 Found 5120 hits with Last Name = 'chang' and Initial = 'h'
Found 5120 hits with Last Name = 'chang' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM110961

(US8614206, 518)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CC[C@@](C)(N)CC(F)(F)C1 |r| Show InChI InChI=1S/C21H23F4N7OS/c1-20(27)6-7-32(10-21(24,25)9-20)19-13(8-28-31(19)2)29-17(33)15-16(26)34-18(30-15)14-11(22)4-3-5-12(14)23/h3-5,8H,6-7,9-10,26-27H2,1-2H3,(H,29,33)/t20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50505052

(CHEMBL3623150)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CC[C@@H](N)CC(F)(F)C1 |r| Show InChI InChI=1S/C20H21F4N7OS/c1-30-19(31-6-5-10(25)7-20(23,24)9-31)13(8-27-30)28-17(32)15-16(26)33-18(29-15)14-11(21)3-2-4-12(14)22/h2-4,8,10H,5-7,9,25-26H2,1H3,(H,28,32)/t10-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505054

(CHEMBL4455188)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCC[C@@H](CC1)NCC(F)F |r| Show InChI InChI=1S/C22H25F4N7OS/c1-32-22(33-8-3-4-12(7-9-33)28-11-16(25)26)15(10-29-32)30-20(34)18-19(27)35-21(31-18)17-13(23)5-2-6-14(17)24/h2,5-6,10,12,16,28H,3-4,7-9,11,27H2,1H3,(H,30,34)/t12-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM227170

(US9328106, 118)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1[C@@]12CC[C@@H](O1)[C@H](N)CC2 |r| Show InChI InChI=1S/C21H22F2N6O2S/c1-29-17(21-7-5-12(24)14(31-21)6-8-21)13(9-26-29)27-19(30)16-18(25)32-20(28-16)15-10(22)3-2-4-11(15)23/h2-4,9,12,14H,5-8,24-25H2,1H3,(H,27,30)/t12-,14-,21-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377655
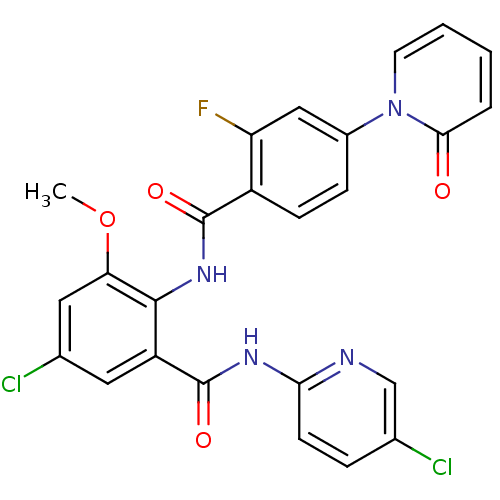
(CHEMBL260160)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1F)-n1ccccc1=O Show InChI InChI=1S/C25H17Cl2FN4O4/c1-36-20-11-15(27)10-18(25(35)30-21-8-5-14(26)13-29-21)23(20)31-24(34)17-7-6-16(12-19(17)28)32-9-3-2-4-22(32)33/h2-13H,1H3,(H,31,34)(H,29,30,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131278
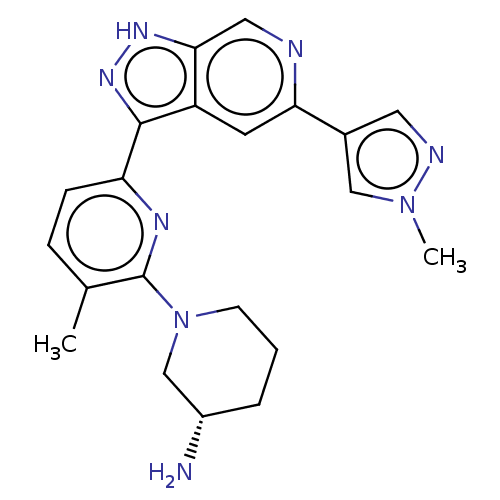
(CHEMBL3634760 | US9260425, 433)Show SMILES Cc1ccc(nc1N1CCC[C@H](N)C1)-c1n[nH]c2cnc(cc12)-c1cnn(C)c1 |r| Show InChI InChI=1S/C21H24N8/c1-13-5-6-17(25-21(13)29-7-3-4-15(22)12-29)20-16-8-18(14-9-24-28(2)11-14)23-10-19(16)26-27-20/h5-6,8-11,15H,3-4,7,12,22H2,1-2H3,(H,26,27)/t15-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM227170

