

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016460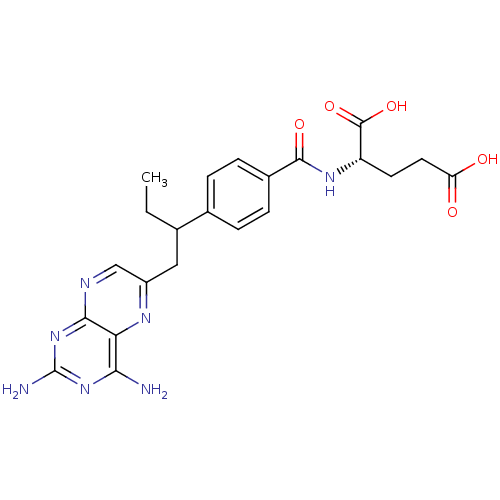 ((S)-2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration of the compound inhibiting dihydrofolate reductase derived from L1210 cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50405400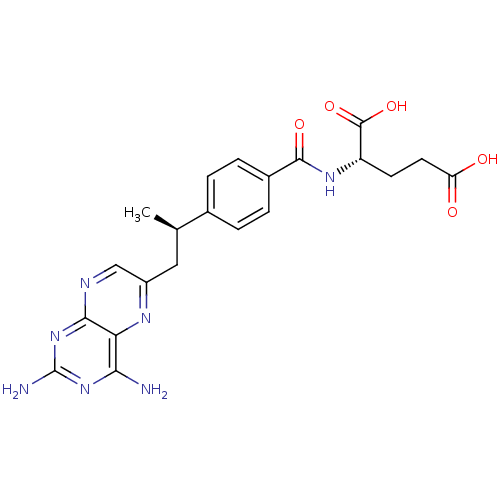 (CHEMBL2051987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50405400 (CHEMBL2051987) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50010932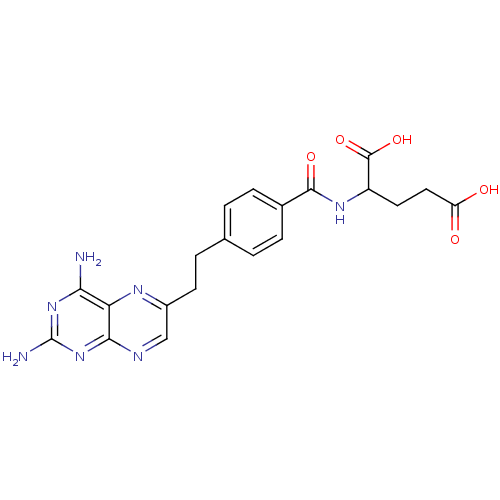 ((10-DA, 10-Deazaminopterin)2-{4-[2-(2,4-Diamino-pt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration of the compound inhibiting dihydrofolate reductase derived from L1210 cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50226274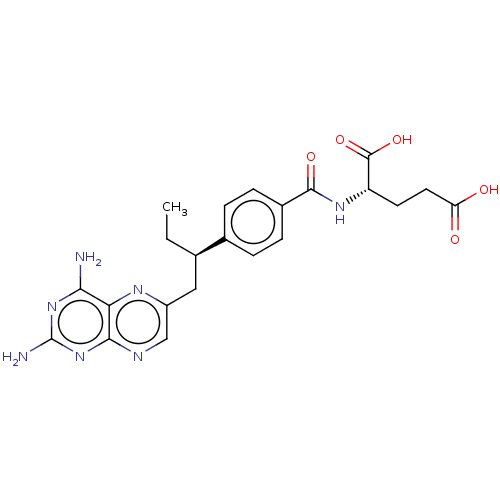 (CHEMBL3349020) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50014707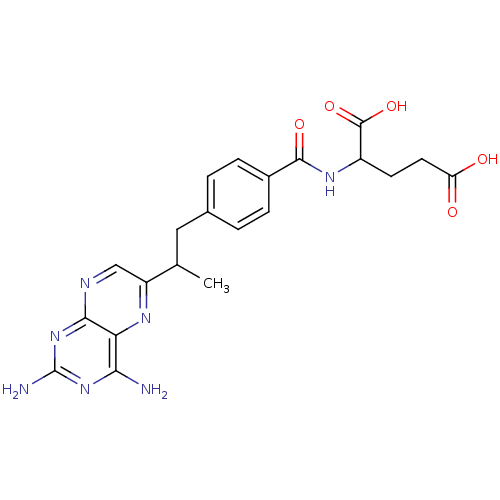 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-propyl]-benzoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration of the compound inhibiting dihydrofolate reductase derived from L1210 cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50014706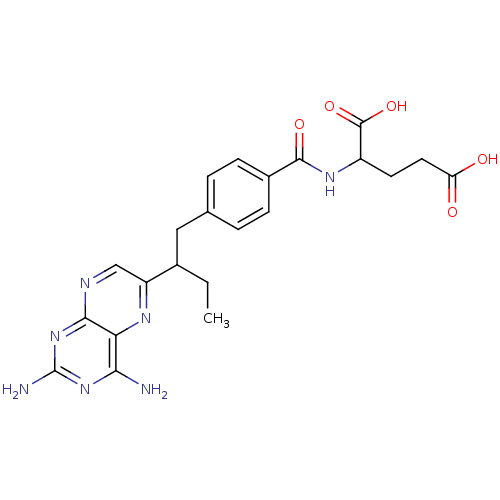 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-butyl]-benzoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration of the compound inhibiting Dihydrofolate reductase derived from L1210 cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of the dihydrofolate reductase enzyme(DHFR) derived from L1210 murine leukemia cells. | J Med Chem 35: 320-4 (1992) BindingDB Entry DOI: 10.7270/Q2WQ02RM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase derived from L1210 cell line. | J Med Chem 33: 673-7 (1990) BindingDB Entry DOI: 10.7270/Q2T43S3F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008287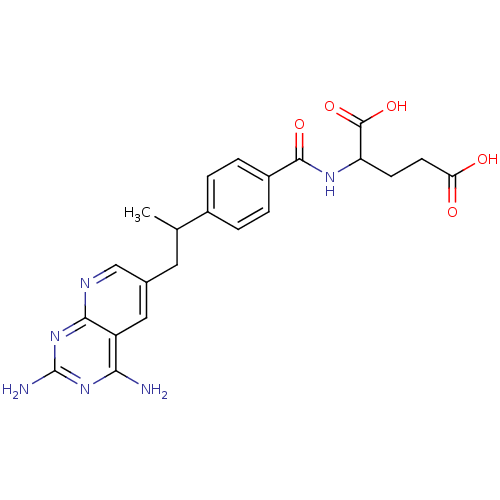 (2-{4-[2-(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase derived from L1210 cell line. | J Med Chem 33: 673-7 (1990) BindingDB Entry DOI: 10.7270/Q2T43S3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008287 (2-{4-[2-(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-yl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of the dihydrofolate reductase enzyme(DHFR) derived from L1210 murine leukemia cells. | J Med Chem 35: 320-4 (1992) BindingDB Entry DOI: 10.7270/Q2WQ02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50405401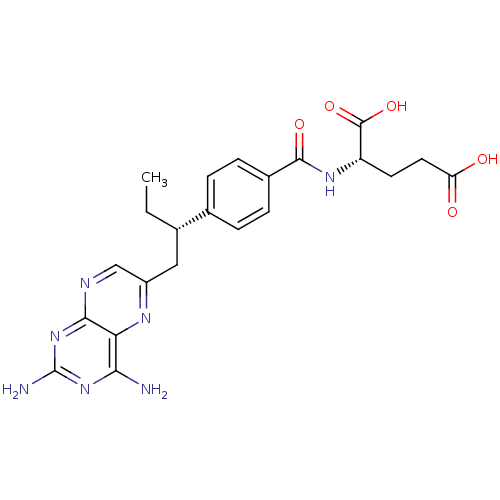 (CHEMBL2051990) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for binding affinity against Dihydrofolate reductase of L1210 cells | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008285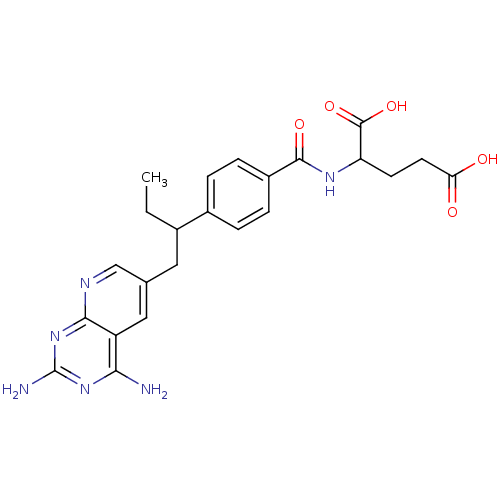 (2-{4-[1-(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of the dihydrofolate reductase enzyme(DHFR) derived from L1210 murine leukemia cells. | J Med Chem 35: 320-4 (1992) BindingDB Entry DOI: 10.7270/Q2WQ02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008285 (2-{4-[1-(2,4-Diamino-pyrido[2,3-d]pyrimidin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase derived from L1210 cell line. | J Med Chem 33: 673-7 (1990) BindingDB Entry DOI: 10.7270/Q2T43S3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50008286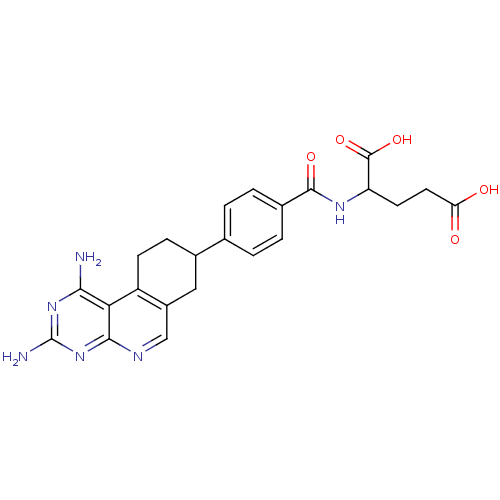 (2-[4-(1,3-Diamino-7,8,9,10-tetrahydro-pyrimido[4,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibition of the dihydrofolate reductase enzyme(DHFR) derived from L1210 murine leukemia cells. | J Med Chem 35: 320-4 (1992) BindingDB Entry DOI: 10.7270/Q2WQ02RM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50016460 ((S)-2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity of the compound towards Dihydrofolate reductase derived from L1210 cells using [3H]- MTX as the radioligand | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50024475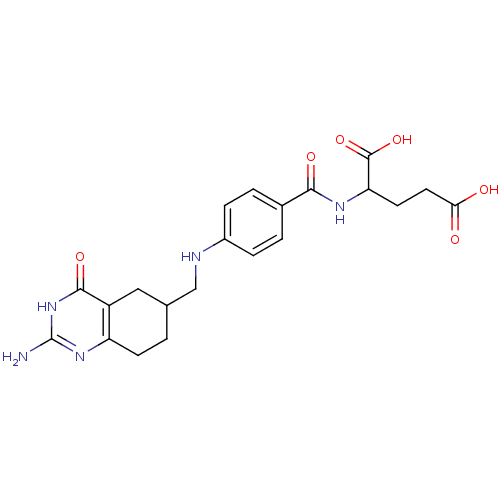 (2-{4-[(2-Amino-4-oxo-1,4,5,6,7,8-hexahydro-quinazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase derived from L1210 cell line. | J Med Chem 33: 673-7 (1990) BindingDB Entry DOI: 10.7270/Q2T43S3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50014935 (2-{4-[2-(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase derived from L1210 cell line. | J Med Chem 33: 673-7 (1990) BindingDB Entry DOI: 10.7270/Q2T43S3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50014936 (2-{4-[1-(2-Amino-4-hydroxy-5,6,7,8-tetrahydro-pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of Dihydrofolate reductase derived from L1210 cell line. | J Med Chem 33: 673-7 (1990) BindingDB Entry DOI: 10.7270/Q2T43S3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50010932 ((10-DA, 10-Deazaminopterin)2-{4-[2-(2,4-Diamino-pt...