

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50146577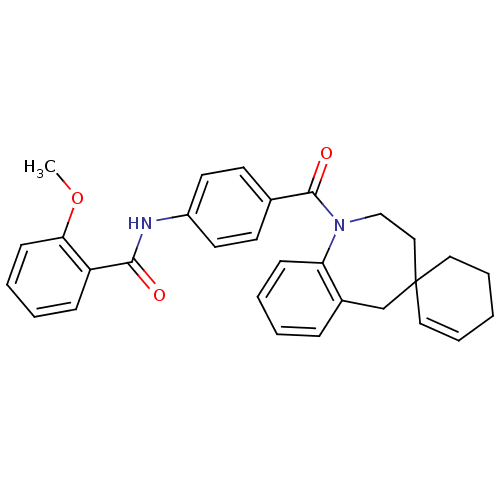 (CHEMBL102311 | spirobenzoxazines analogues) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory concentration against [3H]-AVP binding to cloned human vasopressin receptor | Bioorg Med Chem Lett 14: 2987-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.103 BindingDB Entry DOI: 10.7270/Q2BK1BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50225162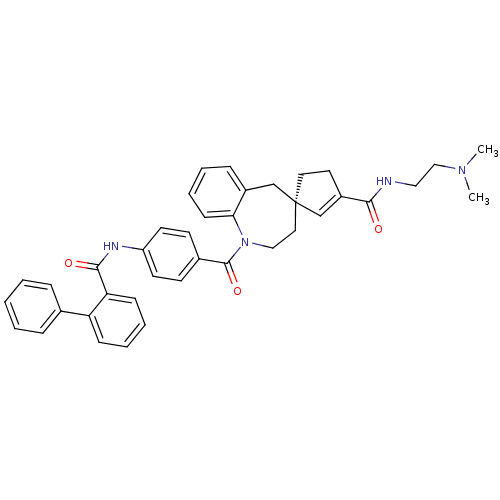 ((4R)-N-[2-(dimethylamino)ethyl]-1-({4-[(2-phenylbe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6623-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.059 BindingDB Entry DOI: 10.7270/Q2T72H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50366915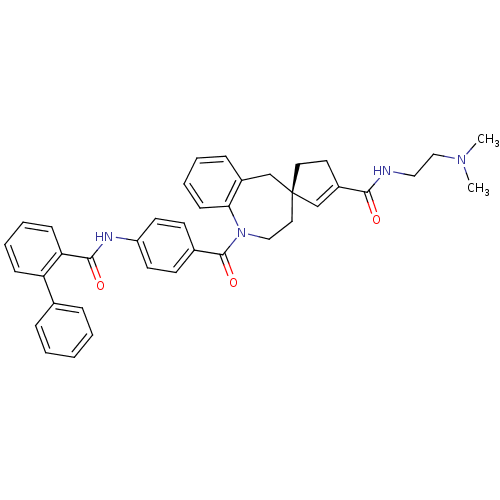 (CHEMBL1788220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50366914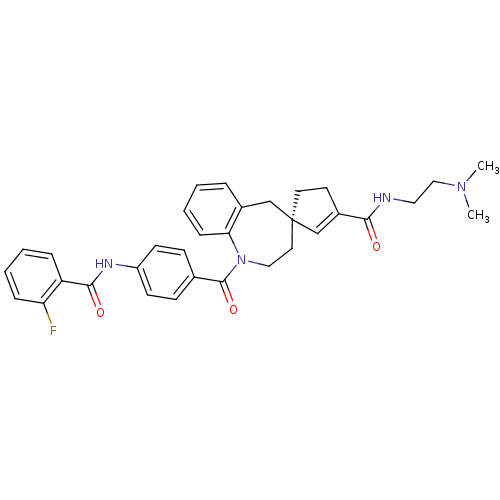 (CHEMBL1788221) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147223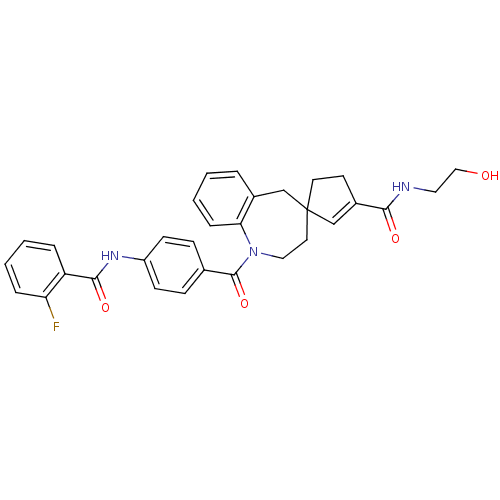 (3'N-(2-hydroxyethyl)-1-[4-(2-fluorophenylcarboxami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147220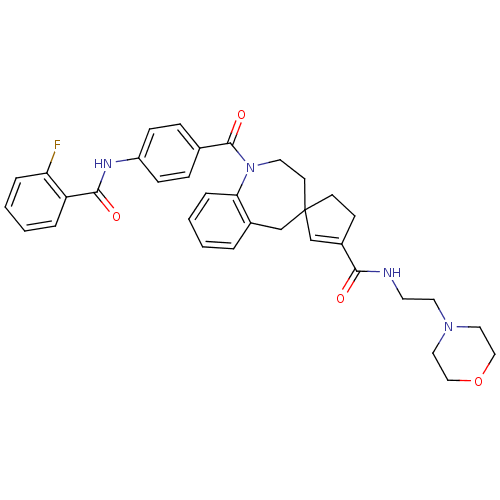 (4-{2-[1-[4-(2-fluorophenylcarboxamido)benzoyl]spir...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147224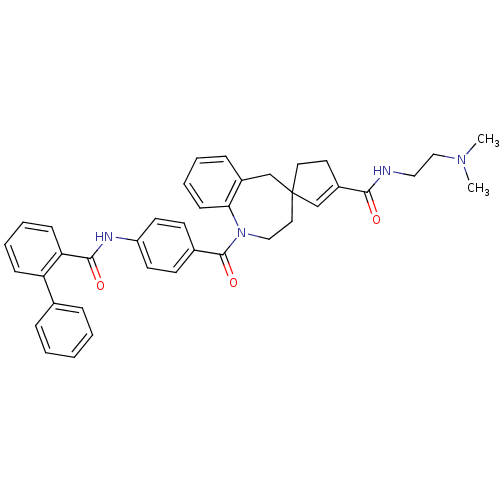 (3'N-(2-dimethylaminoethyl)-1-[4-(2-phenylphenylcar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Evaluated for intracellular calcium mobilization in HEK293 cells transfected to express human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147227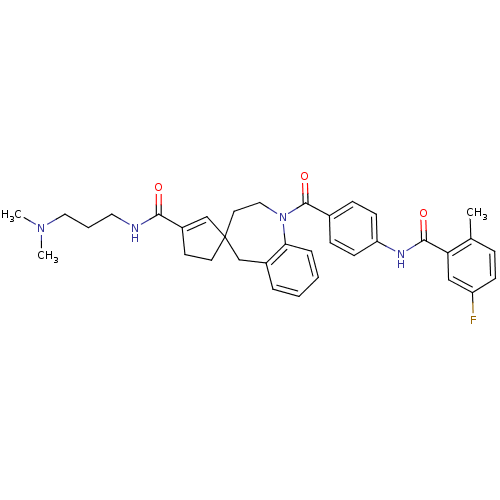 (3'N-(3-dimethylaminopropyl)-1-[4-(5-fluoro-2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Evaluated for intracellular calcium mobilization in HEK293 cells transfected to express human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50225163 ((4R)-1-({4-[(2-chloro-5-fluorobenzene)amido]-3-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6623-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.059 BindingDB Entry DOI: 10.7270/Q2T72H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147219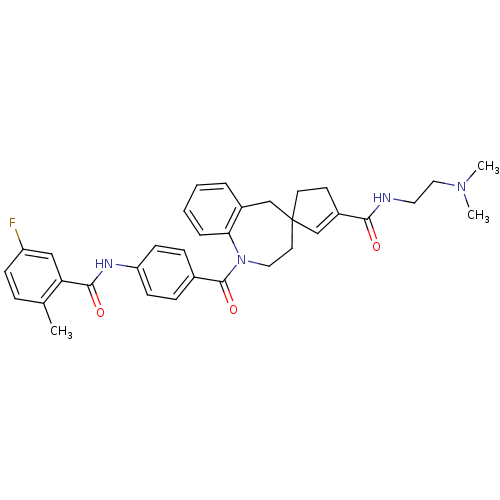 (3'N-(2-dimethylaminoethyl)-1-[4-(5-fluoro-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147224 (3'N-(2-dimethylaminoethyl)-1-[4-(2-phenylphenylcar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Evaluated for intracellular calcium mobilization in HEK293 cells transfected to express human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147227 (3'N-(3-dimethylaminopropyl)-1-[4-(5-fluoro-2-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6623-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.059 BindingDB Entry DOI: 10.7270/Q2T72H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50366915 (CHEMBL1788220) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Evaluated for binding affinity towards vasopressin V2 receptor in rat | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147221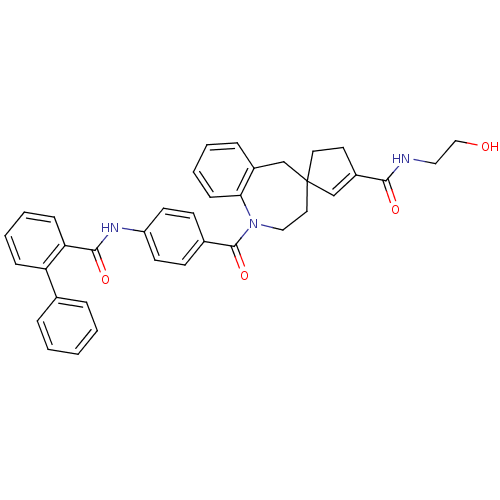 (3'N-(2-hydroxyethyl)-1-[4-(2-phenylphenylcarboxami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Evaluated for intracellular calcium mobilization in HEK293 cells transfected to express human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147228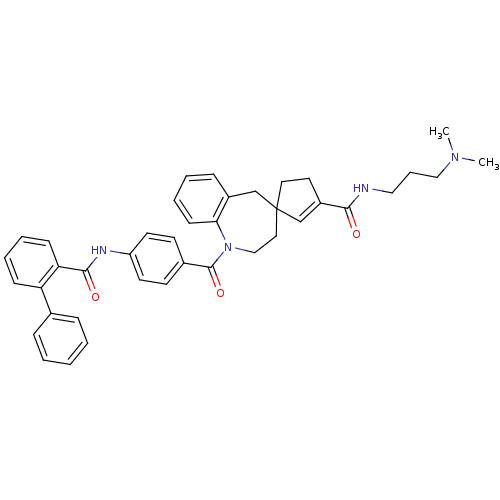 (3'N-(3-dimethylaminopropyl)-1-[4-(2-phenylphenylca...