(US9328106, 118)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1[C@@]12CC[C@@H](O1)[C@H](N)CC2 |r| Show InChI InChI=1S/C21H22F2N6O2S/c1-29-17(21-7-5-12(24)14(31-21)6-8-21)13(9-26-29)27-19(30)16-18(25)32-20(28-16)15-10(22)3-2-4-11(15)23/h2-4,9,12,14H,5-8,24-25H2,1H3,(H,27,30)/t12-,14-,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505051
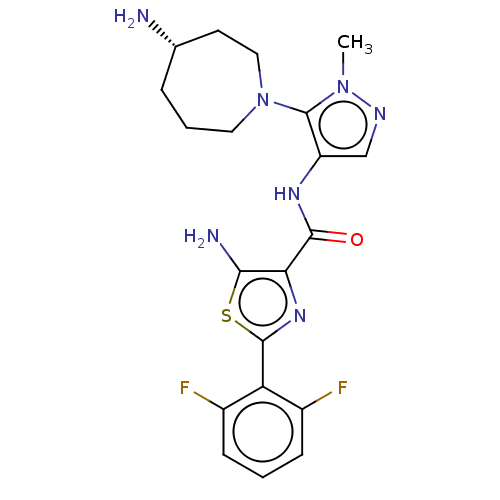
(CHEMBL4437940)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCC[C@H](N)CC1 |r| Show InChI InChI=1S/C20H23F2N7OS/c1-28-20(29-8-3-4-11(23)7-9-29)14(10-25-28)26-18(30)16-17(24)31-19(27-16)15-12(21)5-2-6-13(15)22/h2,5-6,10-11H,3-4,7-9,23-24H2,1H3,(H,26,30)/t11-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50505059

(CHEMBL4459538)Show SMILES CO[C@H]1C[C@H](N)CCN(C1)c1c(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)cnn1C |r| Show InChI InChI=1S/C21H25F2N7O2S/c1-29-21(30-7-6-11(24)8-12(10-30)32-2)15(9-26-29)27-19(31)17-18(25)33-20(28-17)16-13(22)4-3-5-14(16)23/h3-5,9,11-12H,6-8,10,24-25H2,1-2H3,(H,27,31)/t11-,12+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM248955

(US9434725, 186)Show SMILES COc1ccc(nc1N1CCC[C@H](N)C1)-n1ncc2cnc(cc12)-c1cncc(C)n1 |r| Show InChI InChI=1S/C22H24N8O/c1-14-9-24-12-18(27-14)17-8-19-15(10-25-17)11-26-30(19)21-6-5-20(31-2)22(28-21)29-7-3-4-16(23)13-29/h5-6,8-12,16H,3-4,7,13,23H2,1-2H3/t16-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... |
J Med Chem 60: 4458-4473 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00418
BindingDB Entry DOI: 10.7270/Q2H997PN |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505052

(CHEMBL3623150)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CC[C@@H](N)CC(F)(F)C1 |r| Show InChI InChI=1S/C20H21F4N7OS/c1-30-19(31-6-5-10(25)7-20(23,24)9-31)13(8-27-30)28-17(32)15-16(26)33-18(29-15)14-11(21)3-2-4-12(14)22/h2-4,8,10H,5-7,9,25-26H2,1H3,(H,28,32)/t10-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM110700

(US8614206, 120 | US8614206, 125 | US8614206, 400)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCCC(N)C(F)C1 Show InChI InChI=1S/C20H22F3N7OS/c1-29-20(30-7-3-6-13(24)12(23)9-30)14(8-26-29)27-18(31)16-17(25)32-19(28-16)15-10(21)4-2-5-11(15)22/h2,4-5,8,12-13H,3,6-7,9,24-25H2,1H3,(H,27,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131377

(CHEMBL3634781 | US9260425, 162)Show SMILES C1CN(CCN1)c1cccc(n1)-c1n[nH]c2cnc(cc12)-c1cccnc1 Show InChI InChI=1S/C20H19N7/c1-4-16(24-19(5-1)27-9-7-21-8-10-27)20-15-11-17(14-3-2-6-22-12-14)23-13-18(15)25-26-20/h1-6,11-13,21H,7-10H2,(H,25,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505053

(CHEMBL4469964)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1C1CCC(N)C(F)CC1 Show InChI InChI=1S/C21H23F3N6OS/c1-30-18(10-5-7-11(22)14(25)8-6-10)15(9-27-30)28-20(31)17-19(26)32-21(29-17)16-12(23)3-2-4-13(16)24/h2-4,9-11,14H,5-8,25-26H2,1H3,(H,28,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131224
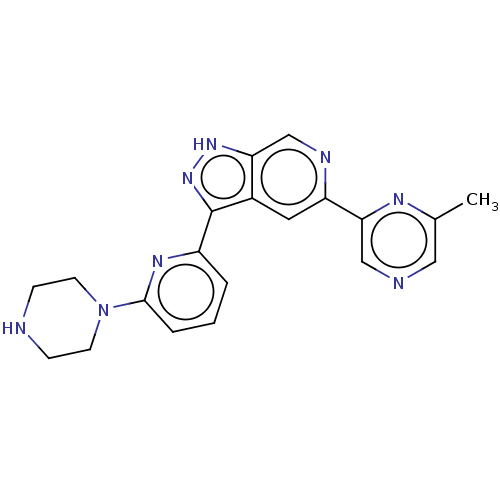
(CHEMBL3634783 | US9260425, 505)Show SMILES Cc1cncc(n1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C20H20N8/c1-13-10-22-11-18(24-13)16-9-14-17(12-23-16)26-27-20(14)15-3-2-4-19(25-15)28-7-5-21-6-8-28/h2-4,9-12,21H,5-8H2,1H3,(H,26,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505061
(CHEMBL4453890)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CC[C@@H](N)[C@@H](O)CC1 |r| Show InChI InChI=1S/C20H23F2N7O2S/c1-28-20(29-7-5-12(23)14(30)6-8-29)13(9-25-28)26-18(31)16-17(24)32-19(27-16)15-10(21)3-2-4-11(15)22/h2-4,9,12,14,30H,5-8,23-24H2,1H3,(H,26,31)/t12-,14+/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM162
(3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...)Show SMILES ONC(=N)c1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3cccc(c3)C(=N)NO)C2=O)c1 Show InChI InChI=1S/C35H38N6O5/c36-33(38-45)27-15-7-13-25(17-27)21-40-29(19-23-9-3-1-4-10-23)31(42)32(43)30(20-24-11-5-2-6-12-24)41(35(40)44)22-26-14-8-16-28(18-26)34(37)39-46/h1-18,29-32,42-43,45-46H,19-22H2,(H2,36,38)(H2,37,39)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company
| |
J Med Chem 39: 3514-25 (1996)
Article DOI: 10.1021/jm9602571
BindingDB Entry DOI: 10.7270/Q2ZW1J3M |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50131224

