 Found 222 hits with Last Name = 'clancy' and Initial = 'dc'
Found 222 hits with Last Name = 'clancy' and Initial = 'dc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27747

((2S,3R)-3-(tert-butoxy)-2-{[2-({[4-(cyclopropylmet...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(cc1NC(=O)Nc1c(C)cc(CC2CC2)cc1C)-c1cccc(F)c1)C(O)=O |r| Show InChI InChI=1S/C34H40FN3O5/c1-19-14-23(16-22-10-11-22)15-20(2)29(19)38-33(42)36-28-18-25(24-8-7-9-26(35)17-24)12-13-27(28)31(39)37-30(32(40)41)21(3)43-34(4,5)6/h7-9,12-15,17-18,21-22,30H,10-11,16H2,1-6H3,(H,37,39)(H,40,41)(H2,36,38,42)/t21-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27730
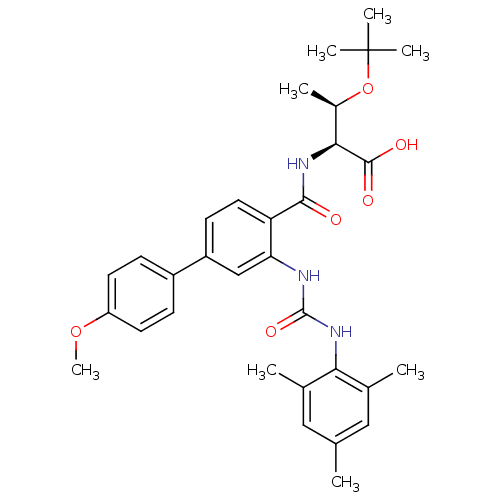
((2S,3R)-3-(tert-butoxy)-2-{[4-(4-methoxyphenyl)-2-...)Show SMILES COc1ccc(cc1)-c1ccc(C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)c(NC(=O)Nc2c(C)cc(C)cc2C)c1 |r| Show InChI InChI=1S/C32H39N3O6/c1-18-15-19(2)27(20(3)16-18)35-31(39)33-26-17-23(22-9-12-24(40-8)13-10-22)11-14-25(26)29(36)34-28(30(37)38)21(4)41-32(5,6)7/h9-17,21,28H,1-8H3,(H,34,36)(H,37,38)(H2,33,35,39)/t21-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27745
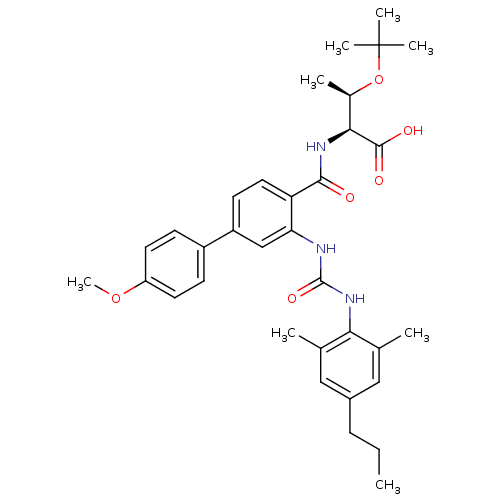
((2S,3R)-3-(tert-butoxy)-2-[(2-{[(2,6-dimethyl-4-pr...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(ccc2C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)-c2ccc(OC)cc2)c(C)c1 |r| Show InChI InChI=1S/C34H43N3O6/c1-9-10-23-17-20(2)29(21(3)18-23)37-33(41)35-28-19-25(24-11-14-26(42-8)15-12-24)13-16-27(28)31(38)36-30(32(39)40)22(4)43-34(5,6)7/h11-19,22,30H,9-10H2,1-8H3,(H,36,38)(H,39,40)(H2,35,37,41)/t22-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27746

((2S,3R)-3-(tert-butoxy)-2-[(2-{[(2,6-dimethyl-4-pr...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(ccc2C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)-c2cccc(F)c2)c(C)c1 |r| Show InChI InChI=1S/C33H40FN3O5/c1-8-10-22-15-19(2)28(20(3)16-22)37-32(41)35-27-18-24(23-11-9-12-25(34)17-23)13-14-26(27)30(38)36-29(31(39)40)21(4)42-33(5,6)7/h9,11-18,21,29H,8,10H2,1-7H3,(H,36,38)(H,39,40)(H2,35,37,41)/t21-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27743

((2S,3R)-3-(tert-butoxy)-2-{[2-({[4-(cyclopropylmet...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(F)cc1NC(=O)Nc1c(C)cc(CC2CC2)cc1C)C(O)=O |r| Show InChI InChI=1S/C28H36FN3O5/c1-15-11-19(13-18-7-8-18)12-16(2)23(15)32-27(36)30-22-14-20(29)9-10-21(22)25(33)31-24(26(34)35)17(3)37-28(4,5)6/h9-12,14,17-18,24H,7-8,13H2,1-6H3,(H,31,33)(H,34,35)(H2,30,32,36)/t17-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50255975

((S)-2-cyclohexyl-2-(3-(3-(2,6-dichloro-4-(trifluor...)Show SMILES OC(=O)[C@@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(Cl)cc(OC(F)(F)F)cc1Cl)C1CCCCC1 |r| Show InChI InChI=1S/C27H24Cl2F3N3O5/c28-19-12-17(40-27(30,31)32)13-20(29)23(19)35-26(39)33-21-11-16-9-5-4-8-15(16)10-18(21)24(36)34-22(25(37)38)14-6-2-1-3-7-14/h4-5,8-14,22H,1-3,6-7H2,(H,34,36)(H,37,38)(H2,33,35,39)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256329