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147221 (3'N-(2-hydroxyethyl)-1-[4-(2-phenylphenylcarboxami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50135417 (((R)-5-{4-[(Biphenyl-2-carbonyl)-amino]-benzoyl}-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibition of vasopressin induced calcium immobilization in human V1a receptor expressing cells | Bioorg Med Chem Lett 13: 4031-4 (2003) BindingDB Entry DOI: 10.7270/Q23T9GM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50135413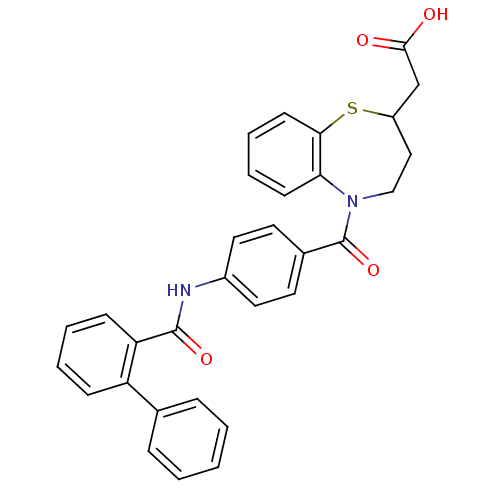 ((5-{4-[(Biphenyl-2-carbonyl)-amino]-benzoyl}-2,3,4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Ability to displace [3H]-arginine vasopressin in cloned human V2 receptor | Bioorg Med Chem Lett 13: 4031-4 (2003) BindingDB Entry DOI: 10.7270/Q23T9GM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147230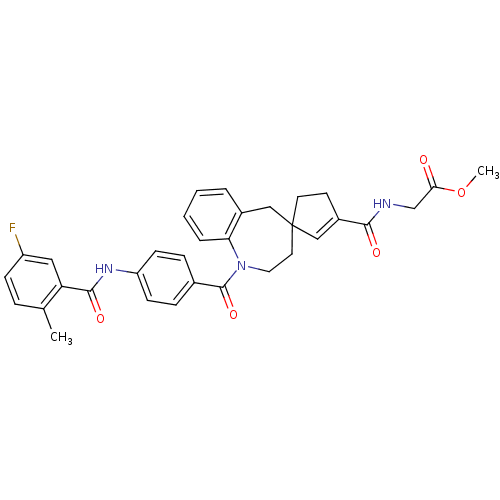 (CHEMBL319636 | methyl 2-[1-[4-(5-fluoro-2-methylph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147225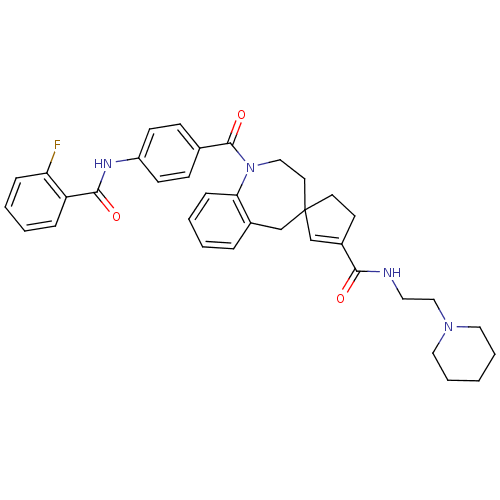 (3'N-(2-hexahydro-1-pyridinylethyl)-1-[4-(2-fluorop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50135420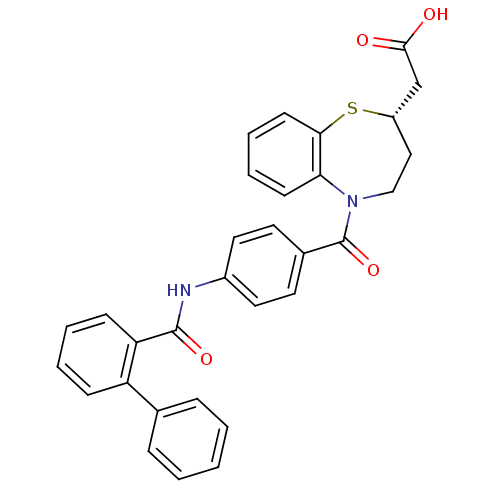 (((S)-5-{4-[(Biphenyl-2-carbonyl)-amino]-benzoyl}-2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Ability to displace [3H]-arginine vasopressin in cloned human V2 receptor | Bioorg Med Chem Lett 13: 4031-4 (2003) BindingDB Entry DOI: 10.7270/Q23T9GM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147226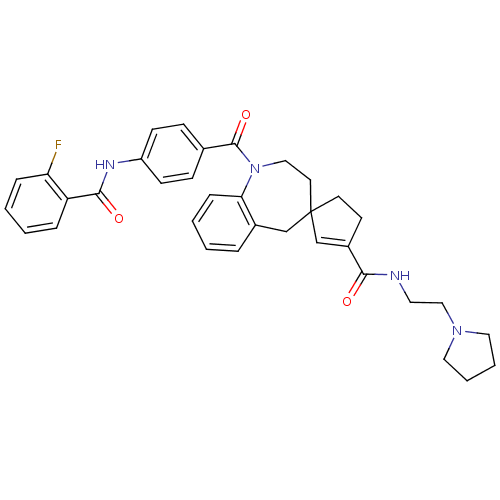 (3'N-(2-tetrahydro-1H-1-pyrrolylethyl)-1-[4-(2-fluo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50147229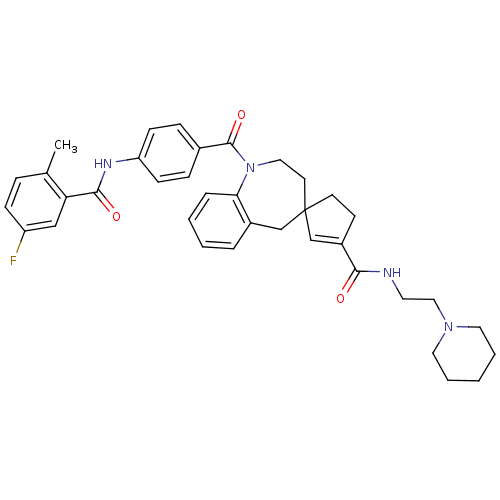 (3'N-(2-hexahydro-1-pyridinylethyl)-1-[4-(5-fluoro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human V2 receptor (Compound 7o) | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147222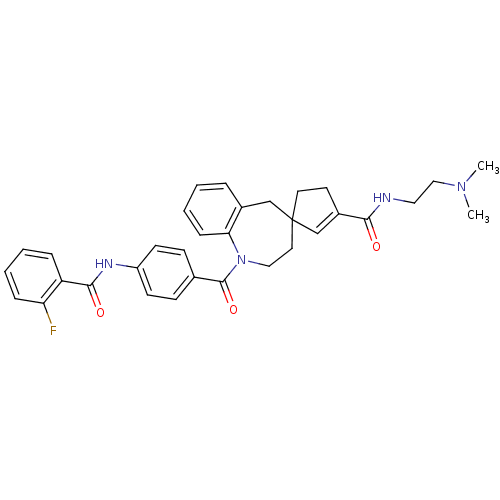 (3'N-(2-dimethylaminoethyl)-1-[4-(2-fluorophenylcar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Evaluated for intracellular calcium mobilization in HEK293 cells transfected to express human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50366915 (CHEMBL1788220) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Evaluated for intracellular calcium mobilization in HEK- 293 cells transfected to express human vasopressin V1a receptor (Compound 7o) | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50225162 ((4R)-N-[2-(dimethylamino)ethyl]-1-({4-[(2-phenylbe...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6623-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.059 BindingDB Entry DOI: 10.7270/Q2T72H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50225166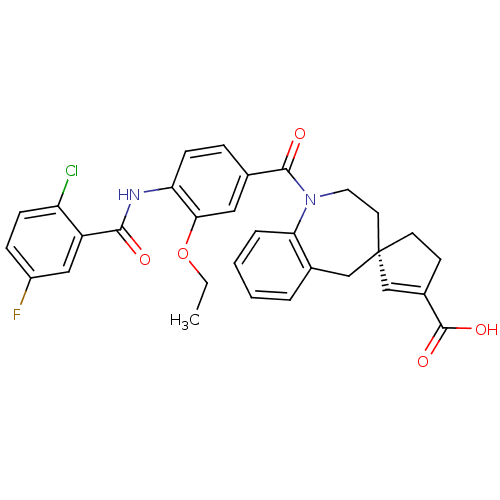 ((4R)-1-({4-[(2-chloro-5-fluorobenzene)amido]-3-eth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6623-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.059 BindingDB Entry DOI: 10.7270/Q2T72H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50135414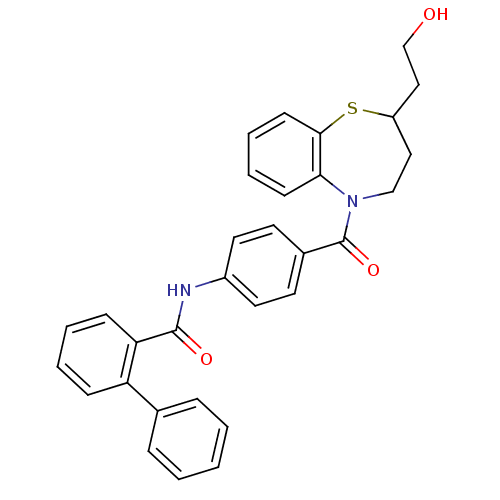 (Biphenyl-2-carboxylic acid {4-[2-(2-hydroxy-ethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Ability