(CHEMBL3634783 | US9260425, 505)Show SMILES Cc1cncc(n1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C20H20N8/c1-13-10-22-11-18(24-13)16-9-14-17(12-23-16)26-27-20(14)15-3-2-4-19(25-15)28-7-5-21-6-8-28/h2-4,9-12,21H,5-8H2,1H3,(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36648
(3-alkylaminoindazole cyclic urea, (H))Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5[nH]nc(N)c5c4)C3=O)cc12 |r| Show InChI InChI=1S/C35H36N8O3/c36-33-25-15-23(11-13-27(25)38-40-33)19-42-29(17-21-7-3-1-4-8-21)31(44)32(45)30(18-22-9-5-2-6-10-22)43(35(42)46)20-24-12-14-28-26(16-24)34(37)41-39-28/h1-16,29-32,44-45H,17-20H2,(H3,36,38,40)(H3,37,39,41)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50214385
(CHEMBL316681)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]c(O)nc3c2)C(=O)N(Cc2ccc3[nH]c(O)nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O5/c42-31-29(17-21-7-3-1-4-8-21)40(19-23-11-13-25-27(15-23)38-33(44)36-25)35(46)41(30(32(31)43)18-22-9-5-2-6-10-22)20-24-12-14-26-28(16-24)39-34(45)37-26/h1-16,29-32,42-43H,17-20H2,(H2,36,38,44)(H2,37,39,45)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM110961

(US8614206, 518)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CC[C@@](C)(N)CC(F)(F)C1 |r| Show InChI InChI=1S/C21H23F4N7OS/c1-20(27)6-7-32(10-21(24,25)9-20)19-13(8-28-31(19)2)29-17(33)15-16(26)34-18(30-15)14-11(22)4-3-5-12(14)23/h3-5,8H,6-7,9-10,26-27H2,1-2H3,(H,29,33)/t20-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50505057

(CHEMBL3676285)Show SMILES N[C@H]1CCCN(C1)c1ccncc1NC(=O)c1nc(sc1N)-c1c(F)cccc1F Show InChI InChI=1S/C20H20F2N6OS/c21-12-4-1-5-13(22)16(12)20-27-17(18(24)30-20)19(29)26-14-9-25-7-6-15(14)28-8-2-3-11(23)10-28/h1,4-7,9,11H,2-3,8,10,23-24H2,(H,26,29)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50505050

(CHEMBL4439756)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCC(N)CC(F)C1 Show InChI InChI=1S/C20H22F3N7OS/c1-29-20(30-6-5-11(24)7-10(21)9-30)14(8-26-29)27-18(31)16-17(25)32-19(28-16)15-12(22)3-2-4-13(15)23/h2-4,8,10-11H,5-7,9,24-25H2,1H3,(H,27,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM1 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50505050

(CHEMBL4439756)Show SMILES Cn1ncc(NC(=O)c2nc(sc2N)-c2c(F)cccc2F)c1N1CCC(N)CC(F)C1 Show InChI InChI=1S/C20H22F3N7OS/c1-29-20(30-6-5-11(24)7-10(21)9-30)14(8-26-29)27-18(31)16-17(25)32-19(28-16)15-12(22)3-2-4-13(15)23/h2-4,8,10-11H,5-7,9,24-25H2,1H3,(H,27,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PIM3 using FAM-pimtide as substrate after 90 mins by Z-LYTE assay |
J Med Chem 62: 2140-2153 (2019)
Article DOI: 10.1021/acs.jmedchem.8b01857
BindingDB Entry DOI: 10.7270/Q2Q52SVB |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM177
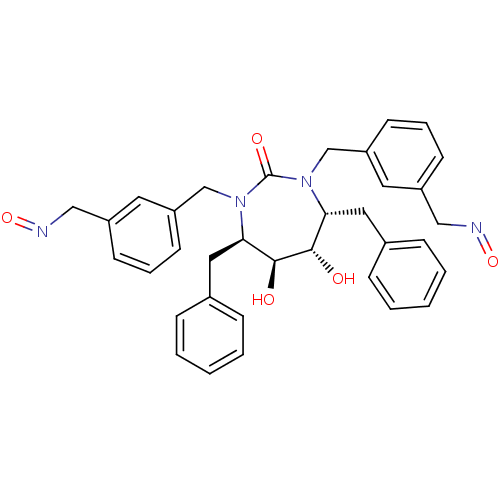
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(CN=O)c2)C(=O)N(Cc2cccc(CN=O)c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H36N4O5/c40-33-31(19-25-9-3-1-4-10-25)38(23-29-15-7-13-27(17-29)21-36-43)35(42)39(24-30-16-8-14-28(18-30)22-37-44)32(34(33)41)20-26-11-5-2-6-12-26/h1-18,31-34,40-41H,19-24H2/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | -65.3 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company
| Assay Description
Ki values were determined with recombinant single-chain dimeric HIV protease and a fluorescent substrate. The use of single-chain dimeric protease a... |
J Med Chem 39: 3514-25 (1996)
Article DOI: 10.1021/jm9602571
BindingDB Entry DOI: 10.7270/Q2ZW1J3M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM177

