 Found 942 hits with Last Name = 'campbell' and Initial = 'rm'
Found 942 hits with Last Name = 'campbell' and Initial = 'rm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Adenosine receptor A1
(Rattus norvegicus (rat)) | BDBM50051653
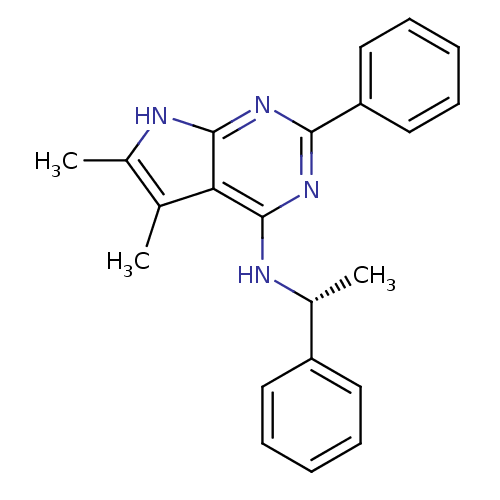
((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...)Show SMILES C[C@@H](Nc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H22N4/c1-14-15(2)23-21-19(14)22(24-16(3)17-10-6-4-7-11-17)26-20(25-21)18-12-8-5-9-13-18/h4-13,16H,1-3H3,(H2,23,24,25,26)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Binding affinity towards rat A1 receptor was determined |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM21173
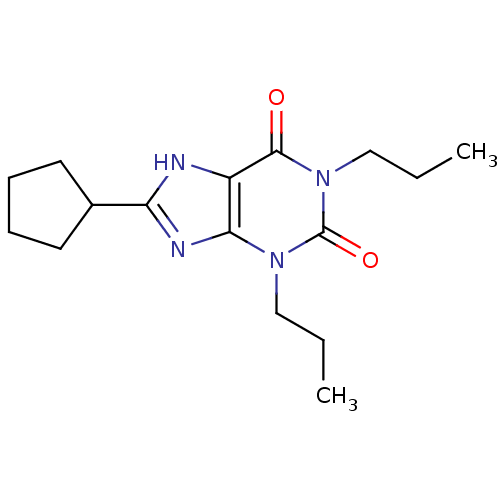
(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-CGS-21,680 to human A2a receptor (hA2a) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080288
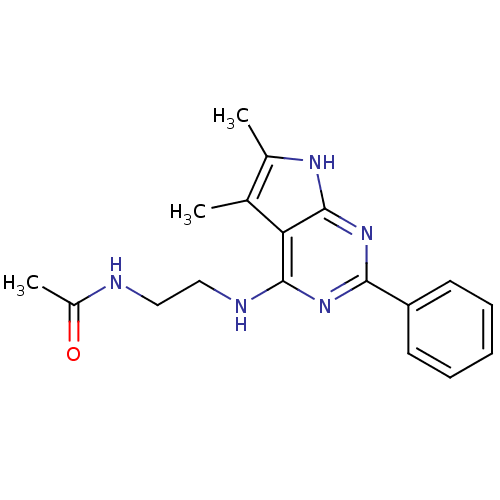
(CHEMBL81616 | N-[2-(5,6-Dimethyl-2-phenyl-7H-pyrro...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C18H21N5O/c1-11-12(2)21-18-15(11)17(20-10-9-19-13(3)24)22-16(23-18)14-7-5-4-6-8-14/h4-8H,9-10H2,1-3H3,(H,19,24)(H2,20,21,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080288

