

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1D receptor beta | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 1D receptor alpha | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469879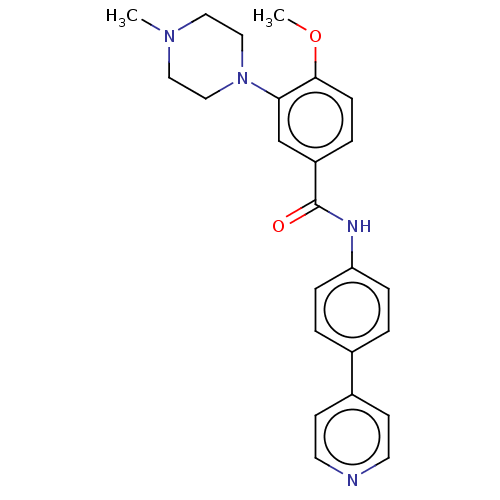 (CHEMBL72088) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1B receptor in rat striatal membrane with [125I]- iodocyanopindolol | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469882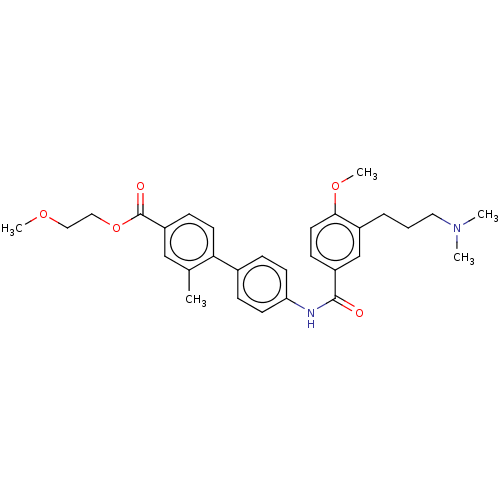 (CHEMBL73446) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469875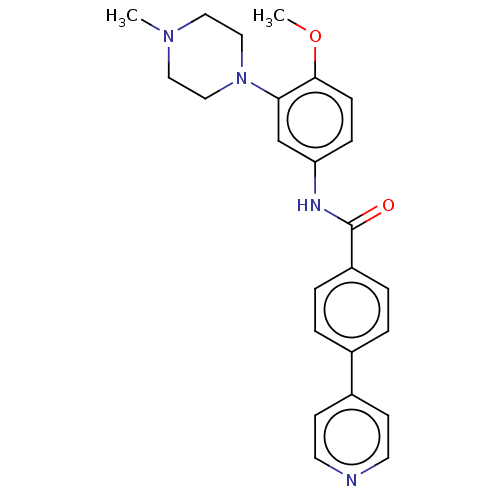 (CHEMBL72981) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469881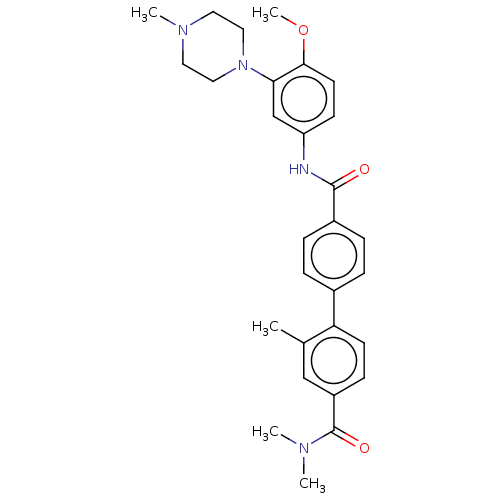 (CHEMBL311150) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50469881 (CHEMBL311150) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1B receptor in rat striatal membrane with [125I]- iodocyanopindolol | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469878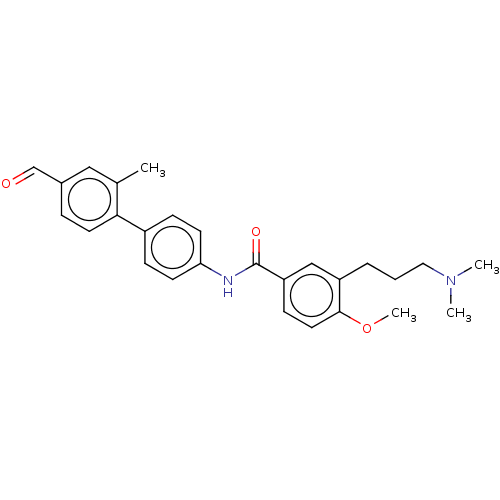 (CHEMBL72700) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469876 (CHEMBL306384) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Rattus norvegicus (Rat)) | BDBM50469876 (CHEMBL306384) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1B receptor in rat striatal membrane with [125I]- iodocyanopindolol | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50060518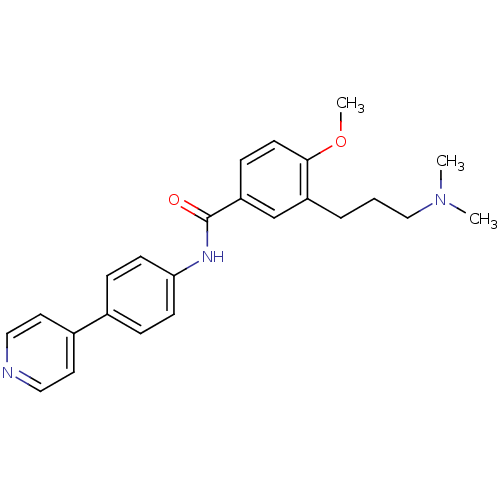 (3-(3-Dimethylamino-propyl)-4-methoxy-N-(4-pyridin-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (GUINEA PIG) | BDBM50469877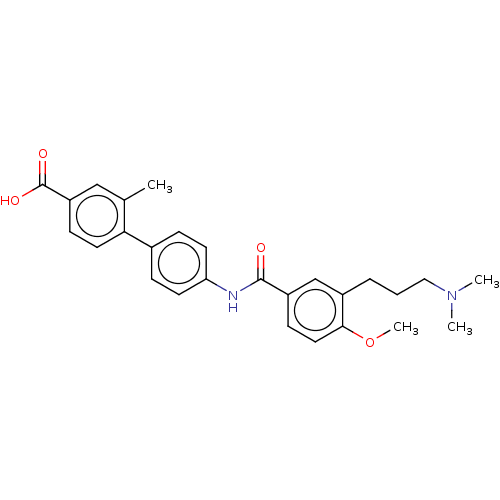 (CHEMBL72092) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description In vitro binding affinity towards 5-hydroxytryptamine 1D receptor in guinea-pig striatum in presence of BMY-7378 and mesulergine | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50469881 (CHEMBL311150) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus with [3H]8-OH-DPAT | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against alpha-1 adrenoceptor | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A/Alpha-2B/Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against alpha-2 adrenoceptor | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against muscarinic M1 receptor | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against muscarinic M3 receptor | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against muscarinic M2 receptor | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50469875 (CHEMBL72981) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus with [3H]8-OH-DPAT | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50469876 (CHEMBL306384) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50469878 (CHEMBL72700) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50469880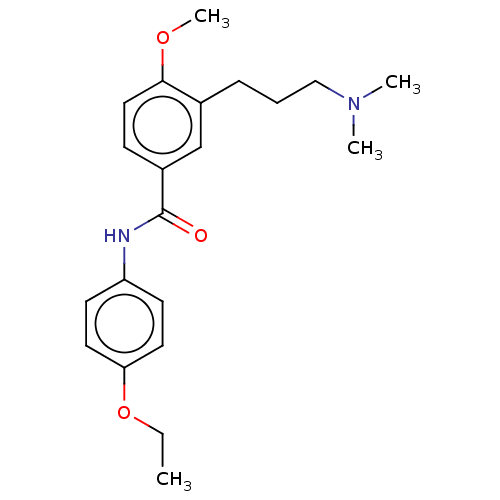 (CHEMBL75771) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine D4 receptor | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50469877 (CHEMBL72092) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine D2 receptor | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine receptor D1 | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against dopamine D3 receptor | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Mus musculus (Mouse)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Tested for 5-hydroxytryptamine receptor uptake | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM79215 (CHEMBL15928 | GR 127935 | GR 127935 hydrochloride ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 4 receptor | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50060518 (3-(3-Dimethylamino-propyl)-4-methoxy-N-(4-pyridin-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research and Development Ltd. Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 1A receptor in rat hippocampus using [3H]8-OH-DPAT | J Med Chem 37: 2253-7 (1994) Article DOI: 10.1021/jm00041a001 BindingDB Entry DOI: 10.7270/Q29889QB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029600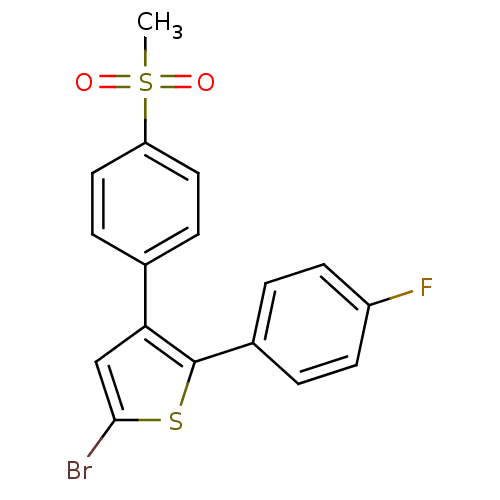 (5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297695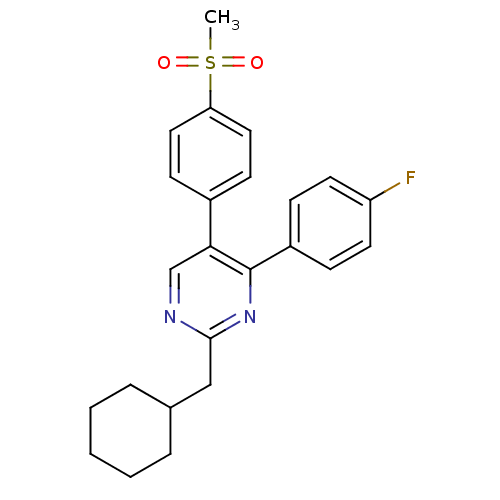 (2-(cyclohexylmethyl)-4-(4-fluorophenyl)-5-(4-(meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297696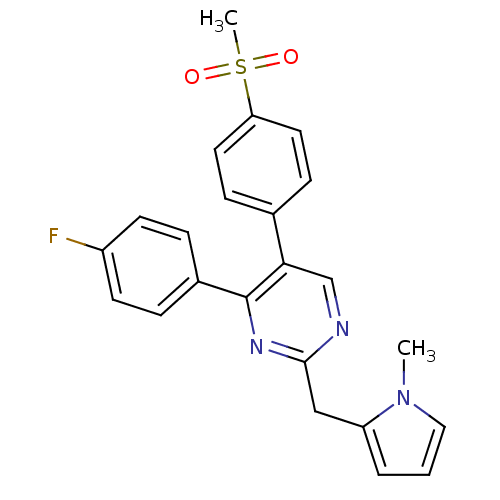 (4-(4-fluorophenyl)-2-((1-methyl-1H-pyrrol-2-yl)met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297709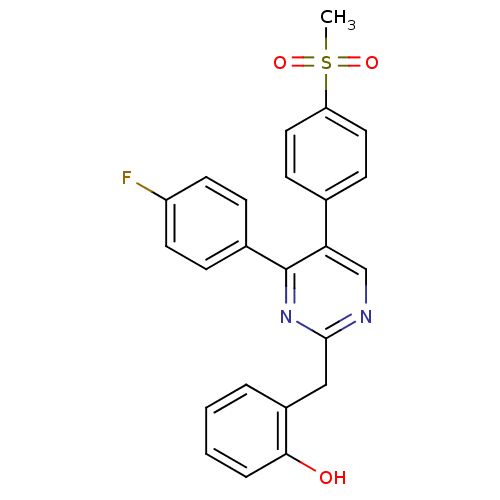 (2-((4-(4-fluorophenyl)-5-(4-(methylsulfonyl)phenyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297697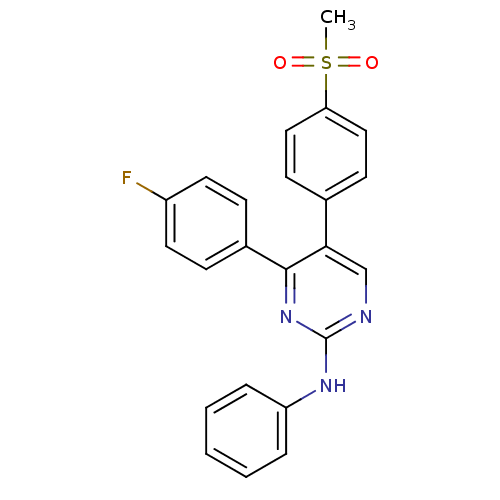 (4-(4-fluorophenyl)-5-(4-(methylsulfonyl)phenyl)-N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297708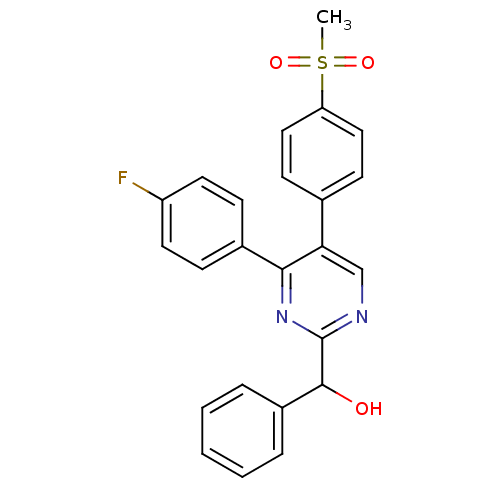 ((4-(4-fluorophenyl)-5-(4-(methylsulfonyl)phenyl)py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50029616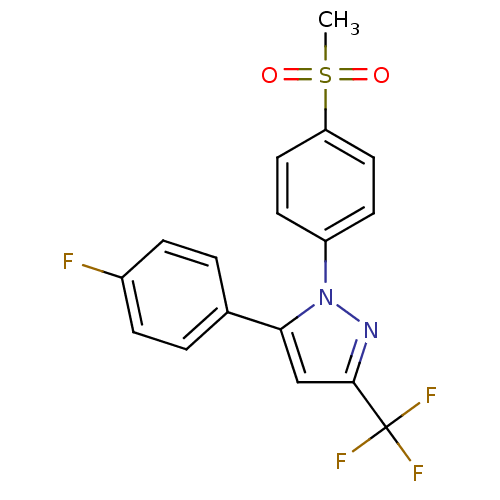 (5-(4-Fluoro-phenyl)-1-(4-methanesulfonyl-phenyl)-3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297698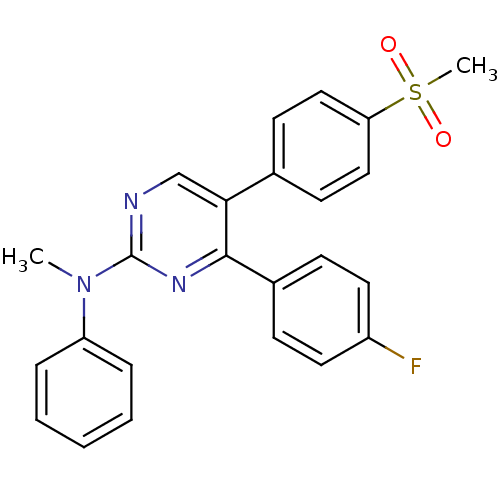 (4-(4-fluorophenyl)-N-methyl-5-(4-(methylsulfonyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297699 (2-benzyl-4-(4-fluorophenyl)-5-(4-(methylsulfonyl)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297700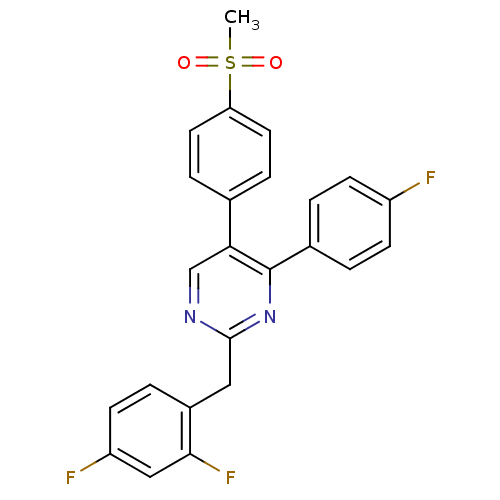 (2-(2,4-difluorobenzyl)-4-(4-fluorophenyl)-5-(4-(me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297701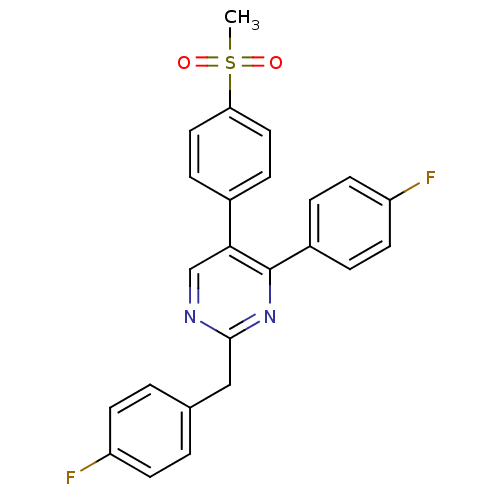 (2-(4-fluorobenzyl)-4-(4-fluorophenyl)-5-(4-(methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 277 