to displace [3H]-arginine vasopressin in cloned human V2 receptor | Bioorg Med Chem Lett 13: 4031-4 (2003) BindingDB Entry DOI: 10.7270/Q23T9GM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50146578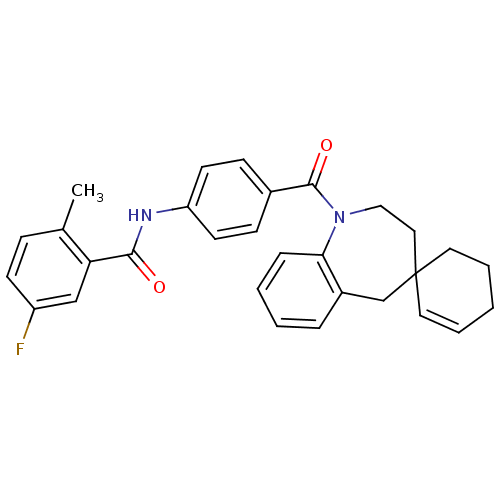 (CHEMBL103406 | spirobenzoxazines analogues) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory concentration against [3H]-AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 2987-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.103 BindingDB Entry DOI: 10.7270/Q2BK1BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50147229 (3'N-(2-hexahydro-1-pyridinylethyl)-1-[4-(5-fluoro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Evaluated for accumulation of cAMP in transfected HEK293 cells expressing human V2 receptor (Compound 7o) | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50225167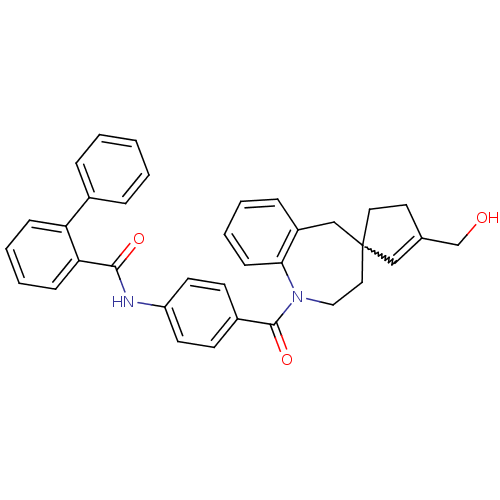 (CHEMBL250346 | N-(4-{[3'-(hydroxymethyl)-1,2,3,5-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6623-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.059 BindingDB Entry DOI: 10.7270/Q2T72H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50225167 (CHEMBL250346 | N-(4-{[3'-(hydroxymethyl)-1,2,3,5-t...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin V1a receptor expressed in HEK293 cells assessed as inhibition of Arg-vasopressin-induced intracllular calciu... | Bioorg Med Chem Lett 17: 6623-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.059 BindingDB Entry DOI: 10.7270/Q2T72H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50146565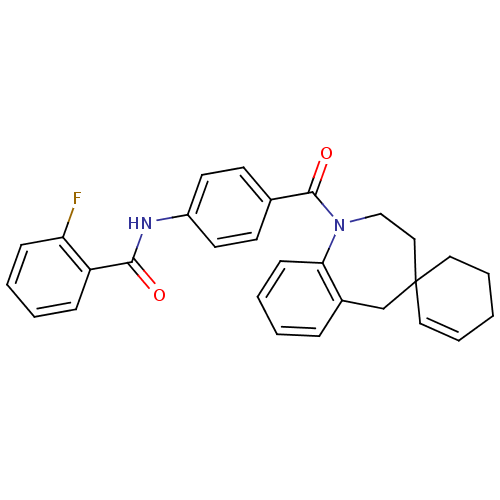 (CHEMBL101157 | spirobenzoxazines