((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(CN=O)c2)C(=O)N(Cc2cccc(CN=O)c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H36N4O5/c40-33-31(19-25-9-3-1-4-10-25)38(23-29-15-7-13-27(17-29)21-36-43)35(42)39(24-30-16-8-14-28(18-30)22-37-44)32(34(33)41)20-26-11-5-2-6-12-26/h1-18,31-34,40-41H,19-24H2/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC |
J Med Chem 41: 2019-28 (1998)
Article DOI: 10.1021/jm9704199
BindingDB Entry DOI: 10.7270/Q29K49B0 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50073223
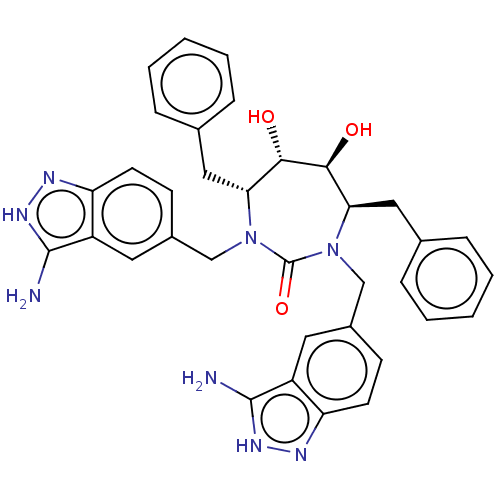
(CHEMBL73240)Show SMILES Nc1[nH]nc2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5n[nH]c(N)c5c4)C3=O)cc12 Show InChI InChI=1S/C41H60N6O6S/c1-26(2)36(44-40(51)53-23-31-25-54-39(42-31)27(3)4)38(50)43-33(18-28-12-9-8-10-13-28)35(48)21-47-17-16-46(20-34(47)37(49)45-41(5,6)7)19-29-14-11-15-30-22-52-24-32(29)30/h8-13,15,25-27,29,33-36,48H,14,16-24H2,1-7H3,(H,43,50)(H,44,51)(H,45,49)/t29?,33-,34-,35+,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50214385

(CHEMBL316681)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]c(O)nc3c2)C(=O)N(Cc2ccc3[nH]c(O)nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O5/c42-31-29(17-21-7-3-1-4-8-21)40(19-23-11-13-25-27(15-23)38-33(44)36-25)35(46)41(30(32(31)43)18-22-9-5-2-6-10-22)20-24-12-14-26-28(16-24)39-34(45)37-26/h1-16,29-32,42-43H,17-20H2,(H2,36,38,44)(H2,37,39,45)/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| <0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of S. cerevisiae glyoxalase-I by using enzymatic assay at each of 6 substrate concentrations between 0.1 mM and... |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36647
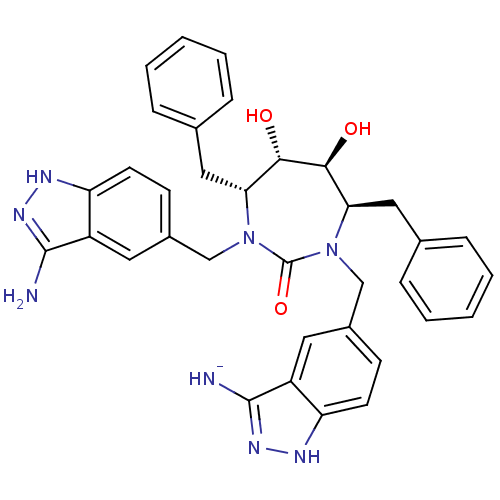
(3-Aminoindazole, 2)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5[nH]nc([NH-])c5c4)C3=O)cc12 |r| Show InChI InChI=1S/C35H35N8O3/c36-33-25-15-23(11-13-27(25)38-40-33)19-42-29(17-21-7-3-1-4-8-21)31(44)32(45)30(18-22-9-5-2-6-10-22)43(35(42)46)20-24-12-14-28-26(16-24)34(37)41-39-28/h1-16,29-32,44-45H,17-20H2,(H5-,36,37,38,39,40,41)/q-1/t29-,30-,31+,32+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131261
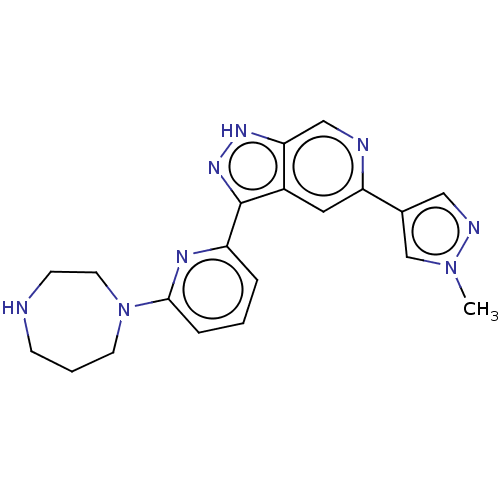
(CHEMBL3634767)Show SMILES Cn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCCNCC1 Show InChI InChI=1S/C20H22N8/c1-27-13-14(11-23-27)17-10-15-18(12-22-17)25-26-20(15)16-4-2-5-19(24-16)28-8-3-6-21-7-9-28/h2,4-5,10-13,21H,3,6-9H2,1H3,(H,25,26) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Transcriptional activation of Retinoid X receptor RXR alpha |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131227