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297702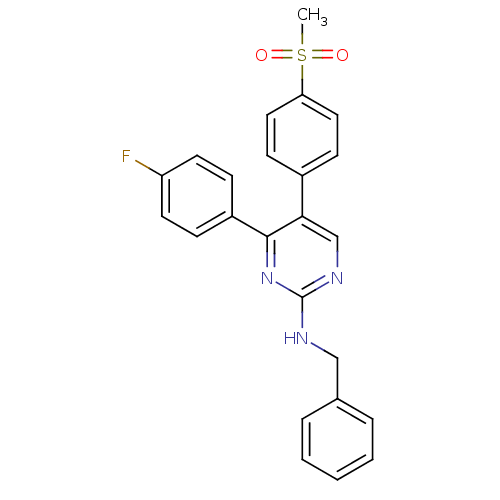 (CHEMBL562768 | N-benzyl-4-(4-fluorophenyl)-5-(4-(m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 686 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297710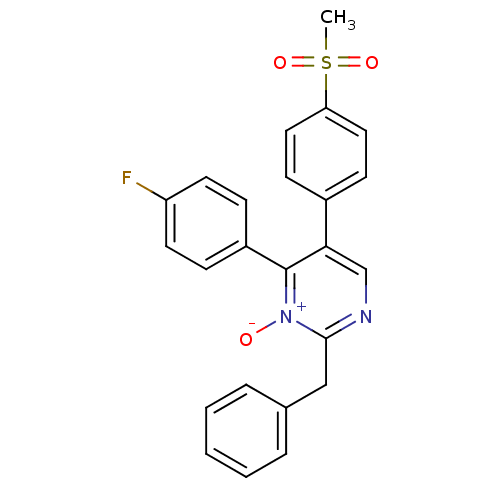 (2-Benzyl-4-(4-fluoro-phenyl)-5-(4-methanesulfonyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 717 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50029600 (5-Bromo-2-(4-fluoro-phenyl)-3-(4-methanesulfonyl-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX1 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50297703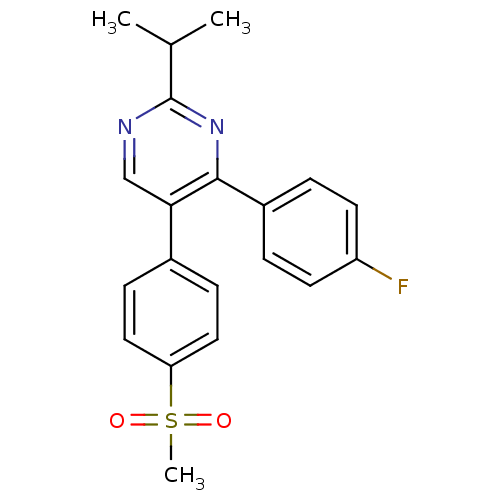 (4-(4-fluorophenyl)-2-isopropyl-5-(4-(methylsulfony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX2 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50297695 (2-(cyclohexylmethyl)-4-(4-fluorophenyl)-5-(4-(meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX1 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50297698 (4-(4-fluorophenyl)-N-methyl-5-(4-(methylsulfonyl)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human COX1 expressed in african green monkey COS cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme ... | Bioorg Med Chem Lett 19: 4509-14 (2009) Article DOI: 10.1016/j.bmcl.2009.02.089 BindingDB Entry DOI: 10.7270/Q2W66KSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 84 total ) | Next | Last >> |