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity of the compound towards Dihydrofolate reductase derived from L1210 cells using [3H]- MTX as the radioligand | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50014707 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-propyl]-benzoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of the compound towards Dihydrofolate reductase derived from human manca leukemia cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity of the compound towards Dihydrofolate reductase derived from L1210 cells using [3H]- MTX as the radioligand | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity of the compound towards Dihydrofolate reductase derived from L1210 cells using [3H]- MTX as the radioligand | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50014706 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-butyl]-benzoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of the compound towards Dihydrofolate reductase derived from human manca leukemia cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50226274 (CHEMBL3349020) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50226274 (CHEMBL3349020) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50004544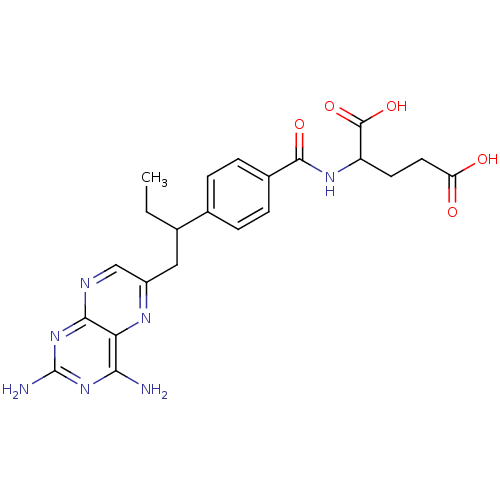 (2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-propyl]-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50405399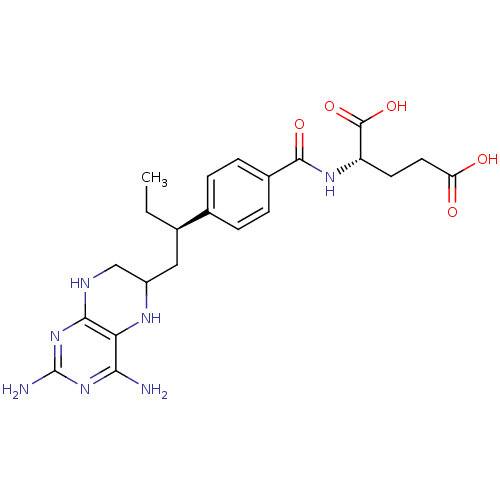 (CHEMBL2051989) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50405399 (CHEMBL2051989) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50004544 (2-{4-[1-(2,4-Diamino-pteridin-6-ylmethyl)-propyl]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50405401 (CHEMBL2051990) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of the compound towards dihydrofolate reductase derived from human manca leukemia cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50025007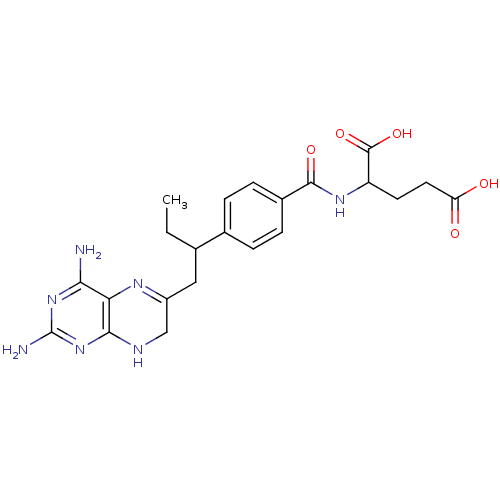 (2-{4-[2-(2,4-Diamino-7,8-dihydro-pteridin-6-yl)-et...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50025008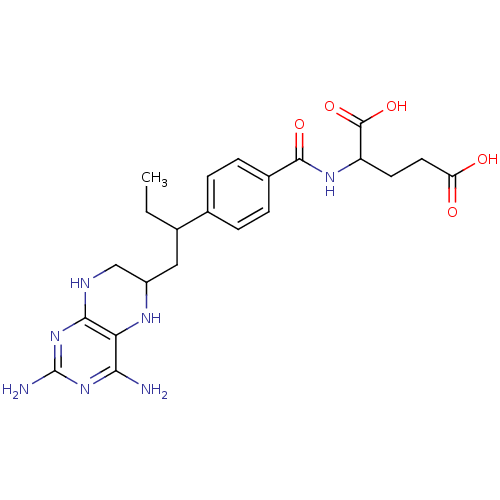 (2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pteridin-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50025007 (2-{4-[2-(2,4-Diamino-7,8-dihydro-pteridin-6-yl)-et...