analogues) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory concentration against [3H]-AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 2987-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.103 BindingDB Entry DOI: 10.7270/Q2BK1BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076666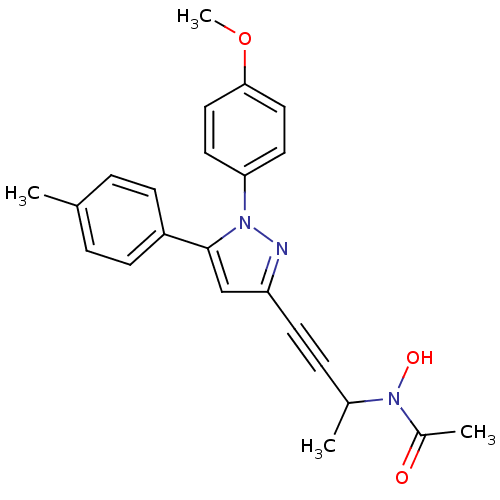 (CHEMBL175153 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50147230 (CHEMBL319636 | methyl 2-[1-[4-(5-fluoro-2-methylph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50147229 (3'N-(2-hexahydro-1-pyridinylethyl)-1-[4-(5-fluoro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076661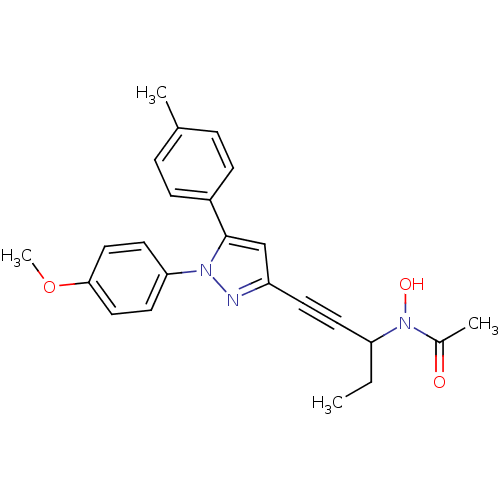 (CHEMBL177727 | N-{1-Ethyl-3-[1-(4-methoxy-phenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50146566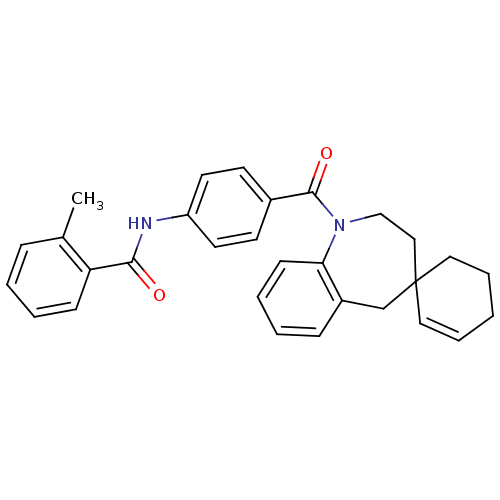 (CHEMBL440147 | spirobenzoxazines analogues) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory concentration against [3H]-AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 2987-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.103 BindingDB Entry DOI: 10.7270/Q2BK1BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076649 (CHEMBL369848 | N-{1-methyl-3-[1-(4-methoxyphenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase(5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50146572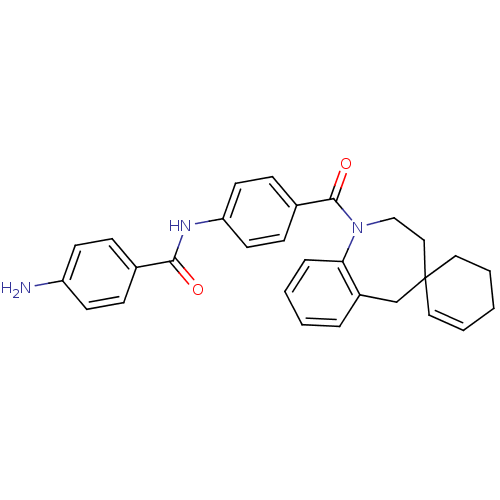 (CHEMBL100402 | spirobenzoxazines analogues) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory concentration against [3H]-AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 2987-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.103 BindingDB Entry DOI: 10.7270/Q2BK1BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50147224 (3'N-(2-dimethylaminoethyl)-1-[4-(2-phenylphenylcar...