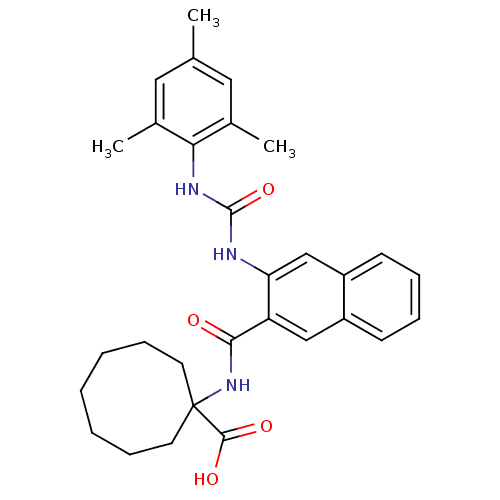
(1-(3-(3-mesitylureido)-2-naphthamido)cyclooctaneca...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)NC2(CCCCCCC2)C(O)=O)c(C)c1 Show InChI InChI=1S/C30H35N3O4/c1-19-15-20(2)26(21(3)16-19)32-29(37)31-25-18-23-12-8-7-11-22(23)17-24(25)27(34)33-30(28(35)36)13-9-5-4-6-10-14-30/h7-8,11-12,15-18H,4-6,9-10,13-14H2,1-3H3,(H,33,34)(H,35,36)(H2,31,32,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50255977
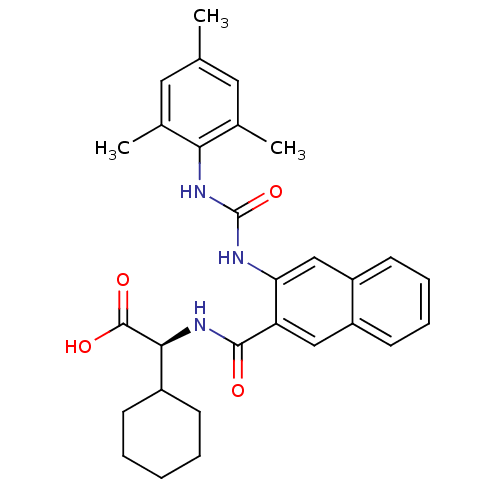
((S)-2-cyclohexyl-2-(3-(3-mesitylureido)-2-naphtham...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)N[C@@H](C2CCCCC2)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C29H33N3O4/c1-17-13-18(2)25(19(3)14-17)32-29(36)30-24-16-22-12-8-7-11-21(22)15-23(24)27(33)31-26(28(34)35)20-9-5-4-6-10-20/h7-8,11-16,20,26H,4-6,9-10H2,1-3H3,(H,31,33)(H,34,35)(H2,30,32,36)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256669
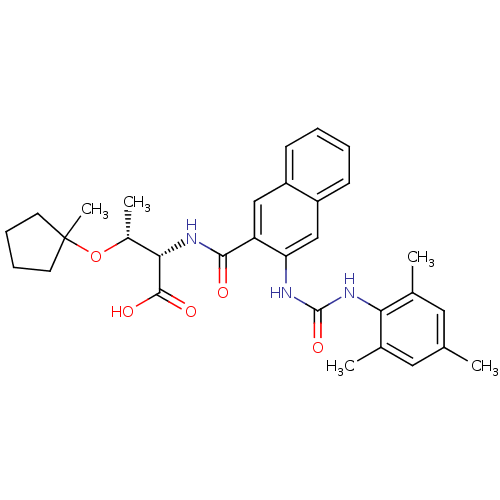
((2S,3R)-2-(3-(3-mesitylureido)-2-naphthamido)-3-(1...)Show SMILES C[C@@H](OC1(C)CCCC1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C31H37N3O5/c1-18-14-19(2)26(20(3)15-18)34-30(38)32-25-17-23-11-7-6-10-22(23)16-24(25)28(35)33-27(29(36)37)21(4)39-31(5)12-8-9-13-31/h6-7,10-11,14-17,21,27H,8-9,12-13H2,1-5H3,(H,33,35)(H,36,37)(H2,32,34,38)/t21-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27734
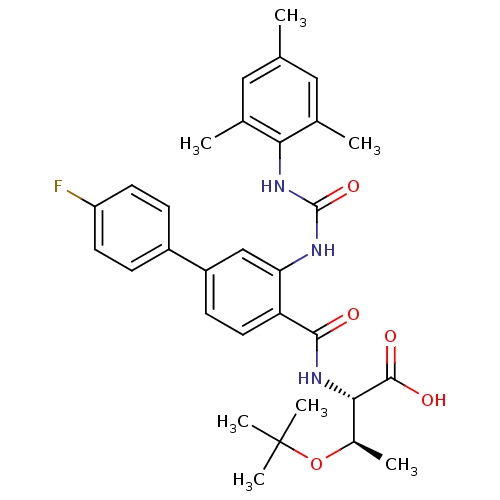
((2S,3R)-3-(tert-butoxy)-2-{[4-(4-fluorophenyl)-2-{...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(cc1NC(=O)Nc1c(C)cc(C)cc1C)-c1ccc(F)cc1)C(O)=O |r| Show InChI InChI=1S/C31H36FN3O5/c1-17-14-18(2)26(19(3)15-17)35-30(39)33-25-16-22(21-8-11-23(32)12-9-21)10-13-24(25)28(36)34-27(29(37)38)20(4)40-31(5,6)7/h8-16,20,27H,1-7H3,(H,34,36)(H,37,38)(H2,33,35,39)/t20-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27726
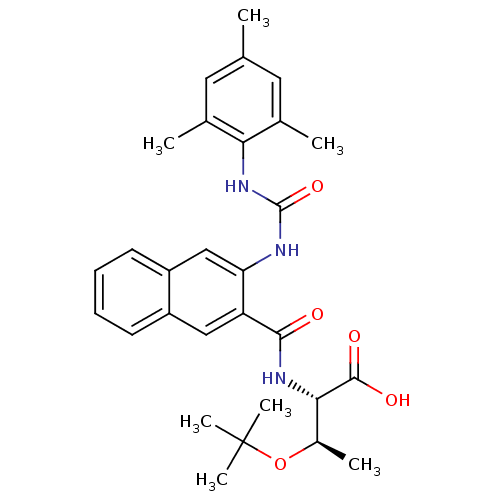
((2S,3R)-3-(tert-butoxy)-2-[(3-{[(2,4,6-trimethylph...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C29H35N3O5/c1-16-12-17(2)24(18(3)13-16)32-28(36)30-23-15-21-11-9-8-10-20(21)14-22(23)26(33)31-25(27(34)35)19(4)37-29(5,6)7/h8-15,19,25H,1-7H3,(H,31,33)(H,34,35)(H2,30,32,36)/t19-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27726

((2S,3R)-3-(tert-butoxy)-2-[(3-{[(2,4,6-trimethylph...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C29H35N3O5/c1-16-12-17(2)24(18(3)13-16)32-28(36)30-23-15-21-11-9-8-10-20(21)14-22(23)26(33)31-25(27(34)35)19(4)37-29(5,6)7/h8-15,19,25H,1-7H3,(H,31,33)(H,34,35)(H2,30,32,36)/t19-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27744
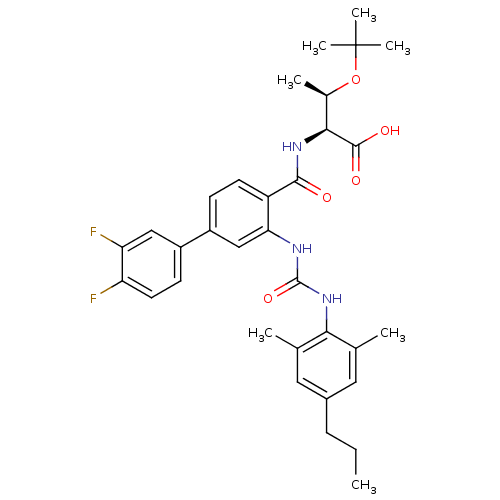
((2S,3R)-3-(tert-butoxy)-2-{[4-(3,4-difluorophenyl)...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(ccc2C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)-c2ccc(F)c(F)c2)c(C)c1 |r| Show InChI InChI=1S/C33H39F2N3O5/c1-8-9-21-14-18(2)28(19(3)15-21)38-32(42)36-27-17-23(22-11-13-25(34)26(35)16-22)10-12-24(27)30(39)37-29(31(40)41)20(4)43-33(5,6)7/h10-17,20,29H,8-9H2,1-7H3,(H,37,39)(H,40,41)(H2,36,38,42)/t20-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27731
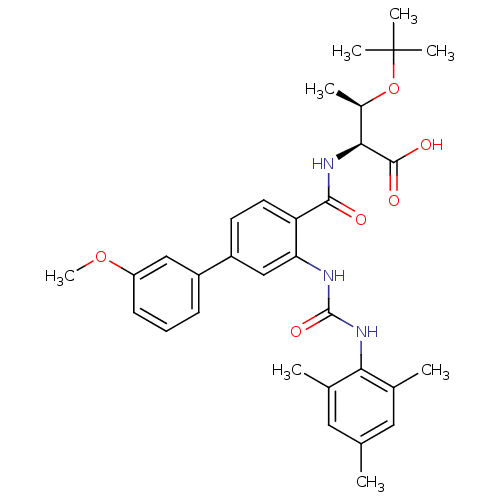
((2S,3R)-3-(tert-butoxy)-2-{[4-(3-methoxyphenyl)-2-...)Show SMILES COc1cccc(c1)-c1ccc(C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)c(NC(=O)Nc2c(C)cc(C)cc2C)c1 |r| Show InChI InChI=1S/C32H39N3O6/c1-18-14-19(2)27(20(3)15-18)35-31(39)33-26-17-23(22-10-9-11-24(16-22)40-8)12-13-25(26)29(36)34-28(30(37)38)21(4)41-32(5,6)7/h9-17,21,28H,1-8H3,(H,34,36)(H,37,38)(H2,33,35,39)/t21-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256573
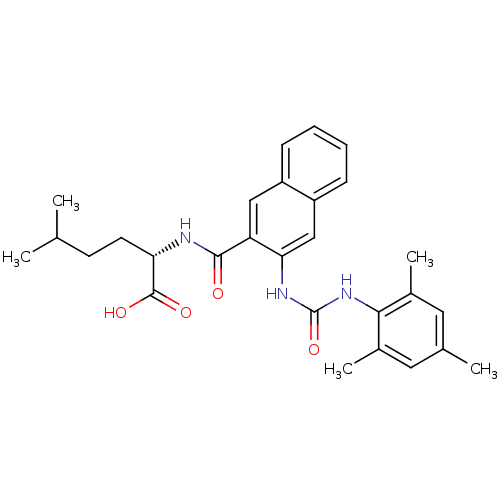
((S)-2-(3-(3-mesitylureido)-2-naphthamido)-5-methyl...)Show SMILES CC(C)CC[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C28H33N3O4/c1-16(2)10-11-23(27(33)34)29-26(32)22-14-20-8-6-7-9-21(20)15-24(22)30-28(35)31-25-18(4)12-17(3)13-19(25)5/h6-9,12-16,23H,10-11H2,1-5H3,(H,29,32)(H,33,34)(H2,30,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27742
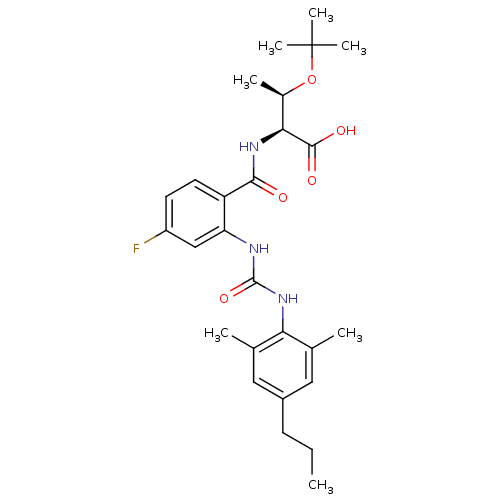
((2S,3R)-3-(tert-butoxy)-2-[(2-{[(2,6-dimethyl-4-pr...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(F)ccc2C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C27H36FN3O5/c1-8-9-18-12-15(2)22(16(3)13-18)31-26(35)29-21-14-19(28)10-11-20(21)24(32)30-23(25(33)34)17(4)36-27(5,6)7/h10-14,17,23H,8-9H2,1-7H3,(H,30,32)(H,33,34)(H2,29,31,35)/t17-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256169
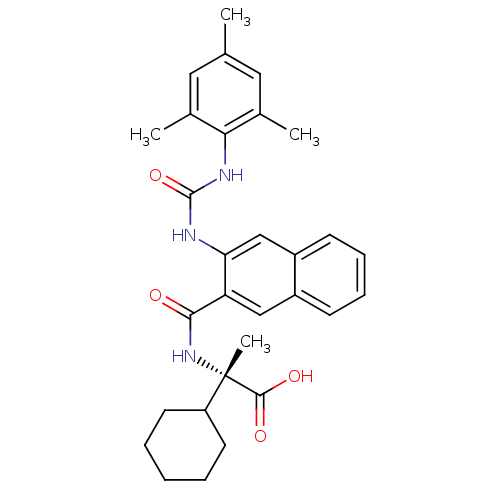
((S)-2-cyclohexyl-2-(3-(3-mesitylureido)-2-naphtham...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)N[C@@](C)(C2CCCCC2)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C30H35N3O4/c1-18-14-19(2)26(20(3)15-18)32-29(37)31-25-17-22-11-9-8-10-21(22)16-24(25)27(34)33-30(4,28(35)36)23-12-6-5-7-13-23/h8-11,14-17,23H,5-7,12-13H2,1-4H3,(H,33,34)(H,35,36)(H2,31,32,37)/t30-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27729
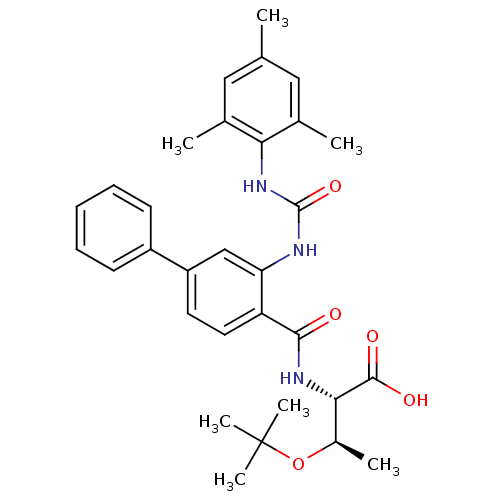
((2S,3R)-3-(tert-butoxy)-2-[(4-phenyl-2-{[(2,4,6-tr...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(cc1NC(=O)Nc1c(C)cc(C)cc1C)-c1ccccc1)C(O)=O |r| Show InChI InChI=1S/C31H37N3O5/c1-18-15-19(2)26(20(3)16-18)34-30(38)32-25-17-23(22-11-9-8-10-12-22)13-14-24(25)28(35)33-27(29(36)37)21(4)39-31(5,6)7/h8-17,21,27H,1-7H3,(H,33,35)(H,36,37)(H2,32,34,38)/t21-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27735
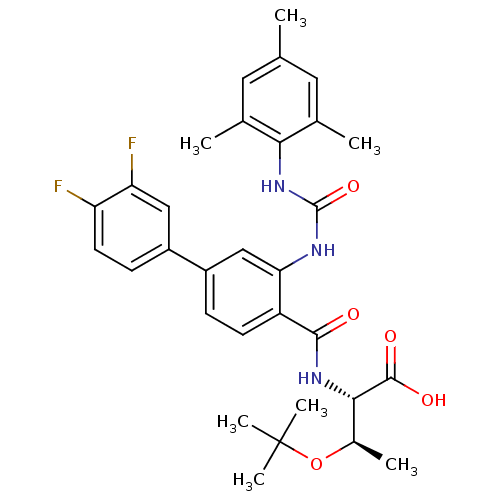
((2S,3R)-3-(tert-butoxy)-2-{[4-(3,4-difluorophenyl)...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(cc1NC(=O)Nc1c(C)cc(C)cc1C)-c1ccc(F)c(F)c1)C(O)=O |r| Show InChI InChI=1S/C31H35F2N3O5/c1-16-12-17(2)26(18(3)13-16)36-30(40)34-25-15-21(20-9-11-23(32)24(33)14-20)8-10-22(25)28(37)35-27(29(38)39)19(4)41-31(5,6)7/h8-15,19,27H,1-7H3,(H,35,37)(H,38,39)(H2,34,36,40)/t19-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256071
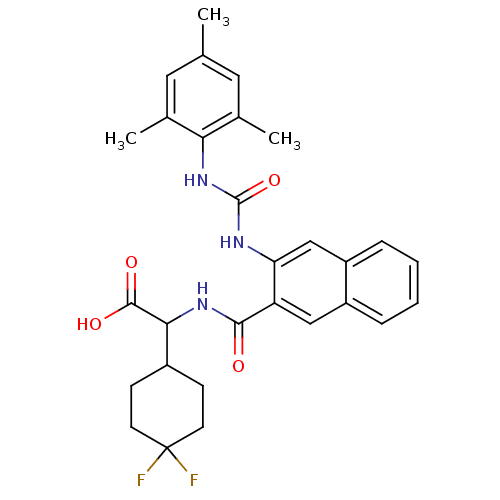