(CHEMBL3634771 | US9260425, 473)Show SMILES N#CCn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C20H19N9/c21-4-7-29-13-14(11-24-29)17-10-15-18(12-23-17)26-27-20(15)16-2-1-3-19(25-16)28-8-5-22-6-9-28/h1-3,10-13,22H,5-9H2,(H,26,27) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50377635
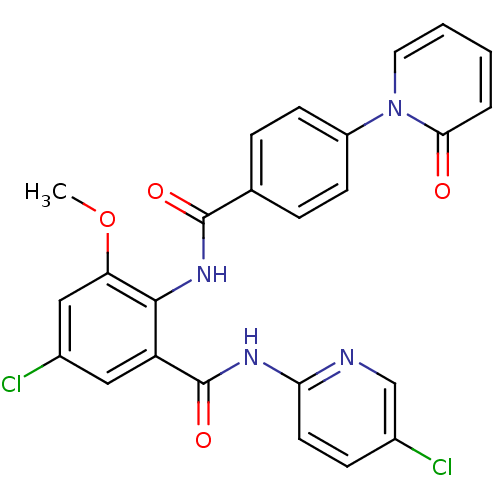
(CHEMBL402980)Show SMILES COc1cc(Cl)cc(C(=O)Nc2ccc(Cl)cn2)c1NC(=O)c1ccc(cc1)-n1ccccc1=O Show InChI InChI=1S/C25H18Cl2N4O4/c1-35-20-13-17(27)12-19(25(34)29-21-10-7-16(26)14-28-21)23(20)30-24(33)15-5-8-18(9-6-15)31-11-3-2-4-22(31)32/h2-14H,1H3,(H,30,33)(H,28,29,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human factor 10a |
Bioorg Med Chem Lett 18: 2845-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.092
BindingDB Entry DOI: 10.7270/Q2611169 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50131278

(CHEMBL3634760 | US9260425, 433)Show SMILES Cc1ccc(nc1N1CCC[C@H](N)C1)-c1n[nH]c2cnc(cc12)-c1cnn(C)c1 |r| Show InChI InChI=1S/C21H24N8/c1-13-5-6-17(25-21(13)29-7-3-4-15(22)12-29)20-16-8-18(14-9-24-28(2)11-14)23-10-19(16)26-27-20/h5-6,8-11,15H,3-4,7,12,22H2,1-2H3,(H,26,27)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM160
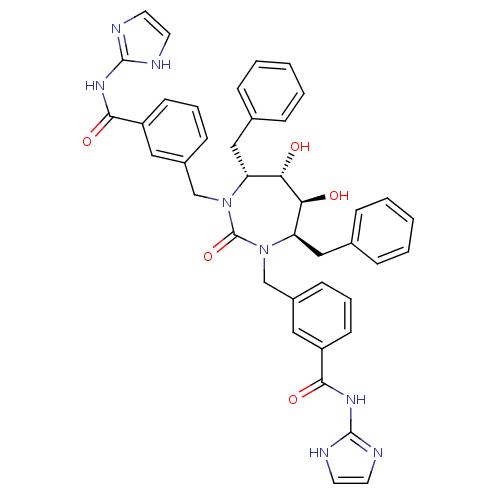
(3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)C(=O)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C41H40N8O5/c50-35-33(23-27-9-3-1-4-10-27)48(25-29-13-7-15-31(21-29)37(52)46-39-42-17-18-43-39)41(54)49(34(36(35)51)24-28-11-5-2-6-12-28)26-30-14-8-16-32(22-30)38(53)47-40-44-19-20-45-40/h1-22,33-36,50-51H,23-26H2,(H2,42,43,46,52)(H2,44,45,47,53)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0140 | -64.4 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company
| |
J Med Chem 39: 3514-25 (1996)
Article DOI: 10.1021/jm9602571
BindingDB Entry DOI: 10.7270/Q2ZW1J3M |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM160