(CHEMBL81616 | N-[2-(5,6-Dimethyl-2-phenyl-7H-pyrro...)Show SMILES CC(=O)NCCNc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C18H21N5O/c1-11-12(2)21-18-15(11)17(20-10-9-19-13(3)24)22-16(23-18)14-7-5-4-6-8-14/h4-8H,9-10H2,1-3H3,(H,19,24)(H2,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-CGS-21,680 to human A2a receptor (hA2a) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080292
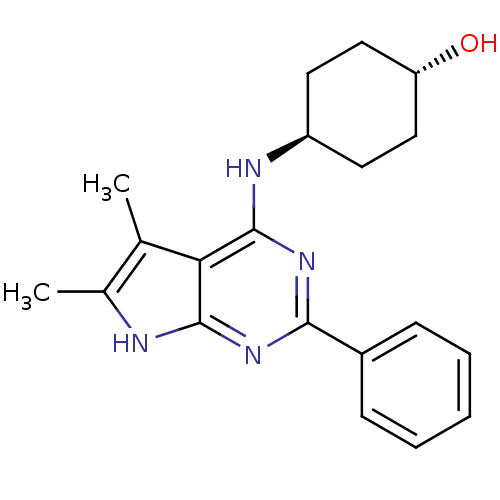
(4-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidi...)Show SMILES Cc1[nH]c2nc(nc(N[C@H]3CC[C@H](O)CC3)c2c1C)-c1ccccc1 |wU:9.8,wD:12.12,(7.76,-9.44,;6.22,-9.46,;5.3,-10.73,;3.85,-10.24,;2.5,-11.04,;1.17,-10.27,;1.17,-8.73,;2.5,-7.93,;2.48,-6.39,;1.15,-5.65,;-.16,-6.42,;-1.5,-5.65,;-1.5,-4.11,;-2.84,-3.34,;-.16,-3.34,;1.17,-4.09,;3.83,-8.7,;5.3,-8.22,;5.75,-6.75,;-.16,-11.04,;-.15,-12.55,;-1.5,-13.32,;-2.82,-12.55,;-2.82,-11.01,;-1.5,-10.24,)| Show InChI InChI=1S/C20H24N4O/c1-12-13(2)21-19-17(12)20(22-15-8-10-16(25)11-9-15)24-18(23-19)14-6-4-3-5-7-14/h3-7,15-16,25H,8-11H2,1-2H3,(H2,21,22,23,24)/t15-,16- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM21173

(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080291
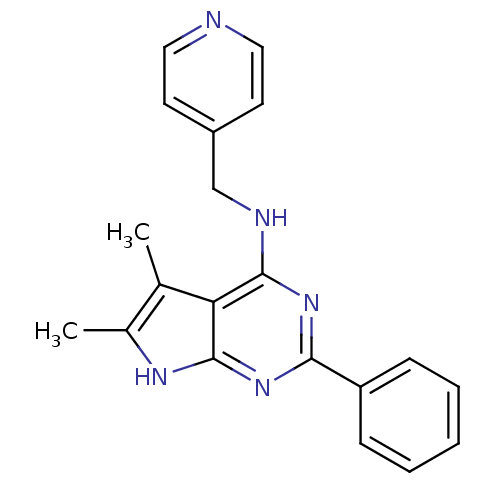
((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...)Show InChI InChI=1S/C20H19N5/c1-13-14(2)23-20-17(13)19(22-12-15-8-10-21-11-9-15)24-18(25-20)16-6-4-3-5-7-16/h3-11H,12H2,1-2H3,(H2,22,23,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 132 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080287
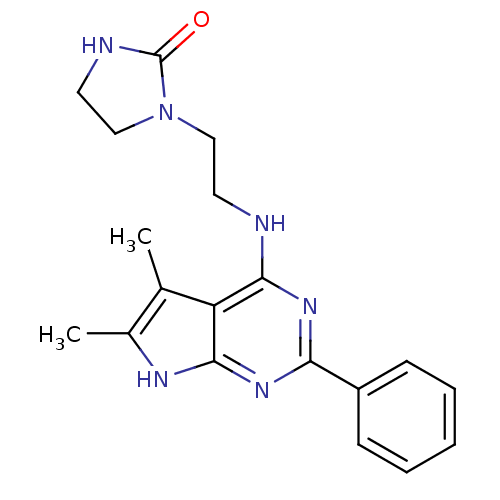
(1-[2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrim...)Show SMILES Cc1[nH]c2nc(nc(NCCN3CCNC3=O)c2c1C)-c1ccccc1 Show InChI InChI=1S/C19H22N6O/c1-12-13(2)22-18-15(12)17(20-8-10-25-11-9-21-19(25)26)23-16(24-18)14-6-4-3-5-7-14/h3-7H,8-11H2,1-2H3,(H,21,26)(H2,20,22,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080287

(1-[2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrim...)Show SMILES Cc1[nH]c2nc(nc(NCCN3CCNC3=O)c2c1C)-c1ccccc1 Show InChI InChI=1S/C19H22N6O/c1-12-13(2)22-18-15(12)17(20-8-10-25-11-9-21-19(25)26)23-16(24-18)14-6-4-3-5-7-14/h3-7H,8-11H2,1-2H3,(H,21,26)(H2,20,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080292

(4-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidi...)Show SMILES Cc1[nH]c2nc(nc(N[C@H]3CC[C@H](O)CC3)c2c1C)-c1ccccc1 |wU:9.8,wD:12.12,(7.76,-9.44,;6.22,-9.46,;5.3,-10.73,;3.85,-10.24,;2.5,-11.04,;1.17,-10.27,;1.17,-8.73,;2.5,-7.93,;2.48,-6.39,;1.15,-5.65,;-.16,-6.42,;-1.5,-5.65,;-1.5,-4.11,;-2.84,-3.34,;-.16,-3.34,;1.17,-4.09,;3.83,-8.7,;5.3,-8.22,;5.75,-6.75,;-.16,-11.04,;-.15,-12.55,;-1.5,-13.32,;-2.82,-12.55,;-2.82,-11.01,;-1.5,-10.24,)| Show InChI InChI=1S/C20H24N4O/c1-12-13(2)21-19-17(12)20(22-15-8-10-16(25)11-9-15)24-18(23-19)14-6-4-3-5-7-14/h3-7,15-16,25H,8-11H2,1-2H3,(H2,21,22,23,24)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 754 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-CGS-21,680 to human A2a receptor (hA2a) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080289
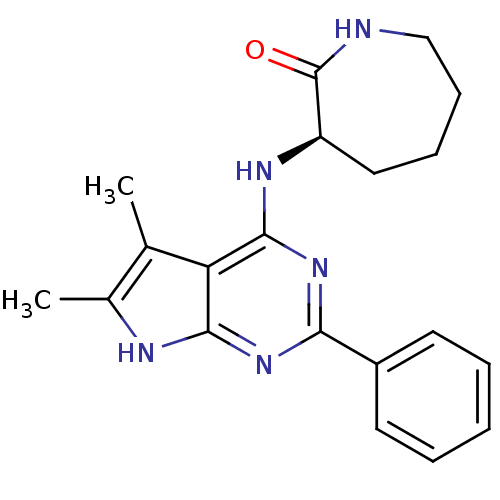
((R)-3-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES Cc1[nH]c2nc(nc(N[C@@H]3CCCCNC3=O)c2c1C)-c1ccccc1 Show InChI InChI=1S/C20H23N5O/c1-12-13(2)22-18-16(12)19(23-15-10-6-7-11-21-20(15)26)25-17(24-18)14-8-4-3-5-9-14/h3-5,8-9,15H,6-7,10-11H2,1-2H3,(H,21,26)(H2,22,23,24,25)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 771 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against membranes from yeast cells transformed with human A1 receptor (hA1) |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080291

((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...)Show InChI InChI=1S/C20H19N5/c1-13-14(2)23-20-17(13)19(22-12-15-8-10-21-11-9-15)24-18(25-20)16-6-4-3-5-7-16/h3-11H,12H2,1-2H3,(H2,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 811 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-CGS-21,680 to human A2a receptor (hA2a) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080290

((1R,2R)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]...)Show SMILES Cc1[nH]c2nc(nc(N[C@@H]3Cc4ccccc4[C@H]3O)c2c1C)-c1ccccc1 Show InChI InChI=1S/C23H22N4O/c1-13-14(2)24-22-19(13)23(27-21(26-22)15-8-4-3-5-9-15)25-18-12-16-10-6-7-11-17(16)20(18)28/h3-11,18,20,28H,12H2,1-2H3,(H2,24,25,26,27)/t18-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 896 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50080286
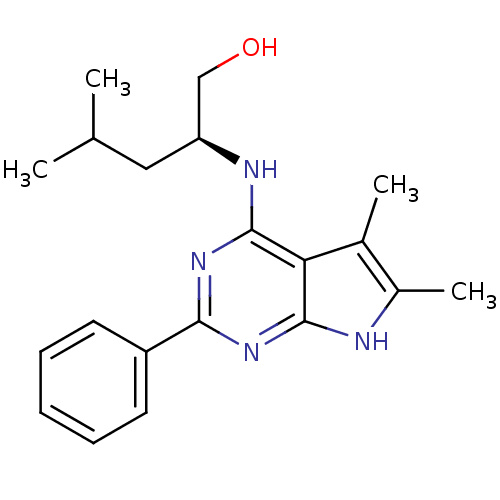
((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES CC(C)C[C@@H](CO)Nc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C20H26N4O/c1-12(2)10-16(11-25)22-20-17-13(3)14(4)21-19(17)23-18(24-20)15-8-6-5-7-9-15/h5-9,12,16,25H,10-11H2,1-4H3,(H2,21,22,23,24)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 951 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of the [3H]-DPCPX to human A1 receptor (hA1) radioligands using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080289

((R)-3-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES Cc1[nH]c2nc(nc(N[C@@H]3CCCCNC3=O)c2c1C)-c1ccccc1 Show InChI InChI=1S/C20H23N5O/c1-12-13(2)22-18-16(12)19(23-15-10-6-7-11-21-20(15)26)25-17(24-18)14-8-4-3-5-9-14/h3-5,8-9,15H,6-7,10-11H2,1-2H3,(H,21,26)(H2,22,23,24,25)/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 962 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50051653

((5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyrimidin-...)Show SMILES C[C@@H](Nc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C22H22N4/c1-14-15(2)23-21-19(14)22(24-16(3)17-10-6-4-7-11-17)26-20(25-21)18-12-8-5-9-13-18/h4-13,16H,1-3H3,(H2,23,24,25,26)/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 981 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50080286

((S)-2-(5,6-Dimethyl-2-phenyl-7H-pyrrolo[2,3-d]pyri...)Show SMILES CC(C)C[C@@H](CO)Nc1nc(nc2[nH]c(C)c(C)c12)-c1ccccc1 Show InChI InChI=1S/C20H26N4O/c1-12(2)10-16(11-25)22-20-17-13(3)14(4)21-19(17)23-18(24-20)15-8-6-5-7-9-15/h5-9,12,16,25H,10-11H2,1-4H3,(H2,21,22,23,24)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cadus Pharmaceutical Corporation
Curated by ChEMBL
| Assay Description
Inhibitory activity against binding of [3H]-DPCPX to human A1 receptor (hA1) using competition binding assay |
Bioorg Med Chem Lett 9: 2413-8 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V5N |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50474994
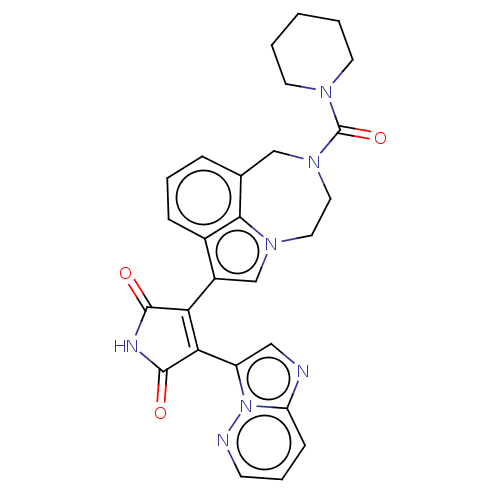
(Bisarylmaleimide 1)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cccnn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-14-28-21-8-5-9-29-34(20)21)19-16-32-12-13-33(27(37)31-10-2-1-3-11-31)15-17-6-4-7-18(19)24(17)32/h4-9,14,16H,1-3,10-13,15H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699
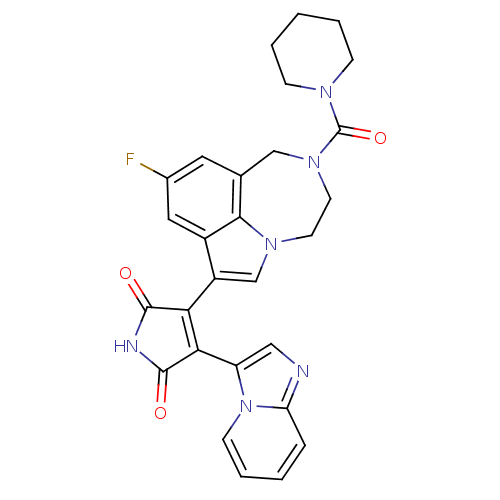
(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475007