(2-(4,4-difluorocyclohexyl)-2-(3-(3-mesitylureido)-...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)NC(C2CCC(F)(F)CC2)C(O)=O)c(C)c1 Show InChI InChI=1S/C29H31F2N3O4/c1-16-12-17(2)24(18(3)13-16)34-28(38)32-23-15-21-7-5-4-6-20(21)14-22(23)26(35)33-25(27(36)37)19-8-10-29(30,31)11-9-19/h4-7,12-15,19,25H,8-11H2,1-3H3,(H,33,35)(H,36,37)(H2,32,34,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27740

((2S)-2-cyclohexyl-2-{[2-({[4-(cyclopropylmethyl)-2...)Show SMILES Cc1cc(CC2CC2)cc(C)c1NC(=O)Nc1cc(F)ccc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C28H34FN3O4/c1-16-12-19(14-18-8-9-18)13-17(2)24(16)32-28(36)30-23-15-21(29)10-11-22(23)26(33)31-25(27(34)35)20-6-4-3-5-7-20/h10-13,15,18,20,25H,3-9,14H2,1-2H3,(H,31,33)(H,34,35)(H2,30,32,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256012

((S)-2-cyclopentyl-2-(3-(3-mesitylureido)-2-naphtha...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)N[C@@H](C2CCCC2)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C28H31N3O4/c1-16-12-17(2)24(18(3)13-16)31-28(35)29-23-15-21-11-7-6-10-20(21)14-22(23)26(32)30-25(27(33)34)19-8-4-5-9-19/h6-7,10-15,19,25H,4-5,8-9H2,1-3H3,(H,30,32)(H,33,34)(H2,29,31,35)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256330
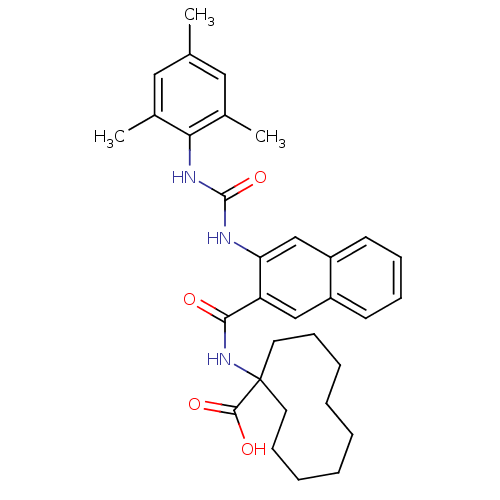
(1-(3-(3-mesitylureido)-2-naphthamido)cyclodecaneca...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)NC2(CCCCCCCCC2)C(O)=O)c(C)c1 Show InChI InChI=1S/C32H39N3O4/c1-21-17-22(2)28(23(3)18-21)34-31(39)33-27-20-25-14-10-9-13-24(25)19-26(27)29(36)35-32(30(37)38)15-11-7-5-4-6-8-12-16-32/h9-10,13-14,17-20H,4-8,11-12,15-16H2,1-3H3,(H,35,36)(H,37,38)(H2,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27733

((2S,3R)-3-(tert-butoxy)-2-{[4-(3-fluorophenyl)-2-{...)Show SMILES C[C@@H](OC(C)(C)C)[C@H](NC(=O)c1ccc(cc1NC(=O)Nc1c(C)cc(C)cc1C)-c1cccc(F)c1)C(O)=O |r| Show InChI InChI=1S/C31H36FN3O5/c1-17-13-18(2)26(19(3)14-17)35-30(39)33-25-16-22(21-9-8-10-23(32)15-21)11-12-24(25)28(36)34-27(29(37)38)20(4)40-31(5,6)7/h8-16,20,27H,1-7H3,(H,34,36)(H,37,38)(H2,33,35,39)/t20-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256710
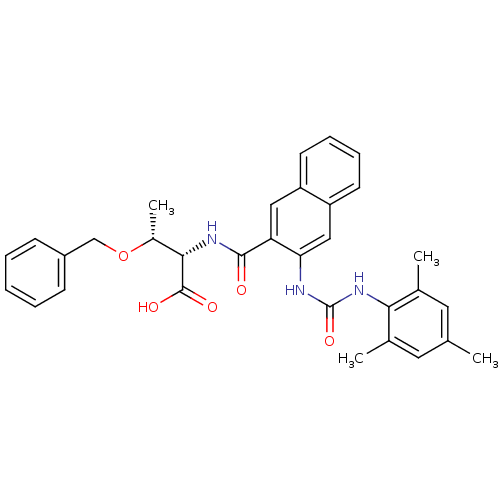
((2S,3R)-3-(benzyloxy)-2-(3-(3-mesitylureido)-2-nap...)Show SMILES C[C@@H](OCc1ccccc1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C32H33N3O5/c1-19-14-20(2)28(21(3)15-19)35-32(39)33-27-17-25-13-9-8-12-24(25)16-26(27)30(36)34-29(31(37)38)22(4)40-18-23-10-6-5-7-11-23/h5-17,22,29H,18H2,1-4H3,(H,34,36)(H,37,38)(H2,33,35,39)/t22-,29+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256667
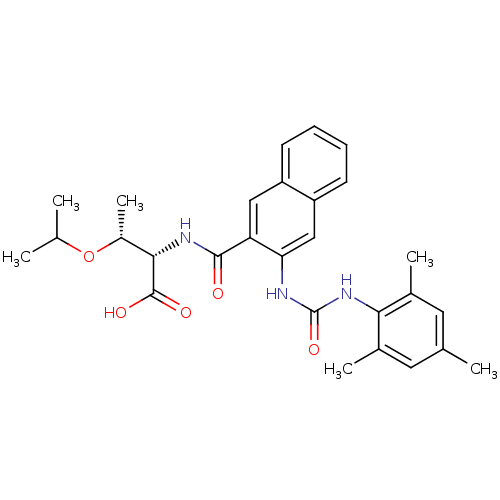
((2S,3R)-3-isopropoxy-2-(3-(3-mesitylureido)-2-naph...)Show SMILES CC(C)O[C@H](C)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C28H33N3O5/c1-15(2)36-19(6)25(27(33)34)30-26(32)22-13-20-9-7-8-10-21(20)14-23(22)29-28(35)31-24-17(4)11-16(3)12-18(24)5/h7-15,19,25H,1-6H3,(H,30,32)(H,33,34)(H2,29,31,35)/t19-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256668
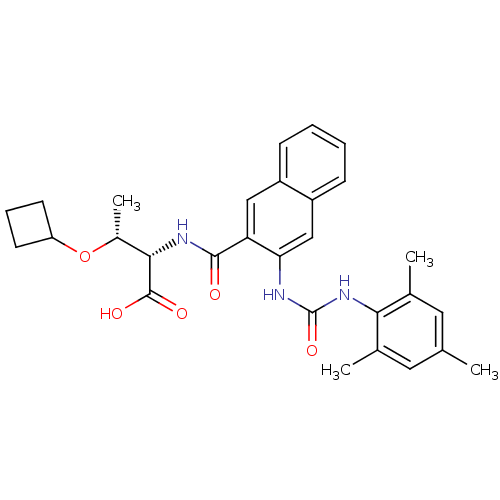
((2S,3R)-3-cyclobutoxy-2-(3-(3-mesitylureido)-2-nap...)Show SMILES C[C@@H](OC1CCC1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C29H33N3O5/c1-16-12-17(2)25(18(3)13-16)32-29(36)30-24-15-21-9-6-5-8-20(21)14-23(24)27(33)31-26(28(34)35)19(4)37-22-10-7-11-22/h5-6,8-9,12-15,19,22,26H,7,10-11H2,1-4H3,(H,31,33)(H,34,35)(H2,30,32,36)/t19-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27739
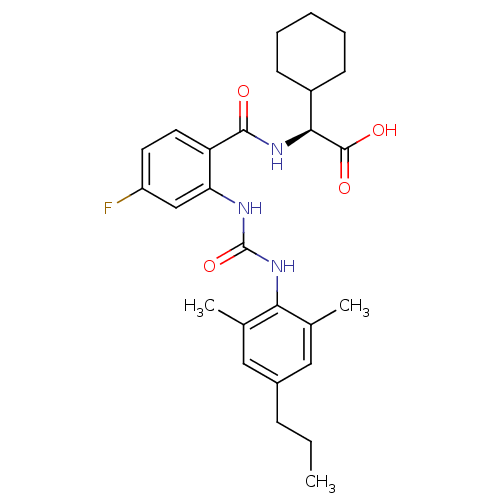
((2S)-2-cyclohexyl-2-[(2-{[(2,6-dimethyl-4-propylph...)Show SMILES CCCc1cc(C)c(NC(=O)Nc2cc(F)ccc2C(=O)N[C@@H](C2CCCCC2)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C27H34FN3O4/c1-4-8-18-13-16(2)23(17(3)14-18)31-27(35)29-22-15-20(28)11-12-21(22)25(32)30-24(26(33)34)19-9-6-5-7-10-19/h11-15,19,24H,4-10H2,1-3H3,(H,30,32)(H,33,34)(H2,29,31,35)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50243599
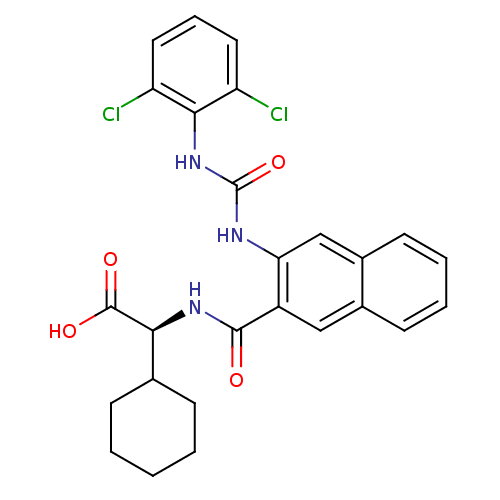
((S)-2-cyclohexyl-2-(2-(3-(2,6-dichlorophenyl)ureid...)Show SMILES OC(=O)[C@@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(Cl)cccc1Cl)C1CCCCC1 |r| Show InChI InChI=1S/C26H25Cl2N3O4/c27-19-11-6-12-20(28)23(19)31-26(35)29-21-14-17-10-5-4-9-16(17)13-18(21)24(32)30-22(25(33)34)15-7-2-1-3-8-15/h4-6,9-15,22H,1-3,7-8H2,(H,30,32)(H,33,34)(H2,29,31,35)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256521
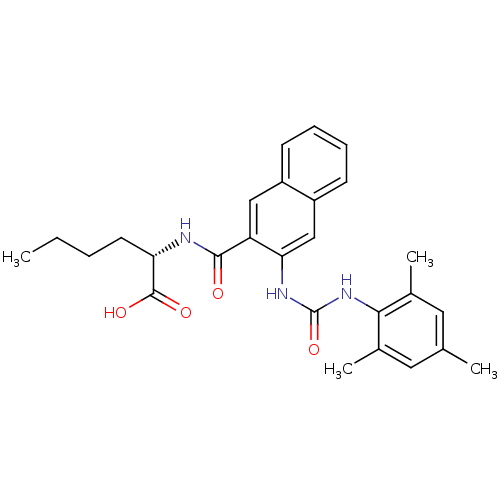
((S)-2-(3-(3-mesitylureido)-2-naphthamido)hexanoic ...)Show SMILES CCCC[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C27H31N3O4/c1-5-6-11-22(26(32)33)28-25(31)21-14-19-9-7-8-10-20(19)15-23(21)29-27(34)30-24-17(3)12-16(2)13-18(24)4/h7-10,12-15,22H,5-6,11H2,1-4H3,(H,28,31)(H,32,33)(H2,29,30,34)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256711
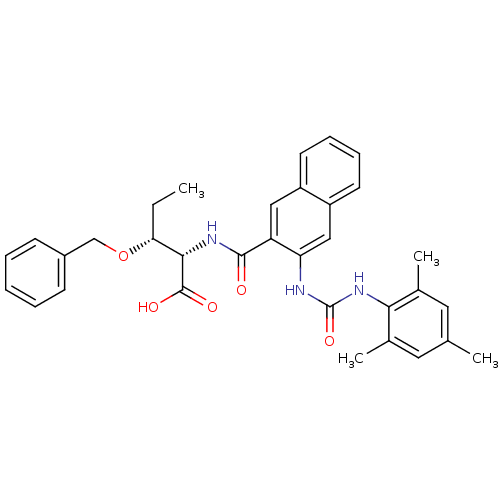
((2S,3R)-3-(benzyloxy)-2-(3-(3-mesitylureido)-2-nap...)Show SMILES CC[C@@H](OCc1ccccc1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C33H35N3O5/c1-5-28(41-19-23-11-7-6-8-12-23)30(32(38)39)35-31(37)26-17-24-13-9-10-14-25(24)18-27(26)34-33(40)36-29-21(3)15-20(2)16-22(29)4/h6-18,28,30H,5,19H2,1-4H3,(H,35,37)(H,38,39)(H2,34,36,40)/t28-,30+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256574
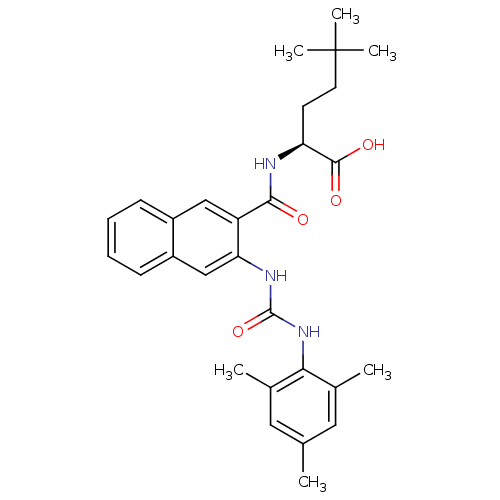
((S)-2-(3-(3-mesitylureido)-2-naphthamido)-5,5-dime...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)N[C@@H](CCC(C)(C)C)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C29H35N3O4/c1-17-13-18(2)25(19(3)14-17)32-28(36)31-24-16-21-10-8-7-9-20(21)15-22(24)26(33)30-23(27(34)35)11-12-29(4,5)6/h7-10,13-16,23H,11-12H2,1-6H3,(H,30,33)(H,34,35)(H2,31,32,36)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256393
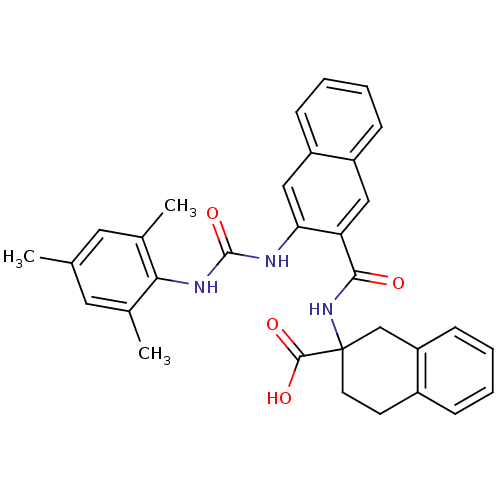
(2-(3-(3-mesitylureido)-2-naphthamido)-1,2,3,4-tetr...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)NC2(CCc3ccccc3C2)C(O)=O)c(C)c1 Show InChI InChI=1S/C32H31N3O4/c1-19-14-20(2)28(21(3)15-19)34-31(39)33-27-17-24-10-6-5-9-23(24)16-26(27)29(36)35-32(30(37)38)13-12-22-8-4-7-11-25(22)18-32/h4-11,14-17H,12-13,18H2,1-3H3,(H,35,36)(H,37,38)(H2,33,34,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27732

((2S,3R)-3-(tert-butoxy)-2-{[4-(2-methoxyphenyl)-2-...)Show SMILES COc1ccccc1-c1ccc(C(=O)N[C@@H]([C@@H](C)OC(C)(C)C)C(O)=O)c(NC(=O)Nc2c(C)cc(C)cc2C)c1 |r| Show InChI InChI=1S/C32H39N3O6/c1-18-15-19(2)27(20(3)16-18)35-31(39)33-25-17-22(23-11-9-10-12-26(23)40-8)13-14-24(25)29(36)34-28(30(37)38)21(4)41-32(5,6)7/h9-17,21,28H,1-8H3,(H,34,36)(H,37,38)(H2,33,35,39)/t21-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
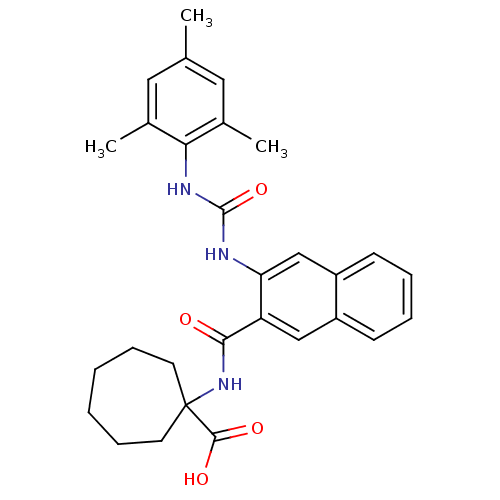
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256328
(1-(3-(3-mesitylureido)-2-naphthamido)cycloheptanec...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)NC2(CCCCCC2)C(O)=O)c(C)c1 Show InChI InChI=1S/C29H33N3O4/c1-18-14-19(2)25(20(3)15-18)31-28(36)30-24-17-22-11-7-6-10-21(22)16-23(24)26(33)32-29(27(34)35)12-8-4-5-9-13-29/h6-7,10-11,14-17H,4-5,8-9,12-13H2,1-3H3,(H,32,33)(H,34,35)(H2,30,31,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
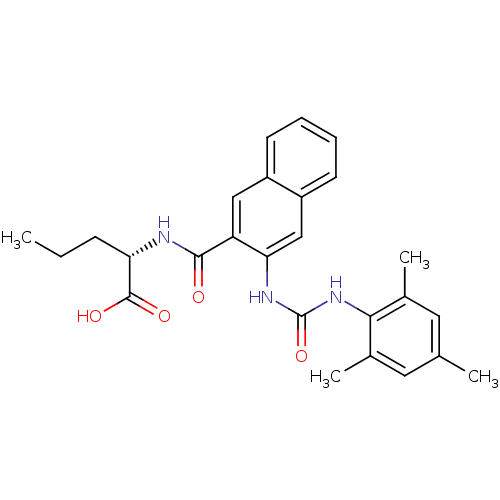
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256443
((S)-2-(3-(3-mesitylureido)-2-naphthamido)pentanoic...)Show SMILES CCC[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C26H29N3O4/c1-5-8-21(25(31)32)27-24(30)20-13-18-9-6-7-10-19(18)14-22(20)28-26(33)29-23-16(3)11-15(2)12-17(23)4/h6-7,9-14,21H,5,8H2,1-4H3,(H,27,30)(H,31,32)(H2,28,29,33)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
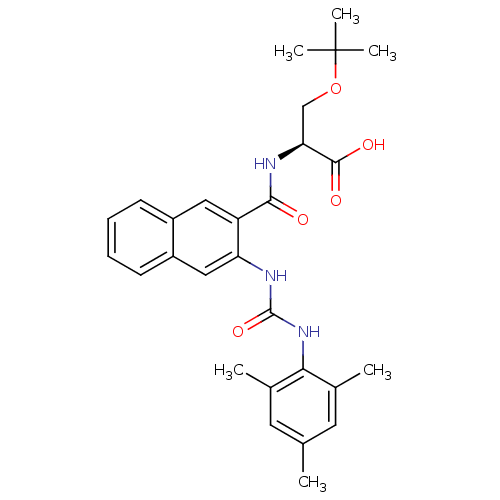
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256620
((S)-3-tert-butoxy-2-(3-(3-mesitylureido)-2-naphtha...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)N[C@@H](COC(C)(C)C)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C28H33N3O5/c1-16-11-17(2)24(18(3)12-16)31-27(35)30-22-14-20-10-8-7-9-19(20)13-21(22)25(32)29-23(26(33)34)15-36-28(4,5)6/h7-14,23H,15H2,1-6H3,(H,29,32)(H,33,34)(H2,30,31,35)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27741
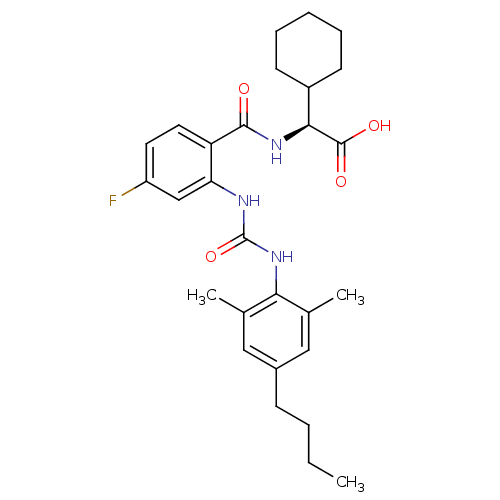
((2S)-2-[(2-{[(4-butyl-2,6-dimethylphenyl)carbamoyl...)Show SMILES CCCCc1cc(C)c(NC(=O)Nc2cc(F)ccc2C(=O)N[C@@H](C2CCCCC2)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C28H36FN3O4/c1-4-5-9-19-14-17(2)24(18(3)15-19)32-28(36)30-23-16-21(29)12-13-22(23)26(33)31-25(27(34)35)20-10-7-6-8-11-20/h12-16,20,25H,4-11H2,1-3H3,(H,31,33)(H,34,35)(H2,30,32,36)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50256712
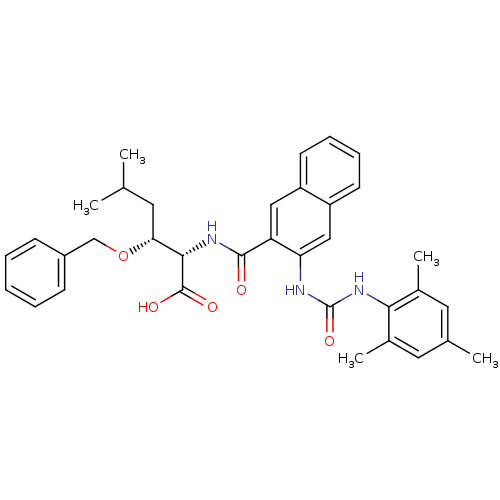
((2S,3R)-3-(benzyloxy)-2-(3-(3-mesitylureido)-2-nap...)Show SMILES CC(C)C[C@@H](OCc1ccccc1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C35H39N3O5/c1-21(2)15-30(43-20-25-11-7-6-8-12-25)32(34(40)41)37-33(39)28-18-26-13-9-10-14-27(26)19-29(28)36-35(42)38-31-23(4)16-22(3)17-24(31)5/h6-14,16-19,21,30,32H,15,20H2,1-5H3,(H,37,39)(H,40,41)(H2,36,38,42)/t30-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256575
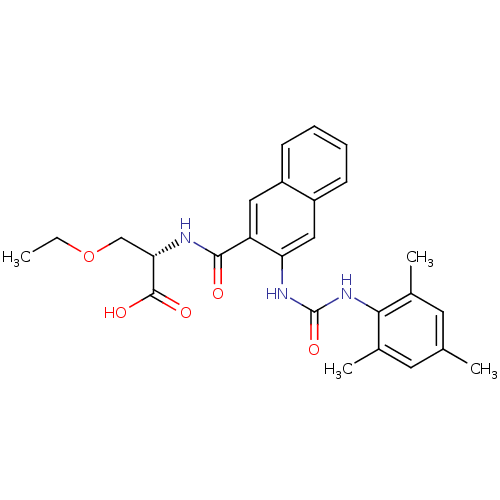