(3-{[(4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-3-{[3...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)C(=O)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C41H40N8O5/c50-35-33(23-27-9-3-1-4-10-27)48(25-29-13-7-15-31(21-29)37(52)46-39-42-17-18-43-39)41(54)49(34(36(35)51)24-28-11-5-2-6-12-28)26-30-14-8-16-32(22-30)38(53)47-40-44-19-20-45-40/h1-22,33-36,50-51H,23-26H2,(H2,42,43,46,52)(H2,44,45,47,53)/t33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to inhibit the purified wild-type HIV-1 Protease |
J Med Chem 40: 181-91 (1997)
Article DOI: 10.1021/jm960586t
BindingDB Entry DOI: 10.7270/Q2ST7NZ3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131265

(CHEMBL3634757 | US9260425, 189)Show SMILES Cn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CC[C@@H](N)C1 |r| Show InChI InChI=1S/C19H20N8/c1-26-10-12(8-22-26)16-7-14-17(9-21-16)24-25-19(14)15-3-2-4-18(23-15)27-6-5-13(20)11-27/h2-4,7-10,13H,5-6,11,20H2,1H3,(H,24,25)/t13-/m1/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131266
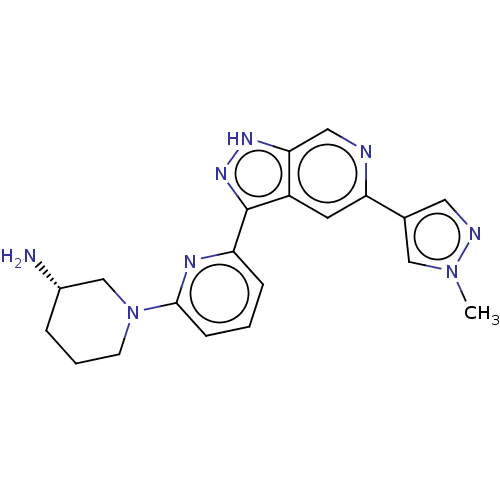
(CHEMBL3634758 | US9260425, 173)Show SMILES Cn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCC[C@H](N)C1 |r| Show InChI InChI=1S/C20H22N8/c1-27-11-13(9-23-27)17-8-15-18(10-22-17)25-26-20(15)16-5-2-6-19(24-16)28-7-3-4-14(21)12-28/h2,5-6,8-11,14H,3-4,7,12,21H2,1H3,(H,25,26)/t14-/m0/s1 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50131225

(CHEMBL3634769 | US9260425, 515)Show SMILES CCn1cncc(-c2cc3c(n[nH]c3cn2)-c2cccc(n2)N2CCNCC2)c1=O Show InChI InChI=1S/C21H22N8O/c1-2-28-13-23-11-15(21(28)30)17-10-14-18(12-24-17)26-27-20(14)16-4-3-5-19(25-16)29-8-6-22-7-9-29/h3-5,10-13,22H,2,6-9H2,1H3,(H,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM36656