(Bisarylmaleimide 2)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H23N5O5/c33-25-21(19-13-28-12-16-4-9-37-24(16)19)22(26(34)29-25)20-15-31-5-6-32(27(35)30-7-10-36-11-8-30)14-17-2-1-3-18(20)23(17)31/h1-4,9,12-13,15H,5-8,10-11,14H2,(H,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475025

(CHEMBL181339)Show SMILES O=C(C1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H25N5O4/c34-26-23(24(27(35)30-26)21-14-29-22-6-1-2-9-33(21)22)20-16-31-10-11-32(28(36)17-7-12-37-13-8-17)15-18-4-3-5-19(20)25(18)31/h1-6,9,14,16-17H,7-8,10-13,15H2,(H,30,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475024

(CHEMBL181371)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H18N4O4/c1-13(29)27-6-7-28-12-18(16-4-2-3-15(11-27)21(16)28)20-19(23(30)26-24(20)31)17-10-25-9-14-5-8-32-22(14)17/h2-5,8-10,12H,6-7,11H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475018
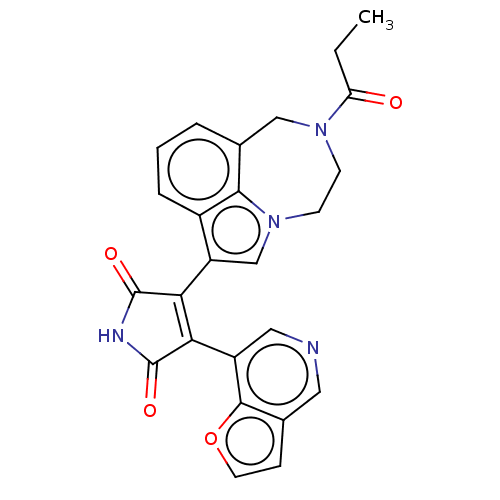
(CHEMBL181518)Show SMILES CCC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:10| Show InChI InChI=1S/C25H20N4O4/c1-2-19(30)28-7-8-29-13-18(16-5-3-4-15(12-28)22(16)29)21-20(24(31)27-25(21)32)17-11-26-10-14-6-9-33-23(14)17/h3-6,9-11,13H,2,7-8,12H2,1H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475031

(CHEMBL359871)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4CCOc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C25H21N3O4/c1-14(29)27-9-10-28-13-19(17-6-3-5-16(12-27)22(17)28)21-20(24(30)26-25(21)31)18-7-2-4-15-8-11-32-23(15)18/h2-7,13H,8-12H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475029