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50405399 (CHEMBL2051989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50405399 (CHEMBL2051989) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50405401 (CHEMBL2051990) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50014707 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-propyl]-benzoy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Inhibitory activity of the compound towards dihydrofolate reductase derived from human manca leukemia cells | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50405398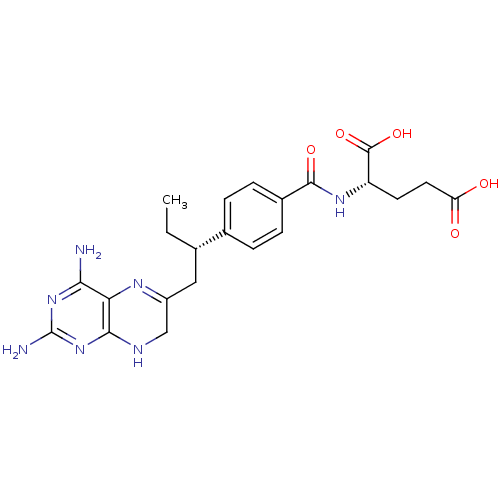 (CHEMBL2051991) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50025008 (2-{4-[2-(2,4-Diamino-5,6,7,8-tetrahydro-pteridin-6...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Gallus gallus (Chicken)) | BDBM50405398 (CHEMBL2051991) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Chicken liver | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50014706 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-butyl]-benzoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Binding affinity of the compound towards Dihydrofolate reductase derived from L1210 cells using [3H]- MTX as the radioligand | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50405398 (CHEMBL2051991) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM50405398 (CHEMBL2051991) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was evaluated for the inhibition of dihydrofolate reductase (DHFR) derived from Lactobacillus casei ATCC 7469 | J Med Chem 29: 1056-61 (1986) BindingDB Entry DOI: 10.7270/Q2P55MHM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50014707 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-propyl]-benzoy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration of the compound inhibiting AICAR formyltransferase enzyme of L. casei | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Mus musculus) | BDBM50014706 (2-{4-[2-(2,4-Diamino-pteridin-6-yl)-butyl]-benzoyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
SRI International Curated by ChEMBL | Assay Description Concentration of the compound inhibiting AICAR formyltransferase enzyme of L. casei | J Med Chem 33: 212-5 (1990) BindingDB Entry DOI: 10.7270/Q2FX78FR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |