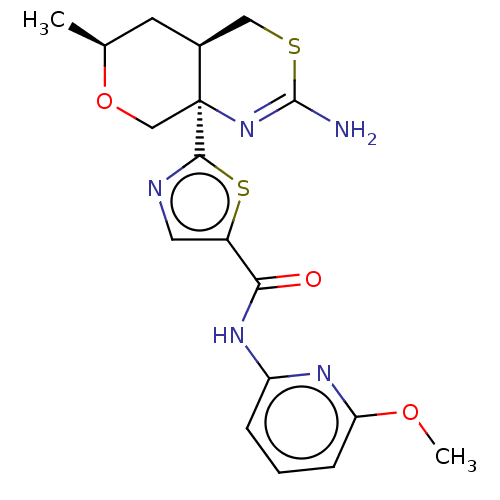 (CHEMBL4212046) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452884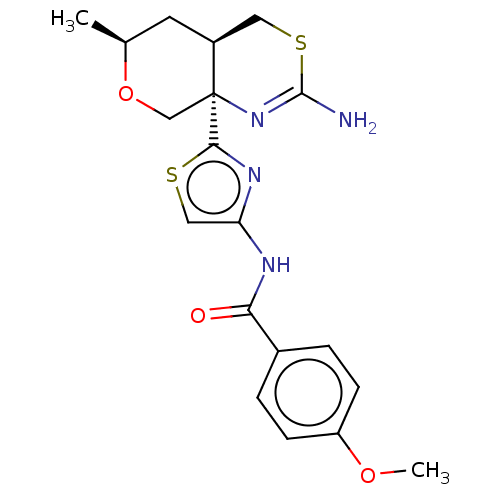 (CHEMBL4217023) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452883 (CHEMBL4203860) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314930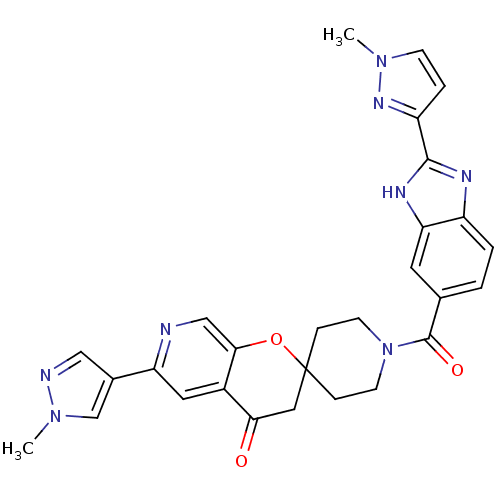 (1-(2-(1-methyl-1H-pyrazol-3-yl)-1H-benzo[d]imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314931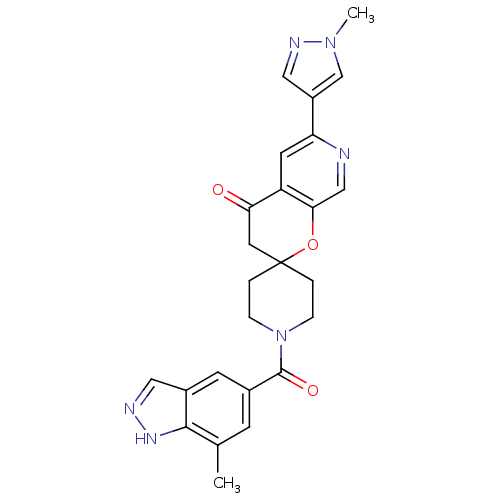 (1-(7-methyl-1H-indazole-5-carbonyl)-6'-(1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314932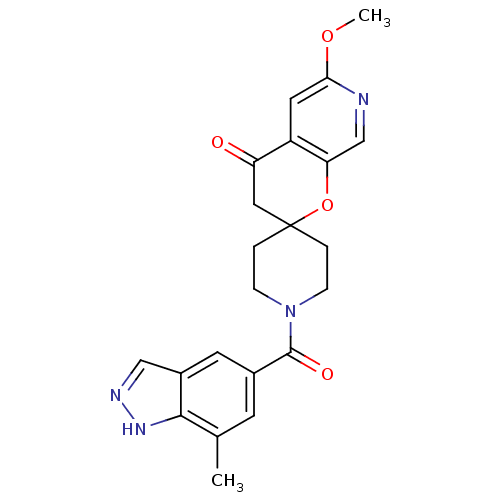 (6'-methoxy-1-(7-methyl-1H-indazole-5-carbonyl)spir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM47353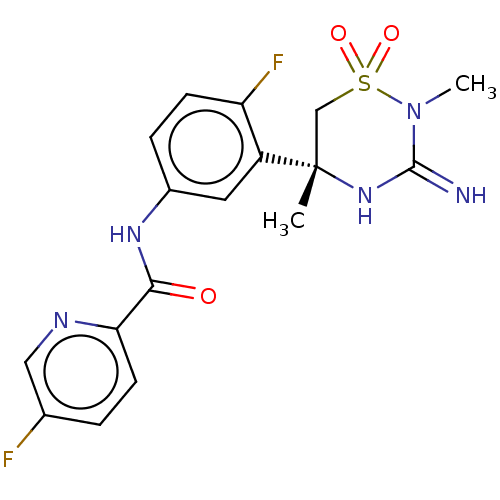 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452874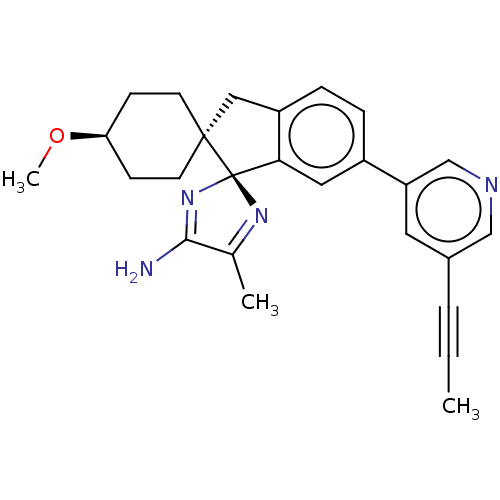 (CHEMBL4217620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312938 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50452874 (CHEMBL4217620) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259964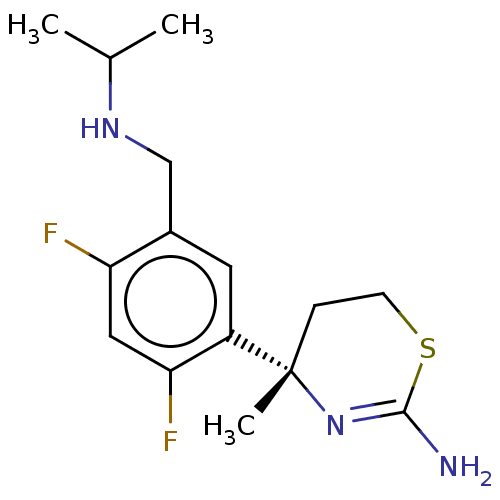 (CHEMBL4083698) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM47353 (BDBM143220 | US9029362, 173 | US9687494, 25) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223400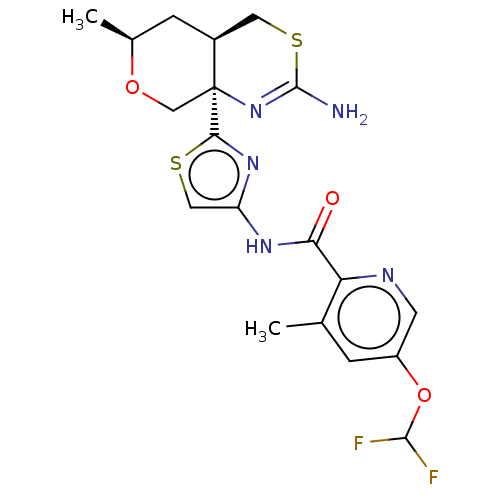 (US9315520, 45 | US9315520, Example 45 | US9605007,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM136576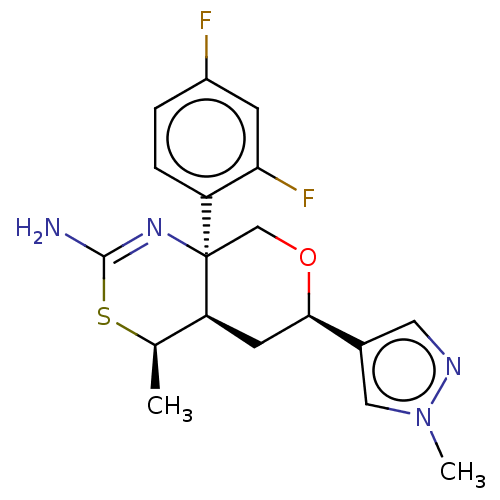 (US8865706, 16) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50081645 (CHEMBL3422237) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length Myc-DDK-tagged BACE2 expressed in HEK293 cell membranes after 30 mins by parallel ... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223396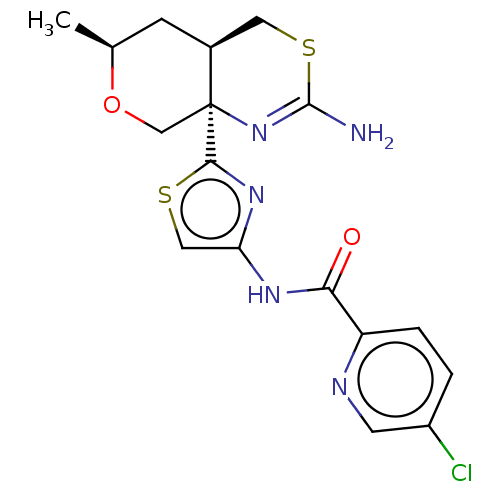 (US9315520, 7 | US9315520, Example 7 | US9605007, E...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312940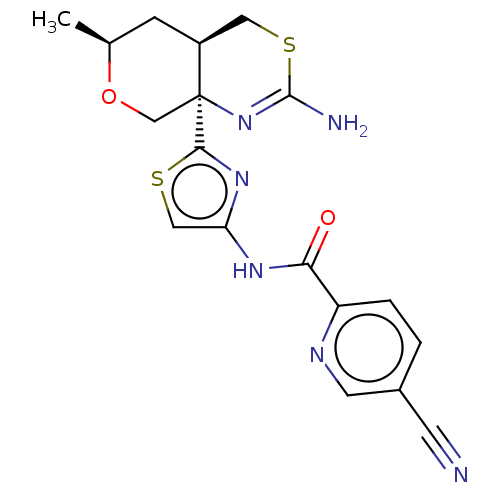 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM312938 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259968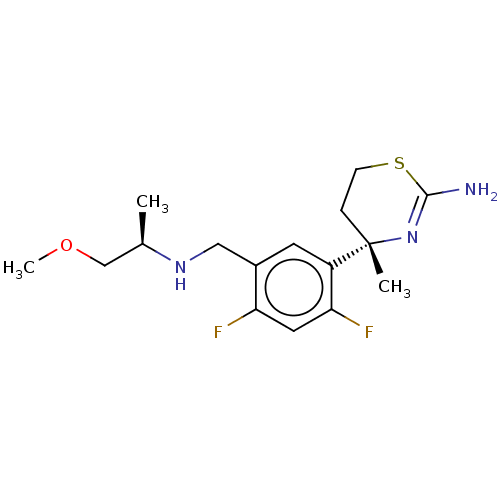 (CHEMBL4084653) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50081646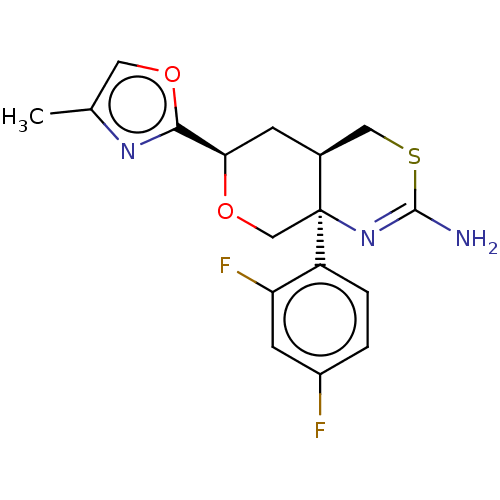 (CHEMBL3422236) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM41536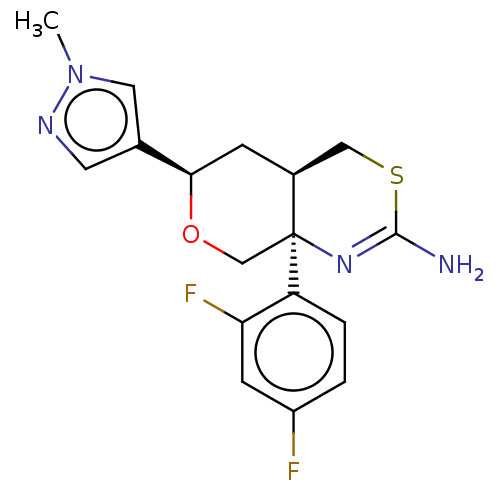 (US8865706, 15) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259974 (CHEMBL4102593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314905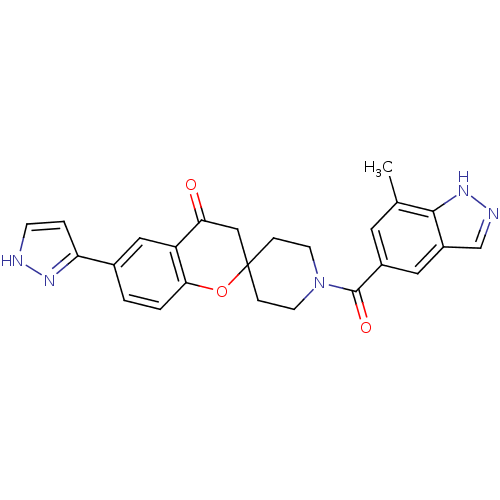 (1'-(7-methyl-1H-indazole-5-carbonyl)-6-(1H-pyrazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314938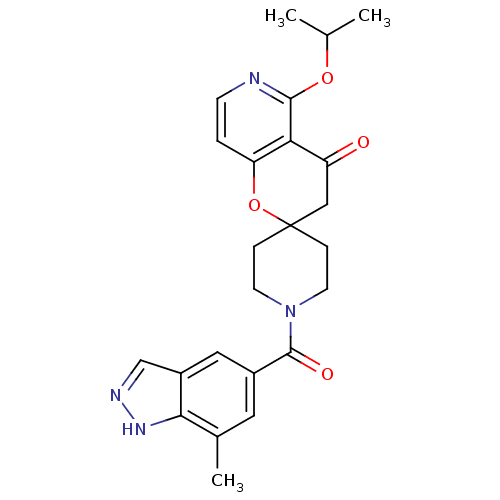 (5'-isopropoxy-1-(7-methyl-1H-indazole-5-carbonyl)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 using biotin-GLTNIKTEEISEISYEVEFR-C[Oregon green]KK-OH as substrate after 3 hrs by fluorescence polarization assay | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223330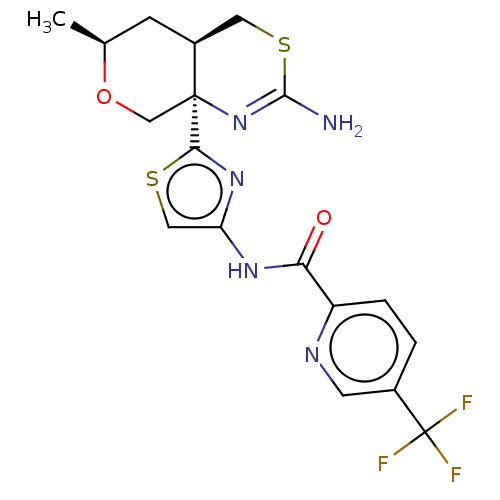 (US9315520, 17 | US9605007, Example 17 | US9744173,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE1 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223395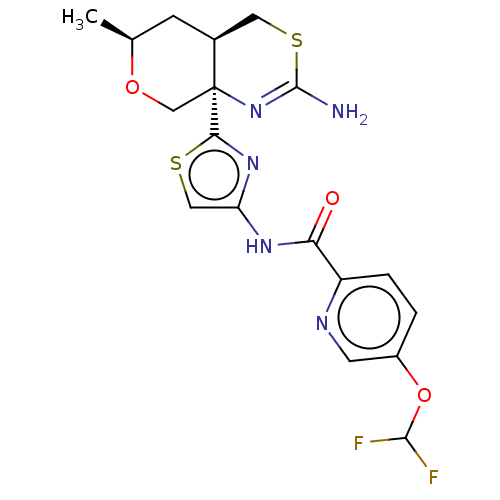 (US9315520, 1 | US9315520, Comparator 1 | US9315520...