(CHEMBL180779)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H21N5O4/c1-28(2)25(33)30-8-7-29-13-18(16-5-3-4-15(12-30)21(16)29)20-19(23(31)27-24(20)32)17-11-26-10-14-6-9-34-22(14)17/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50132988
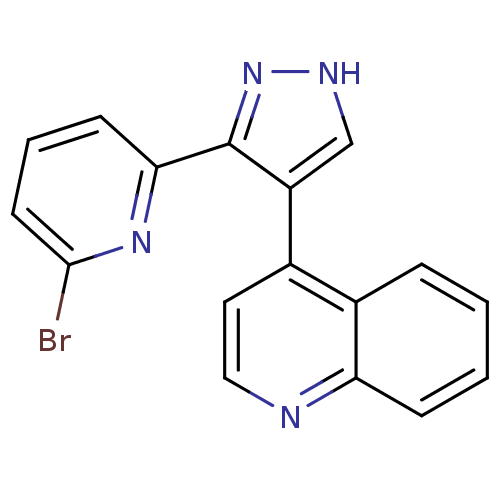
(4-[3-(6-Bromo-pyridin-2-yl)-1H-pyrazol-4-yl]-quino...)Show InChI InChI=1S/C17H11BrN4/c18-16-7-3-6-15(21-16)17-13(10-20-22-17)11-8-9-19-14-5-2-1-4-12(11)14/h1-10H,(H,20,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of transforming growth factor- beta dependent luciferase growth in mouse fibroblasts (NIH 3T3) |
J Med Chem 46: 3953-6 (2003)
Article DOI: 10.1021/jm0205705
BindingDB Entry DOI: 10.7270/Q2RV0N38 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150700
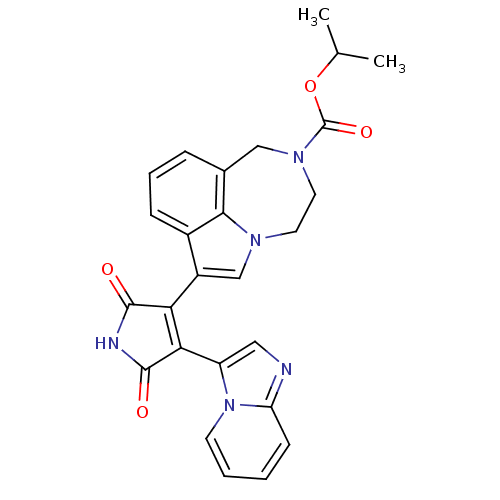
(7-(4-Imidazo[1,2-a]pyridin-3-yl-2,5-dioxo-2,5-dihy...)Show SMILES CC(C)OC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:12| Show InChI InChI=1S/C26H23N5O4/c1-15(2)35-26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698
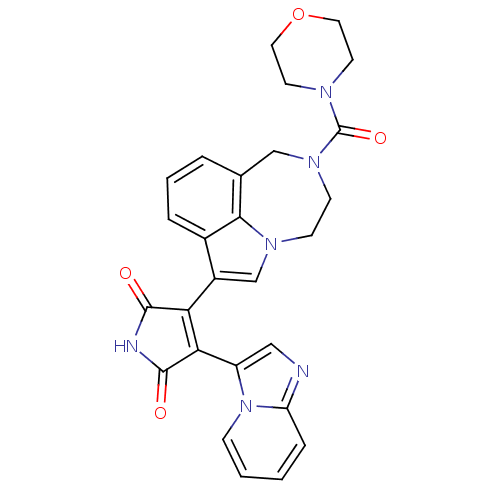
(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475008

(CHEMBL369090)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3ccn4ncccc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(19-6-8-33-21(19)5-2-7-28-33)23(26(35)29-25)20-16-31-9-10-32(27(36)30-11-13-37-14-12-30)15-17-3-1-4-18(20)24(17)31/h1-8,16H,9-15H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475022

(CHEMBL361765)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4ccoc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C25H19N3O4/c1-14(29)27-9-10-28-13-19(17-6-3-5-16(12-27)22(17)28)21-20(24(30)26-25(21)31)18-7-2-4-15-8-11-32-23(15)18/h2-8,11,13H,9-10,12H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150701
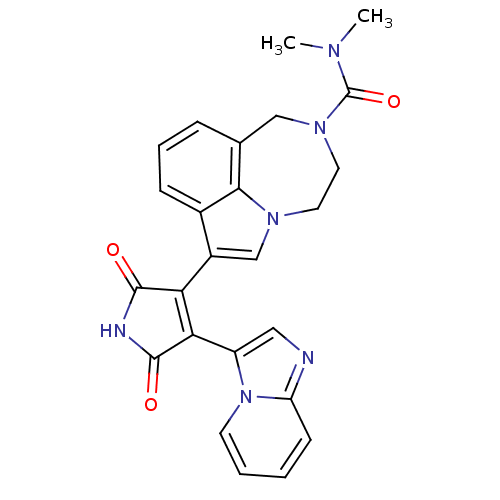
(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50474994

(Bisarylmaleimide 1)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cccnn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-14-28-21-8-5-9-29-34(20)21)19-16-32-12-13-33(27(37)31-10-2-1-3-11-31)15-17-6-4-7-18(19)24(17)32/h4-9,14,16H,1-3,10-13,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150701