((S)-3-ethoxy-2-(3-(3-mesitylureido)-2-naphthamido)...)Show SMILES CCOC[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C26H29N3O5/c1-5-34-14-22(25(31)32)27-24(30)20-12-18-8-6-7-9-19(18)13-21(20)28-26(33)29-23-16(3)10-15(2)11-17(23)4/h6-13,22H,5,14H2,1-4H3,(H,27,30)(H,31,32)(H2,28,29,33)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256072
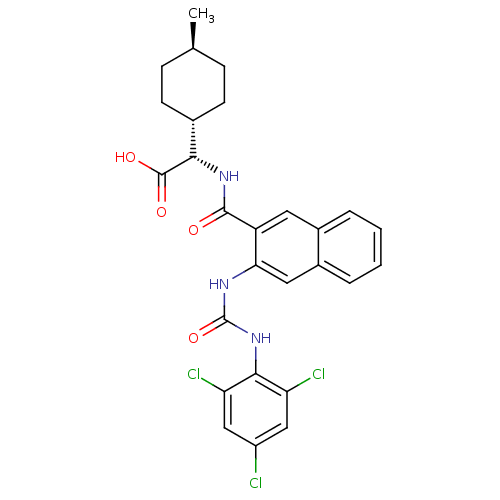
((S)-2-((1r,4S)-4-methylcyclohexyl)-2-(3-(3-(2,4,6-...)Show SMILES C[C@H]1CC[C@@H](CC1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(Cl)cc(Cl)cc1Cl)C(O)=O |r,wU:7.8,4.4,wD:1.0,(-2.28,-28.91,;-2.28,-30.45,;-3.61,-31.23,;-3.61,-32.77,;-2.28,-33.53,;-.94,-32.75,;-.95,-31.22,;-2.27,-35.07,;-3.6,-35.84,;-3.59,-37.38,;-2.25,-38.15,;-4.92,-38.16,;-6.26,-37.4,;-7.58,-38.17,;-8.92,-37.41,;-10.25,-38.18,;-10.25,-39.73,;-8.91,-40.5,;-7.58,-39.72,;-6.25,-40.48,;-4.91,-39.71,;-3.57,-40.48,;-3.57,-42.02,;-4.9,-42.79,;-2.23,-42.78,;-2.23,-44.32,;-3.56,-45.09,;-4.89,-44.32,;-3.55,-46.63,;-2.21,-47.4,;-2.21,-48.94,;-.88,-46.61,;-.89,-45.08,;.44,-44.3,;-.93,-35.83,;-.92,-37.37,;.4,-35.05,)| Show InChI InChI=1S/C27H26Cl3N3O4/c1-14-6-8-15(9-7-14)23(26(35)36)32-25(34)19-10-16-4-2-3-5-17(16)11-22(19)31-27(37)33-24-20(29)12-18(28)13-21(24)30/h2-5,10-15,23H,6-9H2,1H3,(H,32,34)(H,35,36)(H2,31,33,37)/t14-,15-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50255976
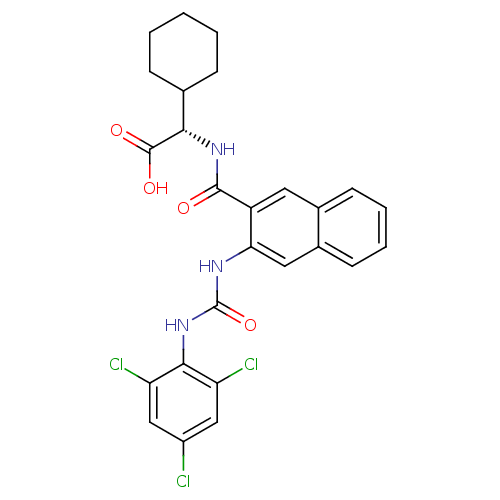
((S)-2-cyclohexyl-2-(3-(3-(2,4,6-trichlorophenyl)ur...)Show SMILES OC(=O)[C@@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(Cl)cc(Cl)cc1Cl)C1CCCCC1 |r| Show InChI InChI=1S/C26H24Cl3N3O4/c27-17-12-19(28)23(20(29)13-17)32-26(36)30-21-11-16-9-5-4-8-15(16)10-18(21)24(33)31-22(25(34)35)14-6-2-1-3-7-14/h4-5,8-14,22H,1-3,6-7H2,(H,31,33)(H,34,35)(H2,30,32,36)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256444
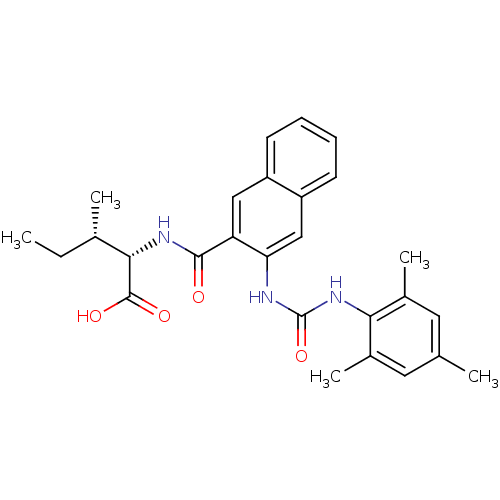
((2S,3S)-2-(3-(3-mesitylureido)-2-naphthamido)-3-me...)Show SMILES CC[C@H](C)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C27H31N3O4/c1-6-16(3)24(26(32)33)29-25(31)21-13-19-9-7-8-10-20(19)14-22(21)28-27(34)30-23-17(4)11-15(2)12-18(23)5/h7-14,16,24H,6H2,1-5H3,(H,29,31)(H,32,33)(H2,28,30,34)/t16-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27725
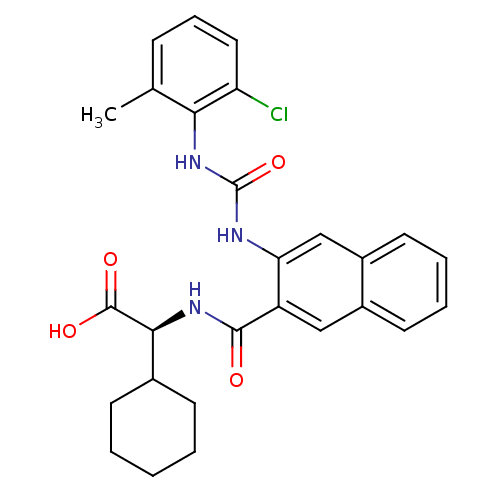
((2S)-2-[(3-{[(2-chloro-6-methylphenyl)carbamoyl]am...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C27H28ClN3O4/c1-16-8-7-13-21(28)23(16)31-27(35)29-22-15-19-12-6-5-11-18(19)14-20(22)25(32)30-24(26(33)34)17-9-3-2-4-10-17/h5-8,11-15,17,24H,2-4,9-10H2,1H3,(H,30,32)(H,33,34)(H2,29,31,35)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256712

((2S,3R)-3-(benzyloxy)-2-(3-(3-mesitylureido)-2-nap...)Show SMILES CC(C)C[C@@H](OCc1ccccc1)[C@H](NC(=O)c1cc2ccccc2cc1NC(=O)Nc1c(C)cc(C)cc1C)C(O)=O |r| Show InChI InChI=1S/C35H39N3O5/c1-21(2)15-30(43-20-25-11-7-6-8-12-25)32(34(40)41)37-33(39)28-18-26-13-9-10-14-27(26)19-29(28)36-35(42)38-31-23(4)16-22(3)17-24(31)5/h6-14,16-19,21,30,32H,15,20H2,1-5H3,(H,37,39)(H,40,41)(H2,36,38,42)/t30-,32+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27725