(Cyclobutylmethyl cyclic urea)Show SMILES Nc1n[nH]c2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(CC4CCC4)C3=O)cc12 |r| Show InChI InChI=1S/C32H37N5O3/c33-31-25-16-24(14-15-26(25)34-35-31)20-37-28(18-22-10-5-2-6-11-22)30(39)29(38)27(17-21-8-3-1-4-9-21)36(32(37)40)19-23-12-7-13-23/h1-6,8-11,14-16,23,27-30,38-39H,7,12-13,17-20H2,(H3,33,34,35)/t27-,28-,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131228
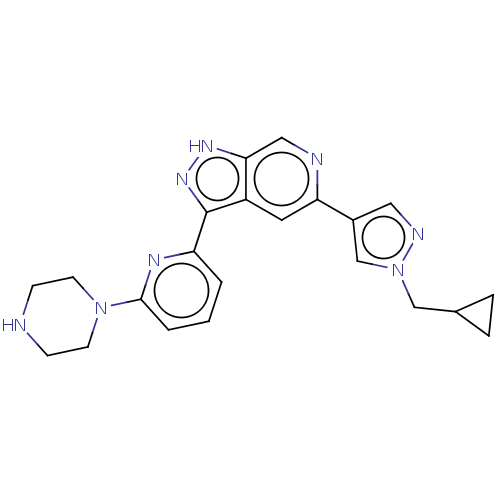
(CHEMBL3634772 | US9260425, 497)Show SMILES C(C1CC1)n1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C22H24N8/c1-2-18(26-21(3-1)29-8-6-23-7-9-29)22-17-10-19(24-12-20(17)27-28-22)16-11-25-30(14-16)13-15-4-5-15/h1-3,10-12,14-15,23H,4-9,13H2,(H,27,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50055590
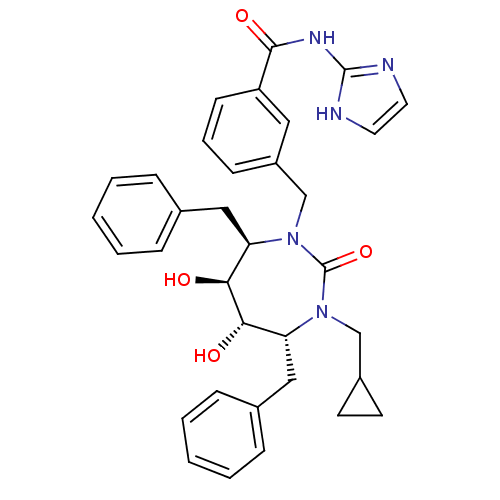
(3-((4R,5S,6S,7R)-4,7-Dibenzyl-3-cyclopropylmethyl-...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2cccc(c2)C(=O)Nc2ncc[nH]2)C(=O)N(CC2CC2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C34H37N5O4/c40-30-28(19-23-8-3-1-4-9-23)38(21-25-14-15-25)34(43)39(29(31(30)41)20-24-10-5-2-6-11-24)22-26-12-7-13-27(18-26)32(42)37-33-35-16-17-36-33/h1-13,16-18,25,28-31,40-41H,14-15,19-22H2,(H2,35,36,37,42)/t28-,29-,30+,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to inhibit the purified wild-type HIV-1 Protease |
J Med Chem 40: 181-91 (1997)
Article DOI: 10.1021/jm960586t
BindingDB Entry DOI: 10.7270/Q2ST7NZ3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50131226
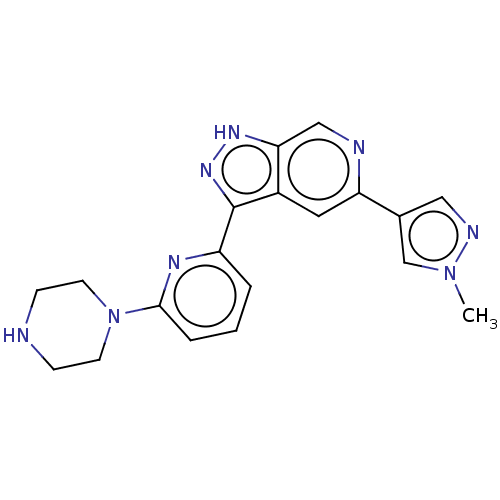
(CHEMBL3634770 | US9260425, 234)Show SMILES Cn1cc(cn1)-c1cc2c(n[nH]c2cn1)-c1cccc(n1)N1CCNCC1 Show InChI InChI=1S/C19H20N8/c1-26-12-13(10-22-26)16-9-14-17(11-21-16)24-25-19(14)15-3-2-4-18(23-15)27-7-5-20-6-8-27/h2-4,9-12,20H,5-8H2,1H3,(H,24,25) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of Pim3 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins |
Bioorg Med Chem Lett 25: 5258-64 (2015)
Article DOI: 10.1016/j.bmcl.2015.09.052
BindingDB Entry DOI: 10.7270/Q2TT4SR7 |
More data for this
Ligand-Target Pair | |
HIV-1 protease
(Human immunodeficiency virus) | BDBM161
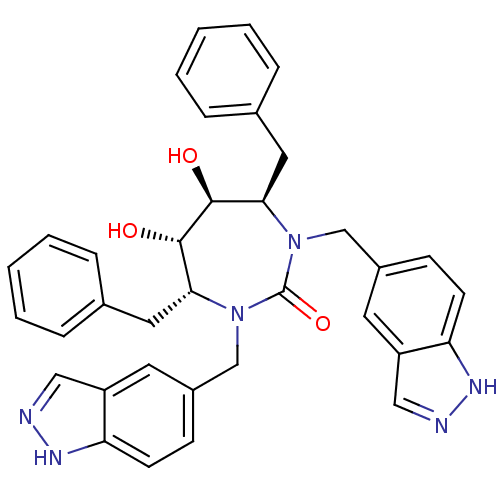
((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N(Cc2ccc3[nH]ncc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-29-27(15-25)19-36-38-29)35(44)41(22-26-12-14-30-28(16-26)20-37-39-30)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | 5.5 | n/a |
DuPont Merck Pharmaceutical Company
| Assay Description
Protease inhibition fluorescence-based assay using cyclic ureas to inhibit HIV-protease. |
Chem Biol 5: 597-608 (1998)
Article DOI: 10.1016/s1074-5521(98)90117-x
BindingDB Entry DOI: 10.7270/Q2R78CK2 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50069033
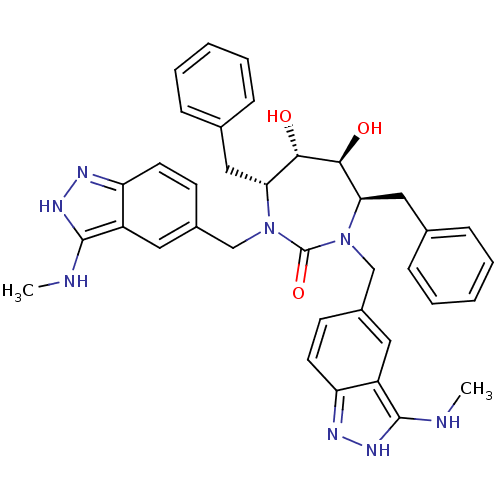
((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...)Show SMILES CNc1[nH]nc2ccc(CN3[C@H](Cc4ccccc4)[C@H](O)[C@@H](O)[C@@H](Cc4ccccc4)N(Cc4ccc5n[nH]c(NC)c5c4)C3=O)cc12 Show InChI InChI=1S/C37H40N8O3/c1-38-35-27-17-25(13-15-29(27)40-42-35)21-44-31(19-23-9-5-3-6-10-23)33(46)34(47)32(20-24-11-7-4-8-12-24)45(37(44)48)22-26-14-16-30-28(18-26)36(39-2)43-41-30/h3-18,31-34,46-47H,19-22H2,1-2H3,(H2,38,40,42)(H2,39,41,43)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
The compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM161

