

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (GUINEA PIG) | BDBM50034711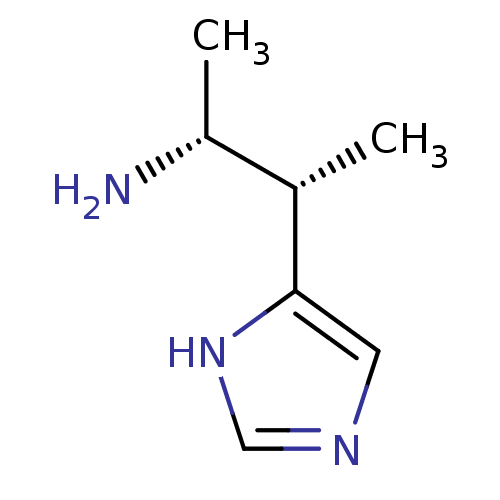 ((1R,2S)-2-(1H-Imidazol-4-yl)-1-methyl-propylamine ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50034708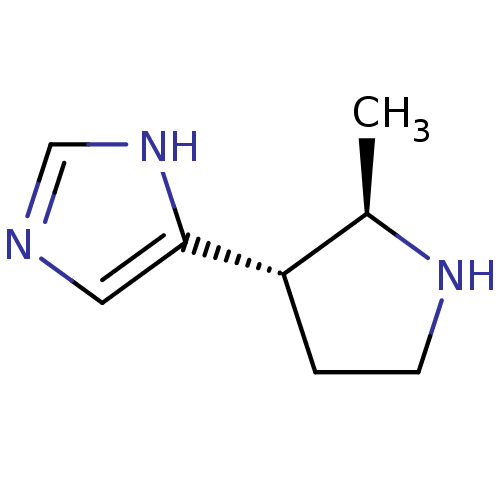 (4-((2R,3S)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50034708 (4-((2R,3S)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50034706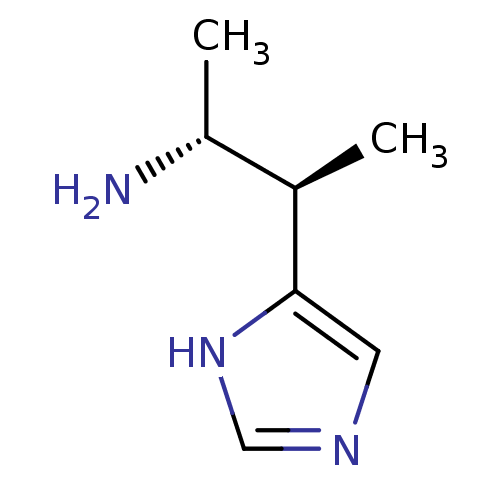 ((1R,2R)-2-(1H-Imidazol-4-yl)-1-methyl-propylamine ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50034707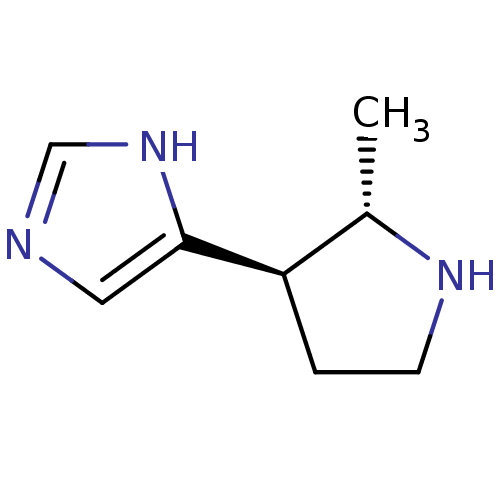 (4-((2S,3R)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50034709 ((1S,2S)-2-(1H-Imidazol-4-yl)-cyclopentylamine | CH...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50568221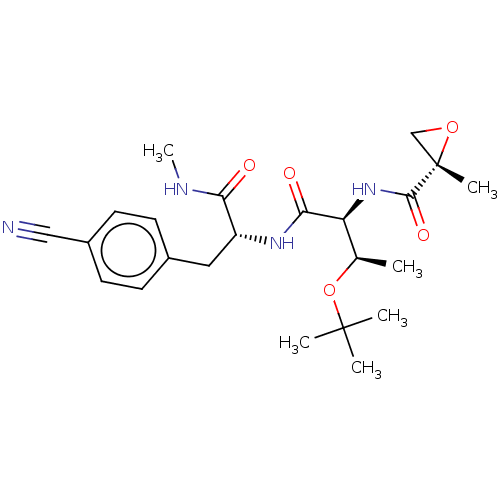 (CHEMBL4854947) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His-tagged BTK expressed in baculovirus infected in Sf9 cells assessed as inhibitory constant incubated fo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116223 BindingDB Entry DOI: 10.7270/Q2M04964 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50568222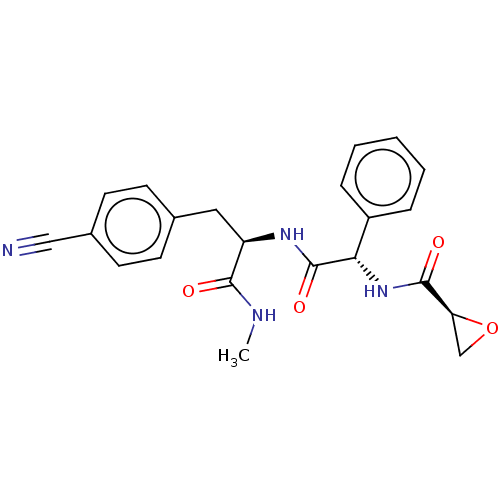 (CHEMBL4867490) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His-tagged BTK expressed in baculovirus infected in Sf9 cells assessed as inhibitory constant incubated fo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116223 BindingDB Entry DOI: 10.7270/Q2M04964 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50034710 ((1R,2S)-2-(1H-Imidazol-4-yl)-1-methyl-cyclohexylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (GUINEA PIG) | BDBM50034713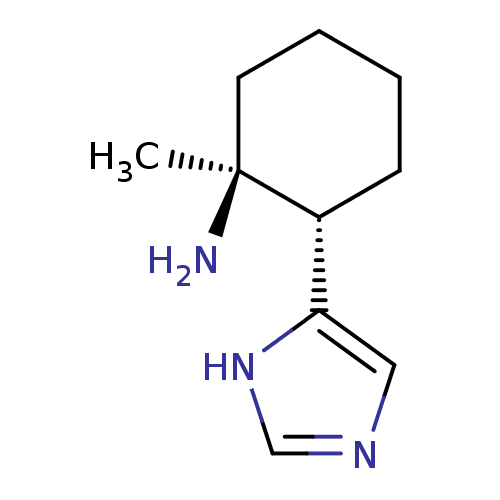 ((1S,2S)-2-(1H-Imidazol-4-yl)-1-methyl-cyclohexylam...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against Histamine H3 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50568220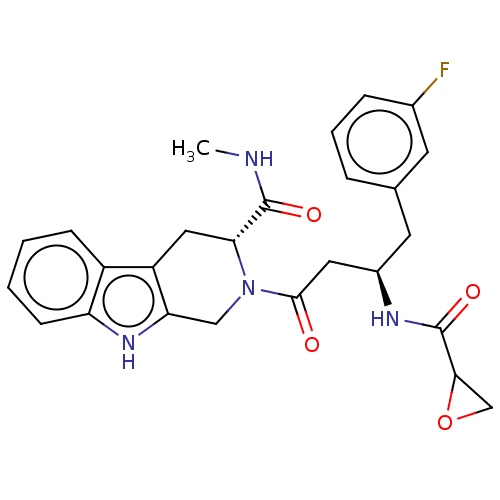 (CHEMBL4873655) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His-tagged BTK expressed in baculovirus infected in Sf9 cells assessed as inhibitory constant incubated fo... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116223 BindingDB Entry DOI: 10.7270/Q2M04964 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50034707 (4-((2S,3R)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against H1 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM22904 ((2R)-1-(1H-imidazol-5-yl)propan-2-amine | (R)-alph...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against H1 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Cavia porcellus (domestic guinea pig)) | BDBM50034708 (4-((2R,3S)-2-Methyl-pyrrolidin-3-yl)-1H-imidazole ...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding afinity against H1 receptor | J Med Chem 38: 1593-9 (1995) BindingDB Entry DOI: 10.7270/Q2BK1BC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM225238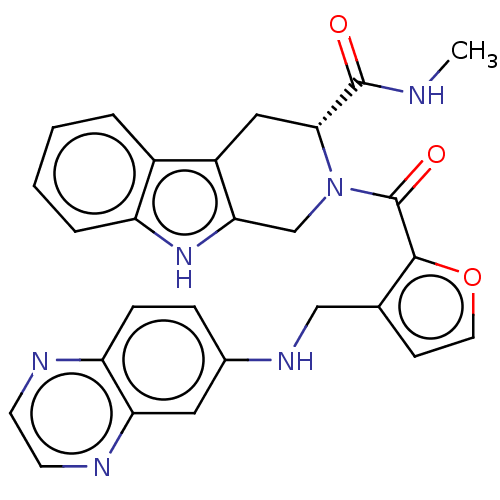 (BTK inhibitor, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant wild type human N-terminal His6-tagged BTK expressed in baculovirus incubated for 20 mins by TR-FRET based competitive bind... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116223 BindingDB Entry DOI: 10.7270/Q2M04964 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM225238 (BTK inhibitor, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | 30 |
X-Chem Pharmaceuticals | Assay Description The BTK time-resolved FRET-based competitive binding assay and cell-based BTK assays have been previously described.[Xu et al., J.Pharmacol. Exp. The... | Chembiochem 18: 864-871 (2017) Article DOI: 10.1002/cbic.201600573 BindingDB Entry DOI: 10.7270/Q22J69Q5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566635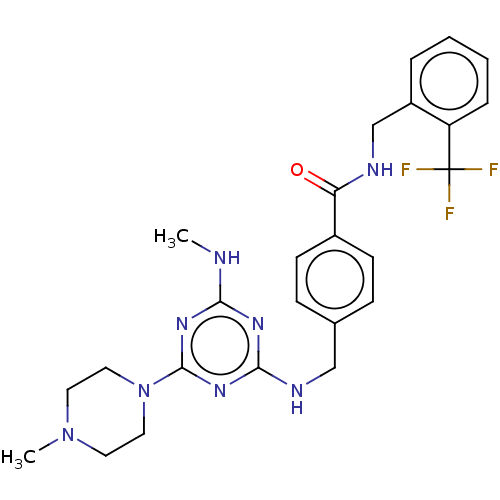 (CHEMBL4875337) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50563635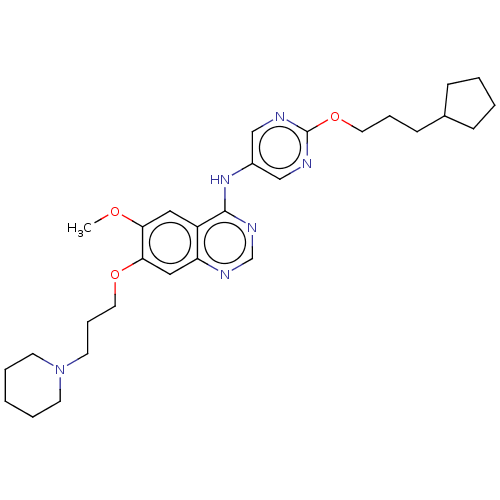 (CHEMBL4777640) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 6His/thrombin cleavage site-fused Avi-tagged dephosphorylated MER (unknown origin) (R528 to M999 residues) using Axltide (CKKSRGDYMTMQJ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01904 BindingDB Entry DOI: 10.7270/Q2XP78NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of MMP-13 using 5-FAM-TPGPLGL[Dap- (DNP)]ARRK(5-TAMRA)-amide as substrate after 45 mins | J Med Chem 55: 7061-79 (2012) Article DOI: 10.1021/jm300449x BindingDB Entry DOI: 10.7270/Q2RX9D6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50559893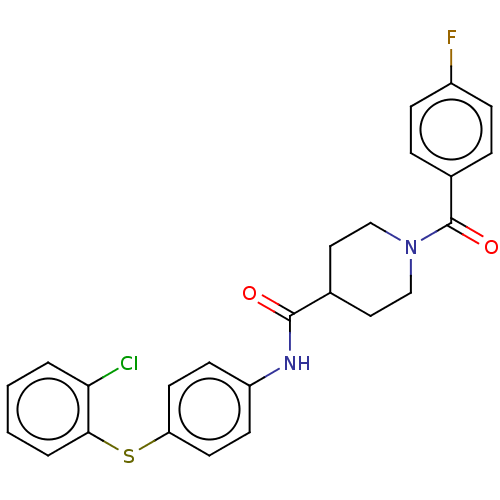 (CHEMBL4749356) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged human recombinant soluble epoxide hydrolase pre-incubated for 15 mins before Epoxy-fluor7 substrate addition and measured af... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00452 BindingDB Entry DOI: 10.7270/Q26H4N4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566638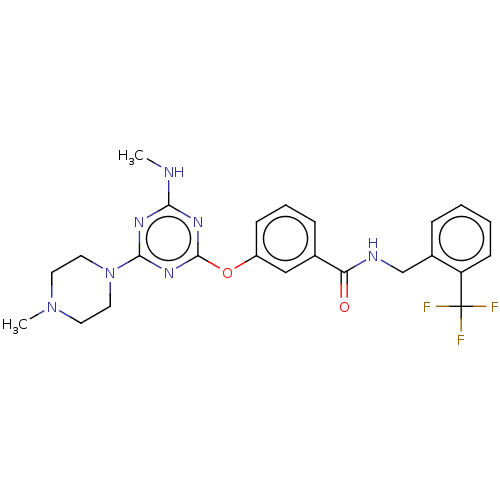 (CHEMBL4862566) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50172077 (CHEMBL3810063) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of 6His/thrombin cleavage site-fused Avi-tagged dephosphorylated MER (unknown origin) (R528 to M999 residues) using Axltide (CKKSRGDYMTMQJ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01904 BindingDB Entry DOI: 10.7270/Q2XP78NC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of TACE using Mca-PLAQAV-Dpa-RSSSR-NH2 as substrate preincubated 15 mins measured every 30 sec for 30 mins | J Med Chem 55: 7061-79 (2012) Article DOI: 10.1021/jm300449x BindingDB Entry DOI: 10.7270/Q2RX9D6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50540028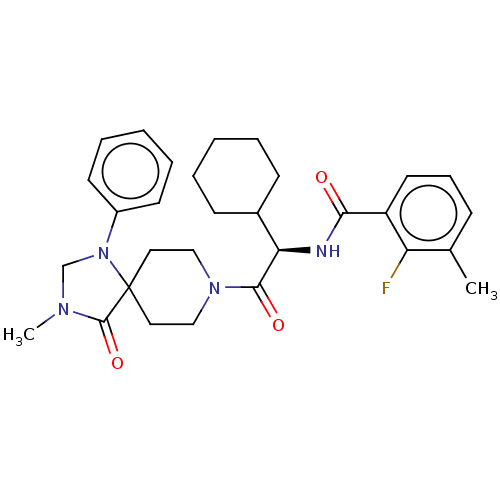 (CHEMBL4635769) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc. Curated