((2S)-2-[(3-{[(2-chloro-6-methylphenyl)carbamoyl]am...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C27H28ClN3O4/c1-16-8-7-13-21(28)23(16)31-27(35)29-22-15-19-12-6-5-11-18(19)14-20(22)25(32)30-24(26(33)34)17-9-3-2-4-10-17/h5-8,11-15,17,24H,2-4,9-10H2,1H3,(H,30,32)(H,33,34)(H2,29,31,35)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256621
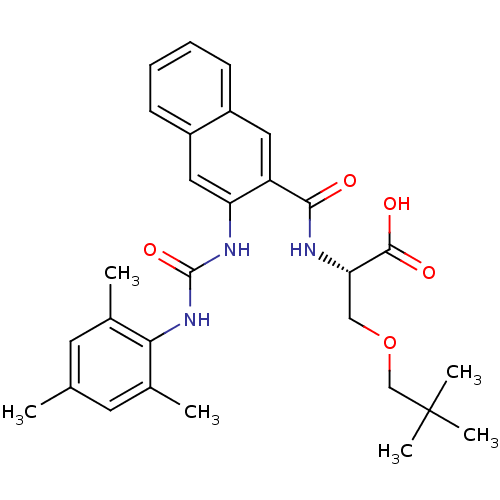
((S)-2-(3-(3-mesitylureido)-2-naphthamido)-3-(neope...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)N[C@@H](COCC(C)(C)C)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C29H35N3O5/c1-17-11-18(2)25(19(3)12-17)32-28(36)31-23-14-21-10-8-7-9-20(21)13-22(23)26(33)30-24(27(34)35)15-37-16-29(4,5)6/h7-14,24H,15-16H2,1-6H3,(H,30,33)(H,34,35)(H2,31,32,36)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence plate reader assay |
Bioorg Med Chem Lett 19: 981-5 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.084
BindingDB Entry DOI: 10.7270/Q2319VRH |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27725

((2S)-2-[(3-{[(2-chloro-6-methylphenyl)carbamoyl]am...)Show SMILES Cc1cccc(Cl)c1NC(=O)Nc1cc2ccccc2cc1C(=O)N[C@@H](C1CCCCC1)C(O)=O |r| Show InChI InChI=1S/C27H28ClN3O4/c1-16-8-7-13-21(28)23(16)31-27(35)29-22-15-19-12-6-5-11-18(19)14-20(22)25(32)30-24(26(33)34)17-9-3-2-4-10-17/h5-8,11-15,17,24H,2-4,9-10H2,1H3,(H,30,32)(H,33,34)(H2,29,31,35)/t24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human glycogen phosphorylase alpha |
Bioorg Med Chem Lett 18: 4068-71 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.102
BindingDB Entry DOI: 10.7270/Q23R0SPM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM27738
((2S)-2-cyclohexyl-2-[(2-{[(4-ethyl-2,6-dimethylphe...)Show SMILES CCc1cc(C)c(NC(=O)Nc2cc(F)ccc2C(=O)N[C@@H](C2CCCCC2)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C26H32FN3O4/c1-4-17-12-15(2)22(16(3)13-17)30-26(34)28-21-14-19(27)10-11-20(21)24(31)29-23(25(32)33)18-8-6-5-7-9-18/h10-14,18,23H,4-9H2,1-3H3,(H,29,31)(H,32,33)(H2,28,30,34)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | 7.6 | 25 |
GSK
| Assay Description
An enzymatic assay was developed to measure the response of the activated form of glycogen phosphorylase to small molecule compounds. The assay was t... |
Bioorg Med Chem Lett 19: 1177-82 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.085
BindingDB Entry DOI: 10.7270/Q22805ZM |
More data for this
Ligand-Target Pair | |
Glycogen phosphorylase, liver form
(Homo sapiens (Human)) | BDBM50256392
(2-(3-(3-mesitylureido)-2-naphthamido)-2,3-dihydro-...)Show SMILES Cc1cc(C)c(NC(=O)Nc2cc3ccccc3cc2C(=O)NC2(Cc3ccccc3C2)C(O)=O)c(C)c1 Show InChI InChI=1S/C31H29N3O4/c1-18-12-19(2)27(20(3)13-18)33-30(38)32-26-15-22-9-5-4-8-21(22)14-25(26)28(35)34-31(29(36)37)16-23-10-6-7-11-24(23)17-31/h4-15H,16-17H2,1-3H3,(H,34,35)(H,36,37)(H2,32,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose |
Bioorg Med Chem Lett 19: 976-80 (2009)
Article DOI: 10.1016/j.bmcl.2008.11.085
BindingDB Entry DOI: 10.7270/Q26T0MGG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data