

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50013847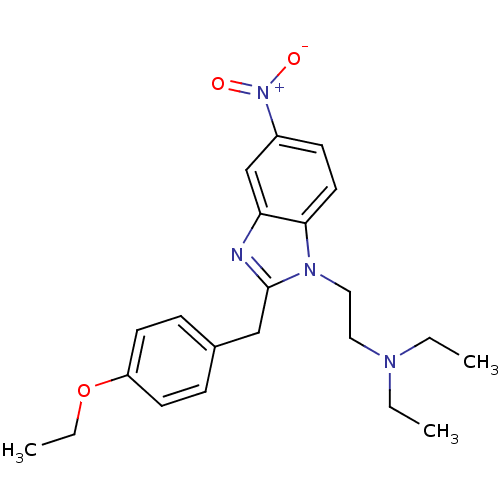 (CHEMBL312040 | Etonitazene | {2-[2-(4-Ethoxy-benzy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL DrugBank PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from human mu opioid receptor expressed in HEK293-TSA cell membranes after 4 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 90 mins | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244276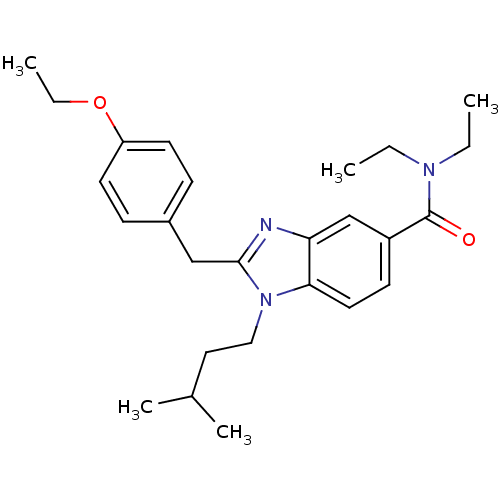 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB1 receptor expressed in CHO cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244242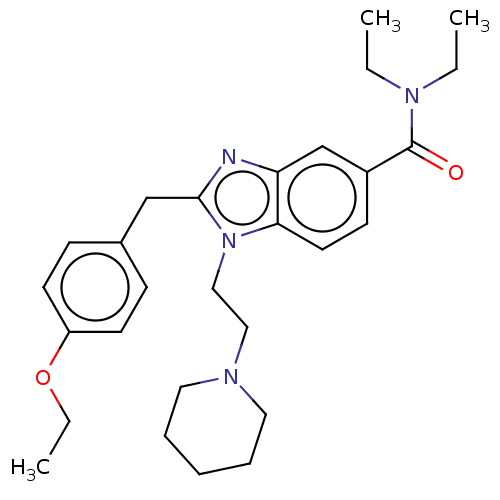 (CHEMBL4082936) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 188 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 254 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB1 receptor expressed in CHO cell membranes after 90 mins | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244310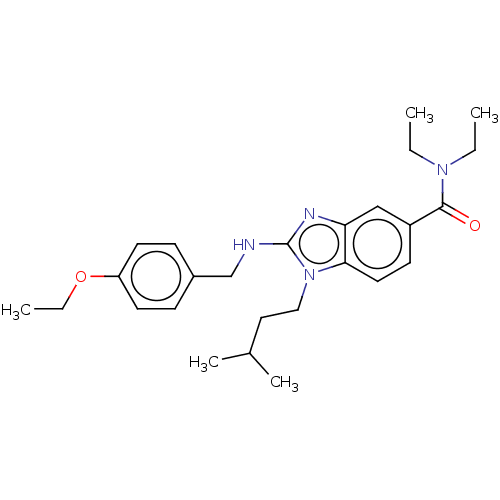 (CHEMBL4075256) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 353 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244244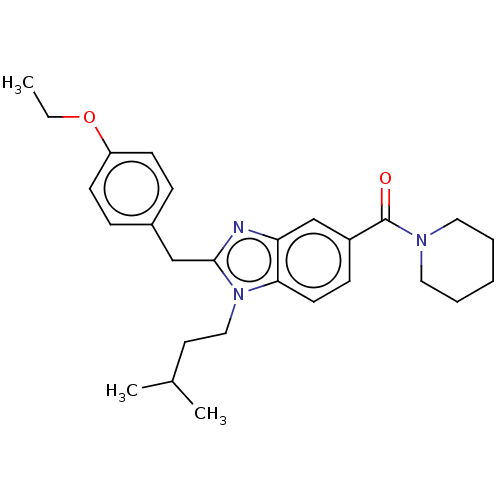 (CHEMBL4104311) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244286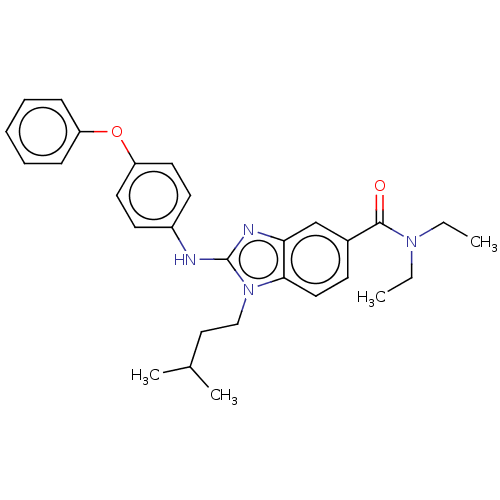 (CHEMBL4100917) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 426 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50244242 (CHEMBL4082936) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 556 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from human mu opioid receptor expressed in HEK293-TSA cell membranes after 4 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50244242 (CHEMBL4082936) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 556 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from human mu opioid receptor expressed in HEK293-TSA cell membranes after 4 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 687 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB1 receptor expressed in CHO cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244302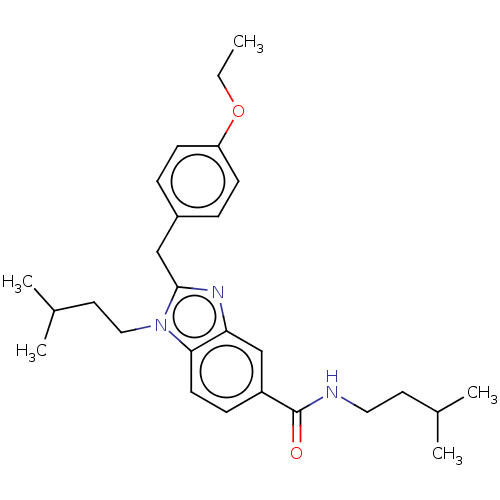 (CHEMBL4061358) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 764 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244254 (CHEMBL4104374) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 962 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244245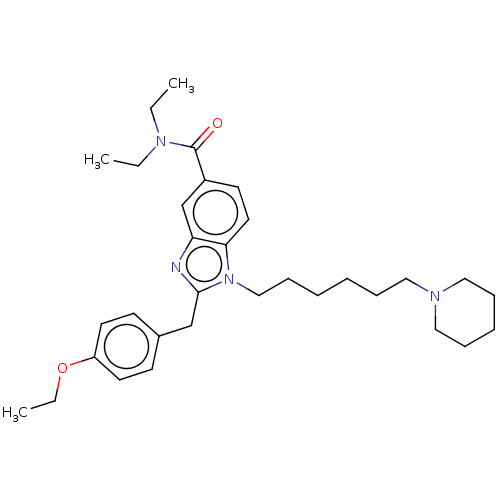 (CHEMBL4082785) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244285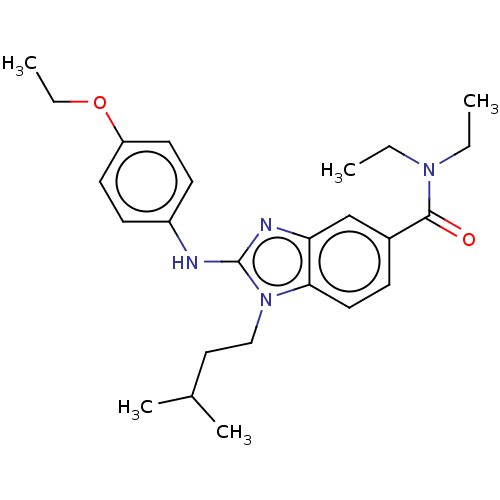 (CHEMBL4079520) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244289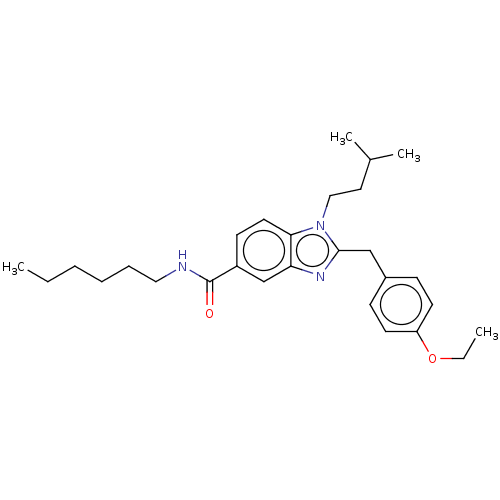 (CHEMBL4086412) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244270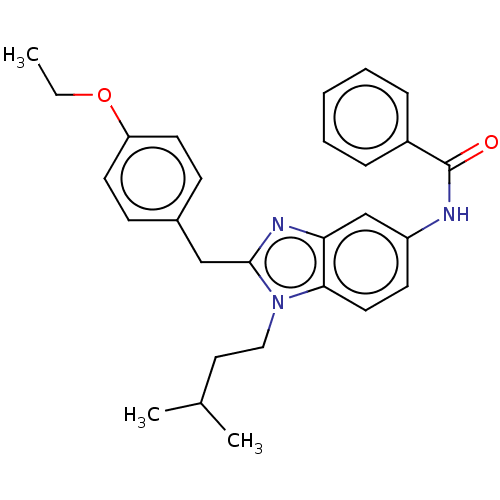 (CHEMBL4099175) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244309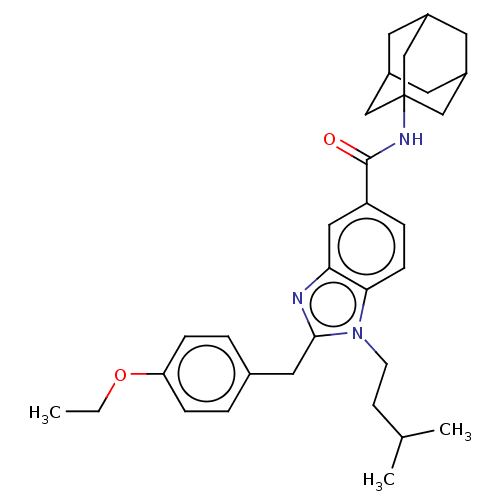 (CHEMBL4063907) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244287 (CHEMBL4085482) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from human mu opioid receptor expressed in HEK293-TSA cell membranes after 4 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from human mu opioid receptor expressed in HEK293-TSA cell membranes after 4 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244253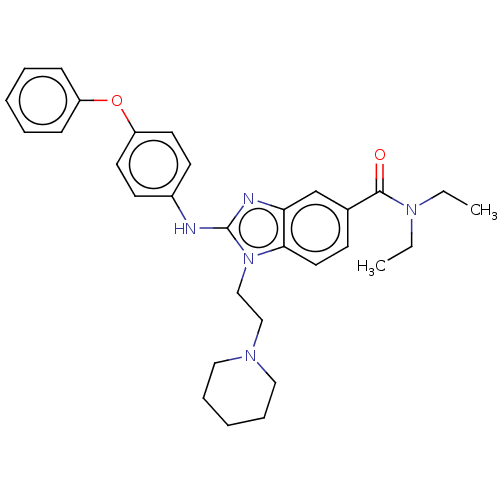 (CHEMBL4074747) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244246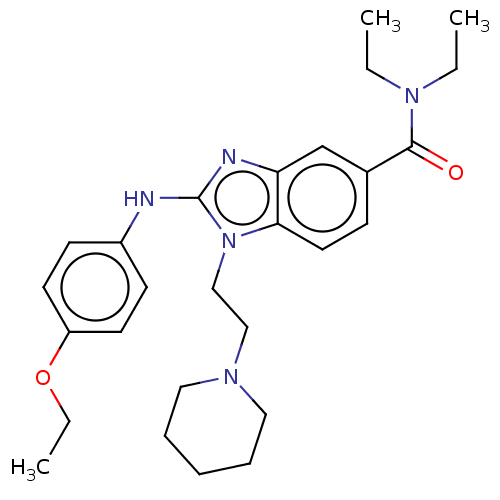 (CHEMBL4095946) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244288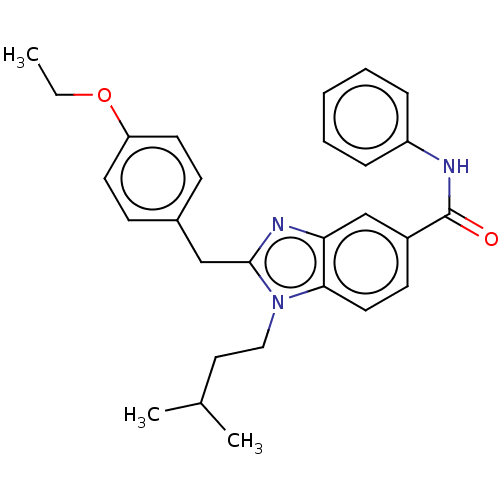 (CHEMBL4076334) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50244244 (CHEMBL4104311) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB1 receptor expressed in CHO cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244311 (CHEMBL4070526) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50244285 (CHEMBL4079520) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from human mu opioid receptor expressed in HEK293-TSA cell membranes after 4 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50244285 (CHEMBL4079520) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-Diprenorphine from human mu opioid receptor expressed in HEK293-TSA cell membranes after 4 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50392999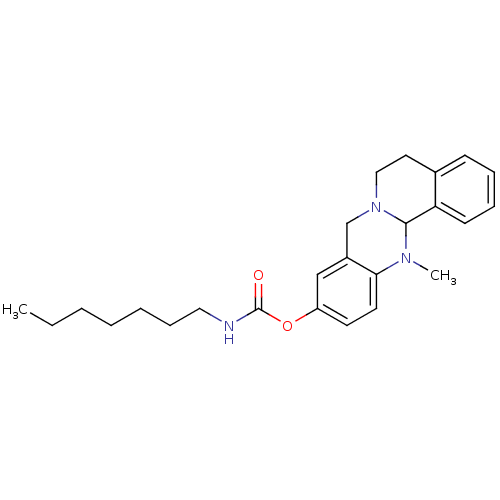 (CHEMBL2152545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate incubated for 30 mins followed by substrate addition and measured after 2... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50392999 (CHEMBL2152545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate incubated for 30 mins followed by substrate addition and measured after 2... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using BTC iodide as substrate preincubated for 4.5 mins followed by substrate addition measured after 2.5 mins by Ellman's m... | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human BChE using BTC iodide as substrate preincubated for 4.5 mins followed by substrate addition measured after 2.5 mins by Ellman's m... | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Tested for binding affinity against human NK-1 receptor transfected on CHO cells using [125I]-Tyr] SP as radioligand | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE using ATC iodide as substrate preincubated for 4.5 mins followed by substrate addition measured after 2.5 mins b... | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50200170 (5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50244276 (2-(4-ethoxybenzyl)-N,N-diethyl-1-isopentyl-1H-benz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB2 receptor expressed in HEK cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50580652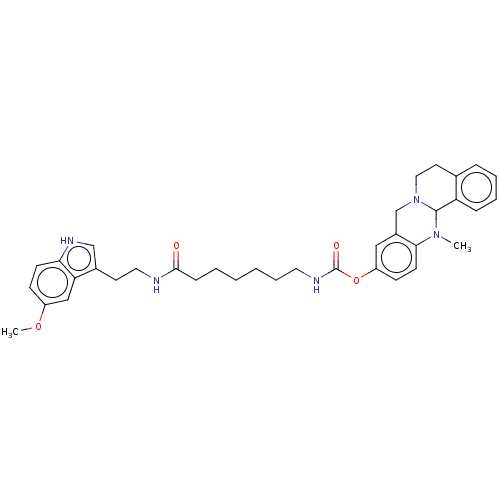 (CHEMBL5078611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50580652 (CHEMBL5078611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 102 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 111 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50580651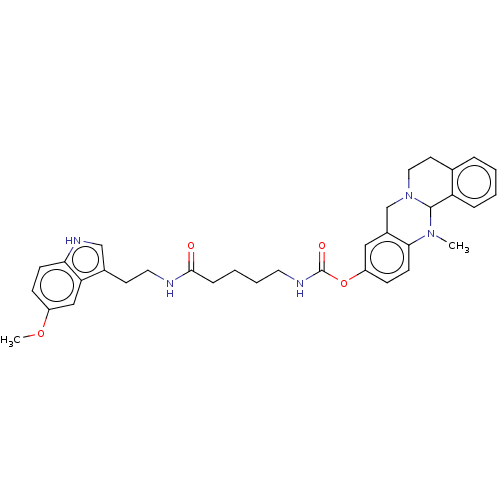 (CHEMBL5081534) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50580651 (CHEMBL5081534) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 127 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50580653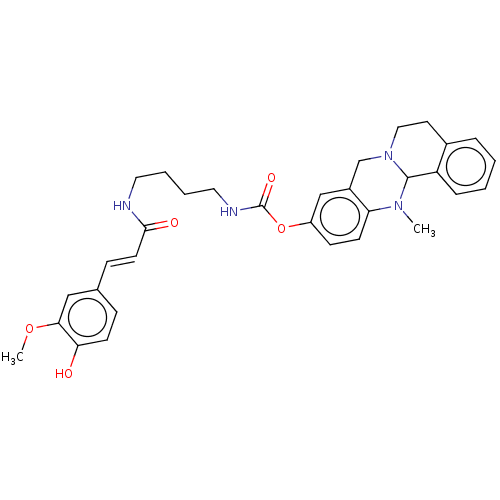 (CHEMBL5084655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50580653 (CHEMBL5084655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 158 | n/a | n/a | n/a | n/a | n/a | n/a |
Julius Maximilian University of W£rzburg Curated by ChEMBL | Assay Description Displacement of [3H]-CP55950 from human CB1 receptor expressed in CHO cell membranes after 3 hrs by scintillation counting method | J Med Chem 61: 1646-1663 (2018) Article DOI: 10.1021/acs.jmedchem.7b01760 BindingDB Entry DOI: 10.7270/Q2RJ4MWK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50580654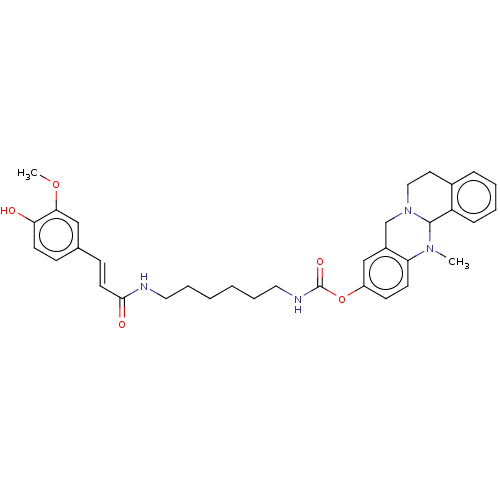 (CHEMBL5086269) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 166 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human BChE using butyrylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellman's method | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00534 BindingDB Entry DOI: 10.7270/Q29027NK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 109 total ) | Next | Last >> |