(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475010
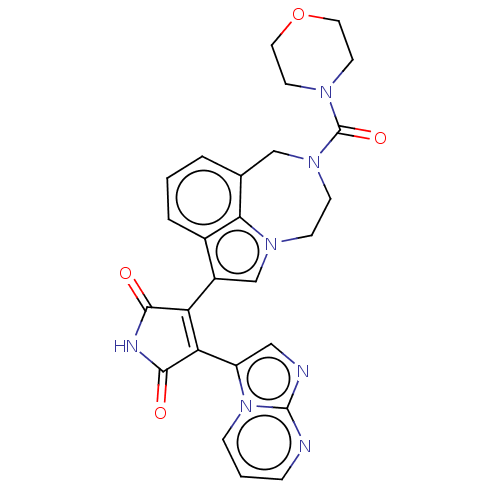
(CHEMBL369316)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ncccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C26H23N7O4/c34-23-20(21(24(35)29-23)19-13-28-25-27-5-2-6-33(19)25)18-15-31-7-8-32(26(36)30-9-11-37-12-10-30)14-16-3-1-4-17(18)22(16)31/h1-6,13,15H,7-12,14H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475001
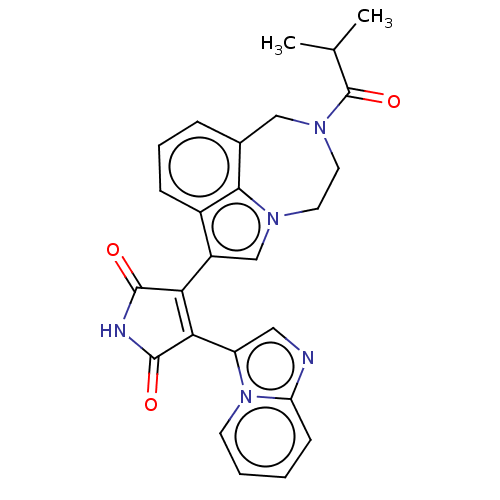
(CHEMBL368246)Show SMILES CC(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C26H23N5O3/c1-15(2)26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475004

(CHEMBL369572)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ncccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-24-21(22(25(36)30-24)20-14-29-26-28-8-5-11-34(20)26)19-16-32-12-13-33(27(37)31-9-2-1-3-10-31)15-17-6-4-7-18(19)23(17)32/h4-8,11,14,16H,1-3,9-10,12-13,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475014
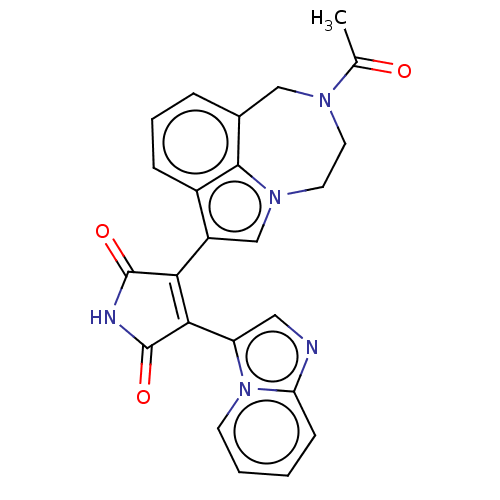
(CHEMBL361948)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H19N5O3/c1-14(30)27-9-10-28-13-17(16-6-4-5-15(12-27)22(16)28)20-21(24(32)26-23(20)31)18-11-25-19-7-2-3-8-29(18)19/h2-8,11,13H,9-10,12H2,1H3,(H,26,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM6811
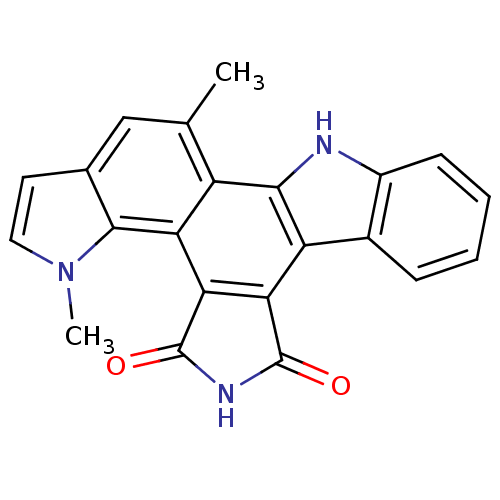
(18,23-dimethyl-3,13,18-triazahexacyclo[14.7.0.0^{2...)Show SMILES Cc1cc2ccn(C)c2c2c3C(=O)NC(=O)c3c3c4ccccc4[nH]c3c12 Show InChI InChI=1S/C22H15N3O2/c1-10-9-11-7-8-25(2)20(11)16-14(10)19-15(12-5-3-4-6-13(12)23-19)17-18(16)22(27)24-21(17)26/h3-9,23H,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 25 |
Lilly Research Laboratories
| Assay Description
In vitro CDK assay using purified enzyme, was incubated at room temperature with substrate, and test compounds in the presence of ATP/[gamma-33P]ATP.... |
Bioorg Med Chem Lett 13: 2261-7 (2003)
Article DOI: 10.1016/s0960-894x(03)00461-x
BindingDB Entry DOI: 10.7270/Q29W0CPW |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475006