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50012653 (CHEMBL3261067) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human BACE2 | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50259966 (CHEMBL4092406) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells expressing wild type APP695 assessed as reduction in soluble APPbeta level after 18 hrs by ELISA | J Med Chem 60: 386-402 (2017) Article DOI: 10.1021/acs.jmedchem.6b01451 BindingDB Entry DOI: 10.7270/Q2154KHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM148176 (US8962616, 22 | US8962616, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM136570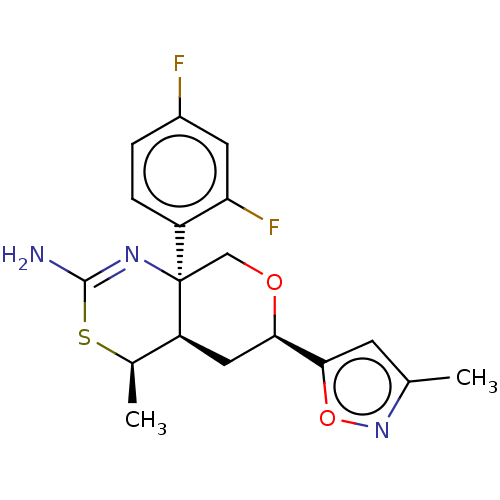 (US8865706, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314906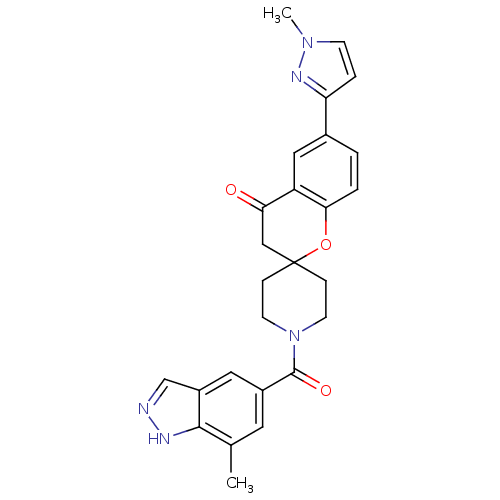 (1'-(7-methyl-1H-indazole-5-carbonyl)-6-(1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM143218 (US8940748, 34 | US9029362, 34 | US9687494, 34) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length FL-tagged BACE1 expressed in HEK293 cell membranes after 30 mins by parallel scint... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314929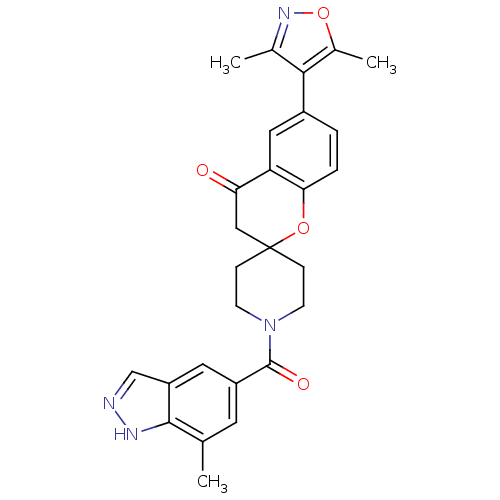 (6-(3,5-dimethylisoxazol-4-yl)-1'-(7-methyl-1H-inda...