((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N(Cc2ccc3[nH]ncc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-29-27(15-25)19-36-38-29)35(44)41(22-26-12-14-30-28(16-26)20-37-39-30)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for inhibition of HIV protease |
Bioorg Med Chem Lett 8: 715-20 (1999)
BindingDB Entry DOI: 10.7270/Q2FQ9VRH |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50288430

((4R,5S,6S,7R)-4,7-Dibenzyl-5,6-dihydroxy-1,3-bis-(...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3c[nH]nc3c2)C(=O)N(Cc2ccc3c[nH]nc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-27-19-36-38-29(27)15-25)35(44)41(22-26-12-14-28-20-37-39-30(28)16-26)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity was evaluated against HIV protease |
Bioorg Med Chem Lett 6: 2919-2924 (1996)
Article DOI: 10.1016/S0960-894X(96)00531-8
BindingDB Entry DOI: 10.7270/Q2QF8SVC |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM178

((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis({...)Show SMILES CC(N=O)c1cccc(CN2[C@H](Cc3ccccc3)[C@H](O)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3cccc(c3)C(C)N=O)C2=O)c1 Show InChI InChI=1S/C37H40N4O5/c1-25(38-45)31-17-9-15-29(19-31)23-40-33(21-27-11-5-3-6-12-27)35(42)36(43)34(22-28-13-7-4-8-14-28)41(37(40)44)24-30-16-10-18-32(20-30)26(2)39-46/h3-20,25-26,33-36,42-43H,21-24H2,1-2H3/t25?,26?,33-,34-,35+,36+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The DuPont Merck Pharmaceutical Company
Curated by ChEMBL
| Assay Description
Inhibition of HIV protease, measured by assaying the cleavage of a fluorescent peptide substrate using HPLC |
J Med Chem 41: 2019-28 (1998)
Article DOI: 10.1021/jm9704199
BindingDB Entry DOI: 10.7270/Q29K49B0 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM248955

(US9434725, 186)Show SMILES COc1ccc(nc1N1CCC[C@H](N)C1)-n1ncc2cnc(cc12)-c1cncc(C)n1 |r| Show InChI InChI=1S/C22H24N8O/c1-14-9-24-12-18(27-14)17-8-19-15(10-25-17)11-26-30(19)21-6-5-20(31-2)22(28-21)29-7-3-4-16(23)13-29/h5-6,8-12,16H,3-4,7,13,23H2,1-2H3/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 (unknown origin) using 5FAM-ARKRRRHPSGPPTA as substrate after 90 mins in presence of ATP by caliper microfluidic mobility shift as... |
J Med Chem 60: 4458-4473 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00418
BindingDB Entry DOI: 10.7270/Q2H997PN |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [501-599]
(Human immunodeficiency virus type 1) | BDBM161

((4R,5S,6S,7R)-4,7-dibenzyl-5,6-dihydroxy-1,3-bis(1...)Show SMILES O[C@@H]1[C@@H](O)[C@@H](Cc2ccccc2)N(Cc2ccc3[nH]ncc3c2)C(=O)N(Cc2ccc3[nH]ncc3c2)[C@@H]1Cc1ccccc1 Show InChI InChI=1S/C35H34N6O3/c42-33-31(17-23-7-3-1-4-8-23)40(21-25-11-13-29-27(15-25)19-36-38-29)35(44)41(22-26-12-14-30-28(16-26)20-37-39-30)32(34(33)43)18-24-9-5-2-6-10-24/h1-16,19-20,31-34,42-43H,17-18,21-22H2,(H,36,38)(H,37,39)/t31-,32-,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | -63.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company
| |
J Med Chem 39: 3514-25 (1996)
Article DOI: 10.1021/jm9602571
BindingDB Entry DOI: 10.7270/Q2ZW1J3M |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1081

((4R,5R,6R)-Tetrahydro-1,3-bis[(3-benzamide oxime)m...)Show SMILES ONC(=N)c1cccc(CN2[C@H](CCc3ccccc3)[C@@H](O)[C@@H](Cc3ccccc3)N(Cc3cccc(c3)C(=N)NO)C2=O)c1 |r| Show InChI InChI=1S/C35H38N6O4/c36-33(38-44)28-15-7-13-26(19-28)22-40-30(18-17-24-9-3-1-4-10-24)32(42)31(21-25-11-5-2-6-12-25)41(35(40)43)23-27-14-8-16-29(20-27)34(37)39-45/h1-16,19-20,30-32,42,44-45H,17-18,21-23H2,(H2,36,38)(H2,37,39)/t30-,31-,32-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0180 | -63.8 | n/a | n/a | n/a | n/a | n/a | 5.5 | 37 |
DuPont Merck Pharmaceutical Company
| Assay Description
Inhibition of HIV protease was measured by assay of the cleavage of a fluorescent peptide substrate. The fluorescent product (2-aminobenzoyl-Ala-Thr-... |
J Med Chem 40: 1707-9 (1997)
Article DOI: 10.1021/jm970081i
BindingDB Entry DOI: 10.7270/Q2CR5RHB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data