(CHEMBL178851)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cnccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-13-29-21-14-28-7-10-34(20)21)19-16-32-11-12-33(27(37)31-8-2-1-3-9-31)15-17-5-4-6-18(19)24(17)32/h4-7,10,13-14,16H,1-3,8-9,11-12,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50474996

(CHEMBL178646)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H25N5O4/c34-26-22(20-14-29-13-17-7-12-37-25(17)20)23(27(35)30-26)21-16-32-10-11-33(28(36)31-8-2-1-3-9-31)15-18-5-4-6-19(21)24(18)32/h4-7,12-14,16H,1-3,8-11,15H2,(H,30,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50373946
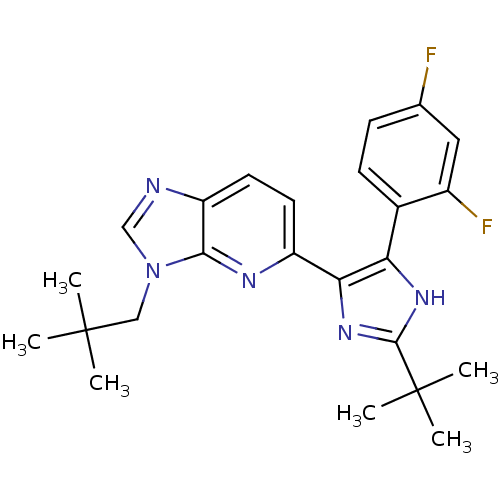
(CHEMBL270657)Show SMILES CC(C)(C)Cn1cnc2ccc(nc12)-c1nc([nH]c1-c1ccc(F)cc1F)C(C)(C)C Show InChI InChI=1S/C24H27F2N5/c1-23(2,3)12-31-13-27-18-10-9-17(28-21(18)31)20-19(29-22(30-20)24(4,5)6)15-8-7-14(25)11-16(15)26/h7-11,13H,12H2,1-6H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha |
Bioorg Med Chem Lett 18: 179-83 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.106
BindingDB Entry DOI: 10.7270/Q2R78G31 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475000

(CHEMBL181296)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4OCOc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H19N3O5/c1-13(28)26-8-9-27-11-17(15-5-2-4-14(10-26)21(15)27)20-19(23(29)25-24(20)30)16-6-3-7-18-22(16)32-12-31-18/h2-7,11H,8-10,12H2,1H3,(H,25,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50373967
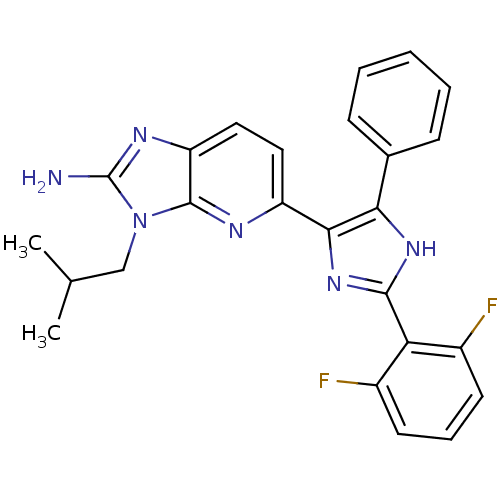
(CHEMBL442972)Show SMILES CC(C)Cn1c(N)nc2ccc(nc12)-c1nc([nH]c1-c1ccccc1)-c1c(F)cccc1F Show InChI InChI=1S/C25H22F2N6/c1-14(2)13-33-24-19(30-25(33)28)12-11-18(29-24)22-21(15-7-4-3-5-8-15)31-23(32-22)20-16(26)9-6-10-17(20)27/h3-12,14H,13H2,1-2H3,(H2,28,30)(H,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Co.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant p38alpha |
Bioorg Med Chem Lett 18: 179-83 (2008)
Article DOI: 10.1016/j.bmcl.2007.10.106
BindingDB Entry DOI: 10.7270/Q2R78G31 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data