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314925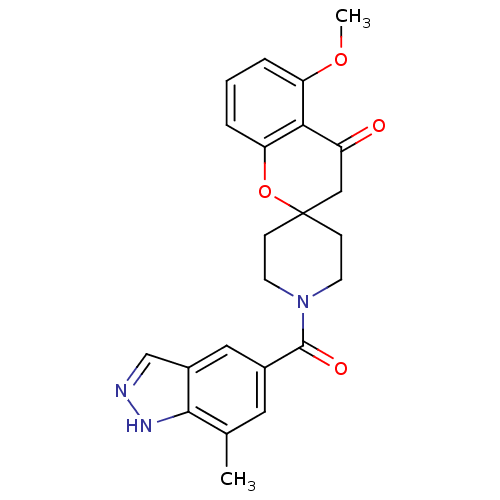 (5-methoxy-1'-(7-methyl-1H-indazole-5-carbonyl)spir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365293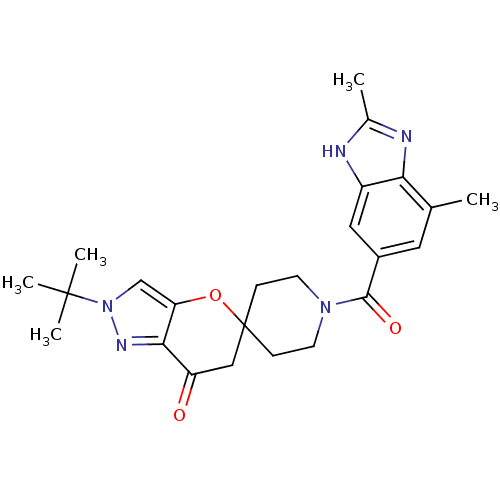 (CHEMBL1958374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365279 (CHEMBL1958360) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 1 (Rattus norvegicus (Rat)) | BDBM50365279 (CHEMBL1958360) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of rat liver Acetyl-CoA carboxylase 1 using acetyl-CoA as substrate preincubated for 10 mins prior substrate addition measured after 20 mi... | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM136583 (US8865706, 22) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) using Biotin-GLTNIKTEEISEISYEVEFR-C[oregon green]KK-OH substrate assessed as fluorescence polarization by cell f... | J Med Chem 58: 3223-52 (2015) Article DOI: 10.1021/acs.jmedchem.5b00191 BindingDB Entry DOI: 10.7270/Q29W0H68 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM312934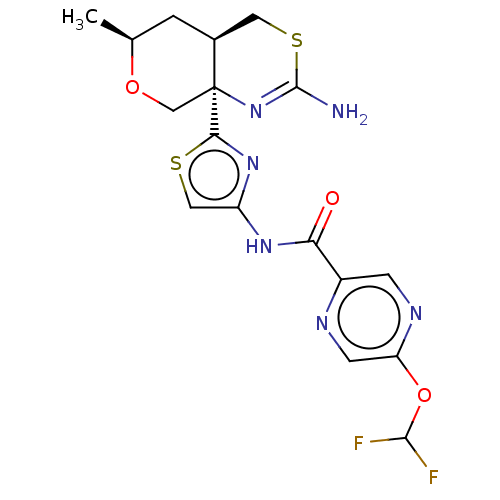 (N-{2-[(4aR,6S,8aR)-2-amino-6-methyl-4,4a,5,6-tetra...