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50225159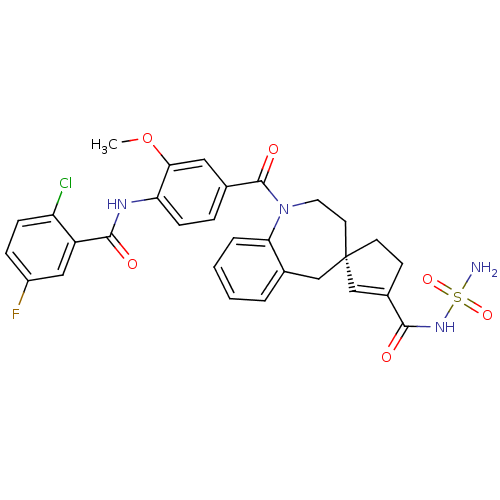 ((4R)-1-({4-[(2-chloro-5-fluorobenzene)amido]-3-met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Antagonist activity at human vasopressin V2 receptor expressed in HEK293 cells assessed as inhibition of Arg-vasopressin-induced cAMP levels | Bioorg Med Chem Lett 17: 6623-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.059 BindingDB Entry DOI: 10.7270/Q2T72H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50147221 (3'N-(2-hydroxyethyl)-1-[4-(2-phenylphenylcarboxami...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Binding affinity measured by inhibition of 3[H] AVP binding to cloned human vasopressin V2 receptor | Bioorg Med Chem Lett 14: 3143-6 (2004) Article DOI: 10.1016/j.bmcl.2004.04.016 BindingDB Entry DOI: 10.7270/Q2Z60PMQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50135419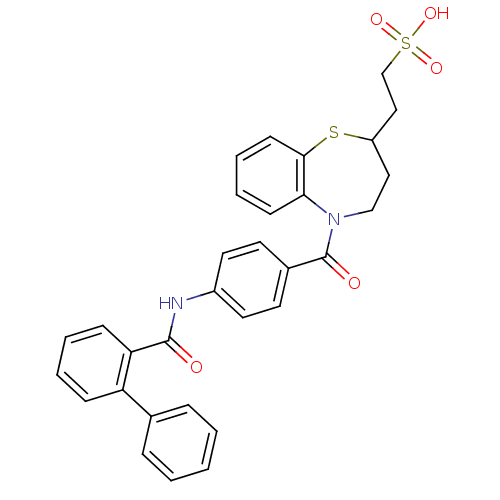 (2-(5-{4-[(Biphenyl-2-carbonyl)-amino]-benzoyl}-2,3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Ability to displace [3H]-arginine vasopressin in cloned human V2 receptor | Bioorg Med Chem Lett 13: 4031-4 (2003) BindingDB Entry DOI: 10.7270/Q23T9GM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50146562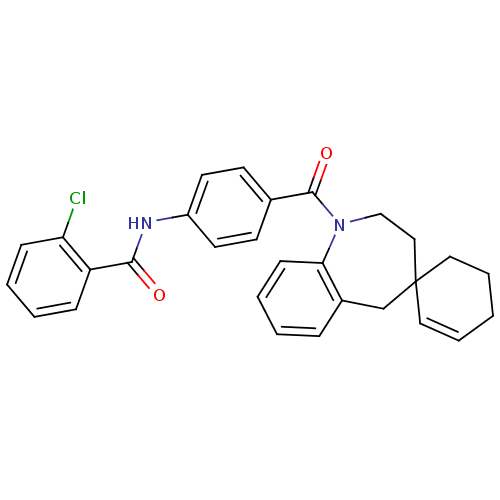 (CHEMBL100893 | spirobenzoxazines analogues) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description In vitro inhibitory concentration against [3H]-AVP binding to cloned human vasopressin V1a receptor | Bioorg Med Chem Lett 14: 2987-9 (2004) Article DOI: 10.1016/j.bmcl.2004.02.103 BindingDB Entry DOI: 10.7270/Q2BK1BSD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50225173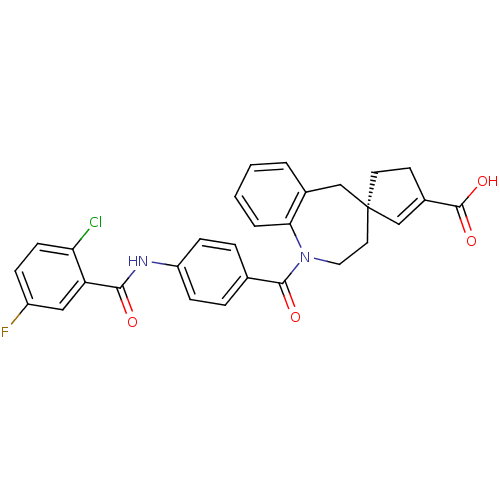 ((4R)-1-({4-[(2-chloro-5-fluorobenzene)amido]phenyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6623-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.059 BindingDB Entry DOI: 10.7270/Q2T72H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50225168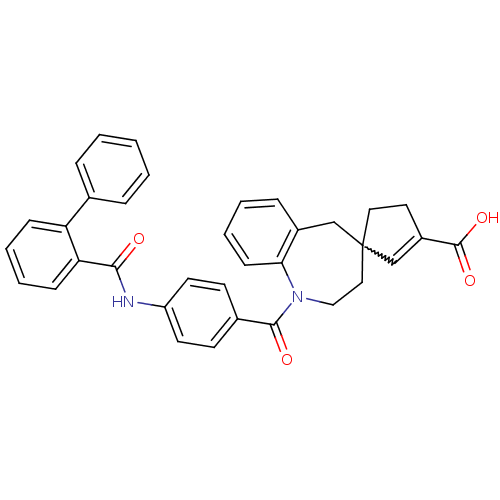 (1-({4-[(2-phenylbenzene)amido]phenyl}carbonyl)-1,2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6623-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.059 BindingDB Entry DOI: 10.7270/Q2T72H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50135418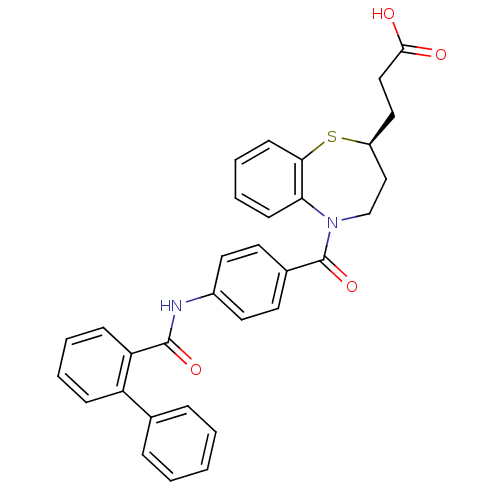 (3-((S)-5-{4-[(Biphenyl-2-carbonyl)-amino]-benzoyl}...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Ability to displace [3H]-arginine vasopressin in cloned human V2 receptor | Bioorg Med Chem Lett 13: 4031-4 (2003) BindingDB Entry DOI: 10.7270/Q23T9GM3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50225172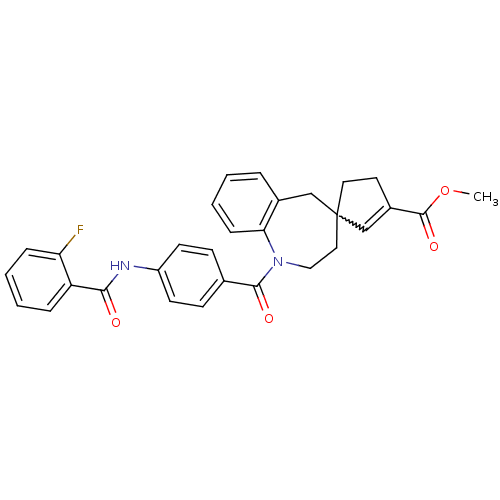 (CHEMBL251771 | methyl 1-({4-[(2-fluorobenzene)amid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development Curated by ChEMBL | Assay Description Displacement of [3H]-Arg-vasopressin from human recombinant vasopressin V1a receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6623-8 (2007) Article DOI: 10.1016/j.bmcl.2007.09.059 BindingDB Entry DOI: 10.7270/Q2T72H5P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 310 total ) | Next | Last >> |