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of BACE1 in human H4 cells transfected with wild type APP assessed as reduction in soluble portion of APPbeta level in cells incubated for... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM223395 (US9315520, 1 | US9315520, Comparator 1 | US9315520...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Displacement of [3H]-PF-6475886 from recombinant human full length FL-tagged BACE1 expressed in HEK293 cell membranes after 30 mins by parallel scint... | J Med Chem 61: 4476-4504 (2018) Article DOI: 10.1021/acs.jmedchem.8b00246 BindingDB Entry DOI: 10.7270/Q23J3GJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50365292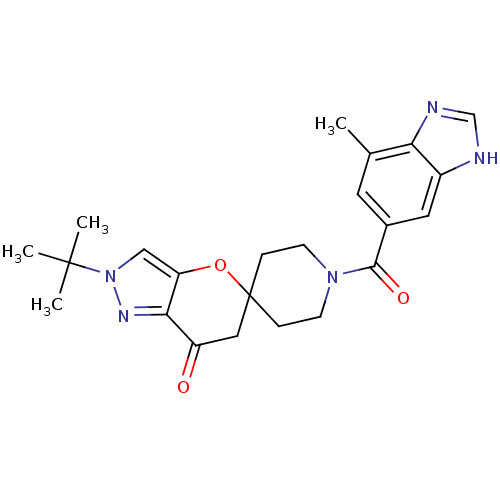 (CHEMBL1958373) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human Acetyl-CoA carboxylase 2 expressed in CHO cells after 1 hr by fluorescence assay | J Med Chem 55: 935-42 (2012) Article DOI: 10.1021/jm201503u BindingDB Entry DOI: 10.7270/Q2CZ37MS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 527 total ) | Next | Last >> |