by ChEMBL | Assay Description Inhibition of C-terminal FLAG-tagged human autotaxin expressed in Freestyle 293 cells using synthetic substrate FS-3 by fluorescence based assay | J Med Chem 63: 7840-7856 (2020) Article DOI: 10.1021/acs.jmedchem.0c00688 BindingDB Entry DOI: 10.7270/Q28D00T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50574409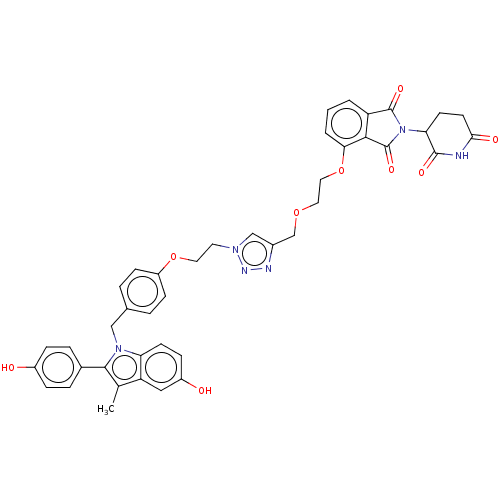 (CHEMBL4873534) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to recombinant His-tagged ERalpha LBD (307 to 554 residue) (unknown origin) expressed in Escherichia coli preincubated for 15 mins f... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00127 BindingDB Entry DOI: 10.7270/Q26T0RG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448867 (CHEMBL3125235) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448871 (CHEMBL3125101) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50540025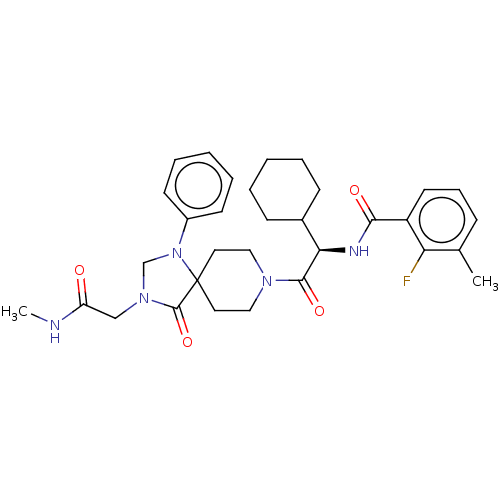 (CHEMBL4639517) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc. Curated by ChEMBL | Assay Description Inhibition of C-terminal FLAG-tagged human autotaxin expressed in Freestyle 293 cells using synthetic substrate FS-3 by fluorescence based assay | J Med Chem 63: 7840-7856 (2020) Article DOI: 10.1021/acs.jmedchem.0c00688 BindingDB Entry DOI: 10.7270/Q28D00T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50540030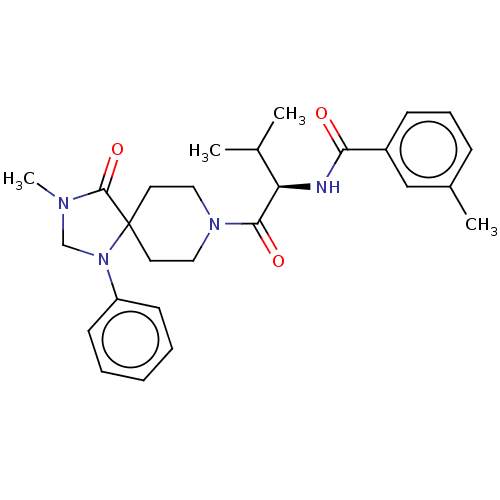 (CHEMBL4633392) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc. Curated by ChEMBL | Assay Description Inhibition of C-terminal FLAG-tagged human autotaxin expressed in Freestyle 293 cells using synthetic substrate FS-3 by fluorescence based assay | J Med Chem 63: 7840-7856 (2020) Article DOI: 10.1021/acs.jmedchem.0c00688 BindingDB Entry DOI: 10.7270/Q28D00T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36463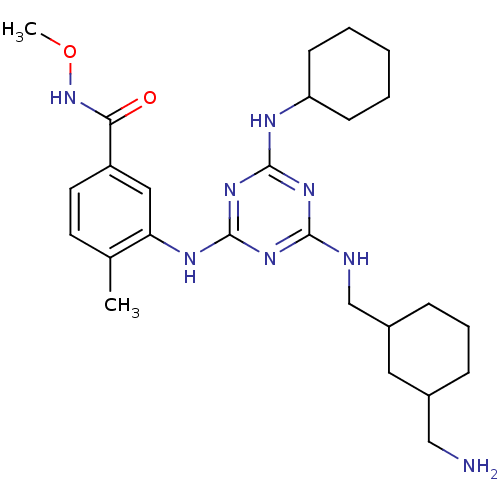 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50559894 (CHEMBL4793243) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His-tagged human recombinant soluble epoxide hydrolase pre-incubated for 15 mins before Epoxy-fluor7 substrate addition and measured af... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c00452 BindingDB Entry DOI: 10.7270/Q26H4N4T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50091691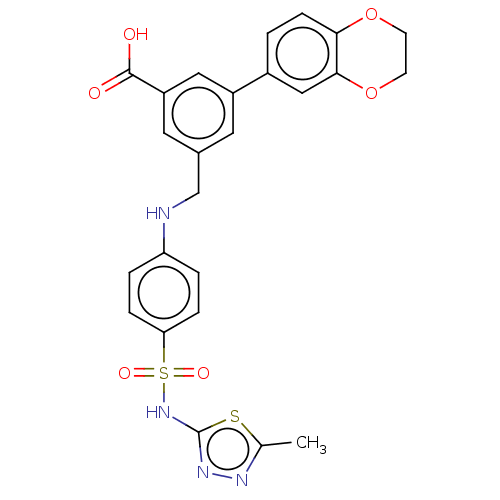 (CHEMBL3582356) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) | ACS Med Chem Lett 6: 531-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00025 BindingDB Entry DOI: 10.7270/Q2BP04JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM36462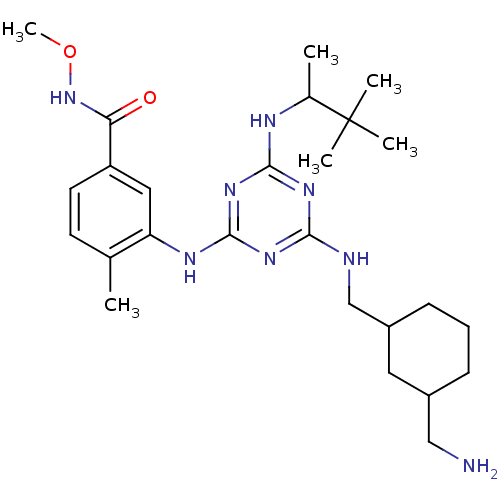 (3-(4-((3-(Aminomethyl)cyclohexyl)methylamino)-6-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <2 | n/a | n/a | n/a | n/a | 9.5 | 16 |
Praecis Pharmaceuticals | Assay Description Selection of DNA-encoded libraries (DELs), which are covalent attachment of encoding double stranded DNA to small-molecule created using a combinatio... | Nat Chem Biol 5: 647-54 (2009) Article DOI: 10.1038/nchembio.211 BindingDB Entry DOI: 10.7270/Q2MP51NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50574409 (CHEMBL4873534) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to ERalpha S463P mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00127 BindingDB Entry DOI: 10.7270/Q26T0RG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50540029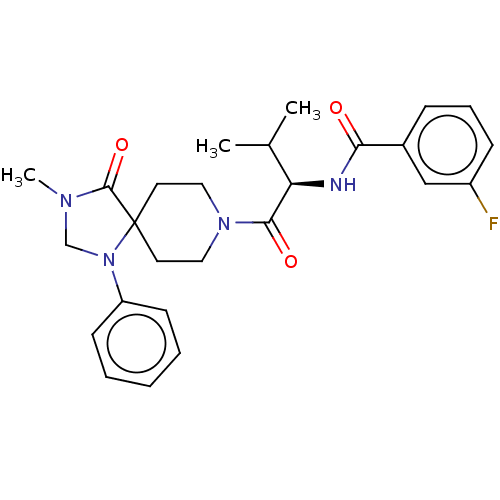 (CHEMBL4648587) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc. Curated by ChEMBL | Assay Description Inhibition of C-terminal FLAG-tagged human autotaxin expressed in Freestyle 293 cells using synthetic substrate FS-3 by fluorescence based assay | J Med Chem 63: 7840-7856 (2020) Article DOI: 10.1021/acs.jmedchem.0c00688 BindingDB Entry DOI: 10.7270/Q28D00T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50091689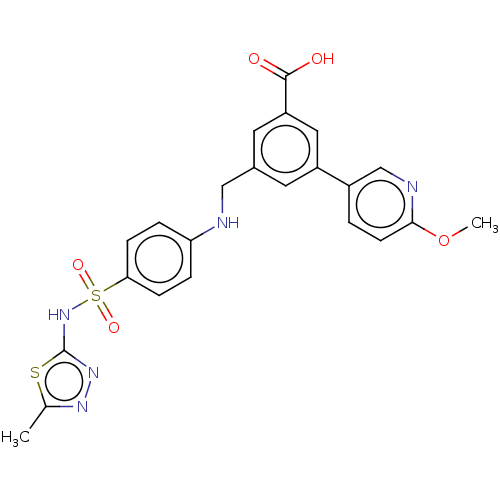 (CHEMBL3582354) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma (unknown origin) | ACS Med Chem Lett 6: 531-6 (2015) Article DOI: 10.1021/acsmedchemlett.5b00025 BindingDB Entry DOI: 10.7270/Q2BP04JF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448888 (CHEMBL3125253) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448868 (CHEMBL3125104) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448875 (CHEMBL3125266) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50539700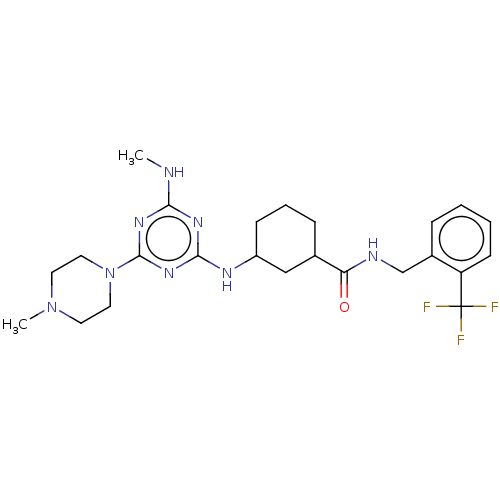 (CHEMBL4637413) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM225238 (BTK inhibitor, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His-tagged BTK C481S mutant expressed in baculovirus infected in Sf9 cells incubated for 1 hr by TR-FRET a... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116223 BindingDB Entry DOI: 10.7270/Q2M04964 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Enoyl-[acyl-carrier-protein] reductase [NADH] (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50448880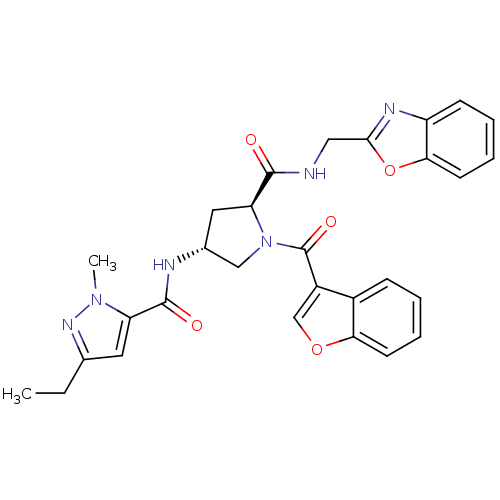 (CHEMBL3125261) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis full length biotinylated InhA (270 amino acids) using DDCoA as substrate assessed as NADH oxidation by fluor... | J Med Chem 57: 1276-88 (2014) Article DOI: 10.1021/jm401326j BindingDB Entry DOI: 10.7270/Q2NP25XB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional epoxide hydrolase 2 (Homo sapiens (Human)) | BDBM50566636 (CHEMBL4870025) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human sEH using EnzChek as substrate preincubated with enzyme for 5 mins followed by substrate addition and measured every 30 secs for ... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116216 BindingDB Entry DOI: 10.7270/Q22J6GNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| A disintegrin and metalloproteinase with thrombospondin motifs 4 (Homo sapiens (Human)) | BDBM50168737 ((2R,3R)-1-[4-(2-Chloro-4-fluoro-benzyloxy)-benzene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of ADAMTS-4 using WAAG-3R as substrate preincubated for 15 mins measured after 1 hr | J Med Chem 55: 7061-79 (2012) Article DOI: 10.1021/jm300449x BindingDB Entry DOI: 10.7270/Q2RX9D6T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50574409 (CHEMBL4873534) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to ERalpha Y537S mutant (unknown origin) expressed in Escherichia coli preincubated for 15 mins followed by ligand addition and meas... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00127 BindingDB Entry DOI: 10.7270/Q26T0RG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase receptor UFO (Homo sapiens (Human)) | BDBM50172077 (CHEMBL3810063) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of GST-tagged AXL (unknown origin) (464 to 485 residues) using Axltide (CKKSRGDYMTMQJG-acid) peptide as substrate preincubated for 30 mins... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01904 BindingDB Entry DOI: 10.7270/Q2XP78NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50563640 (CHEMBL4746916) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of His6/TEV fused-GST-tagged Flt3 (unknown origin) (H564 to S993 residues) using Axltide (CKKSRGDYMTMQJ-acid) peptide as substrate preincu... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01904 BindingDB Entry DOI: 10.7270/Q2XP78NC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50540026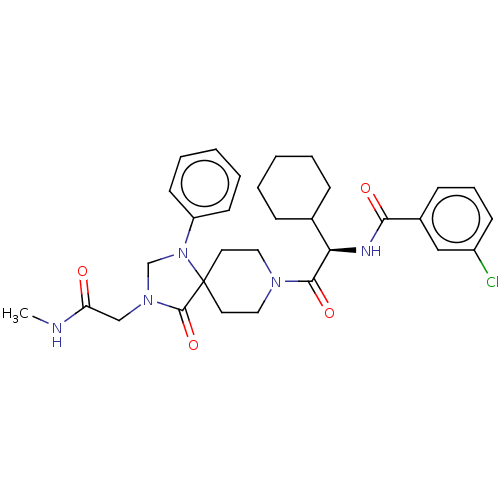 (CHEMBL4645846) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
X-Chem, Inc. Curated by ChEMBL | Assay Description Inhibition of C-terminal FLAG-tagged human autotaxin expressed in Freestyle 293 cells using synthetic substrate FS-3 by fluorescence based assay | J Med Chem 63: 7840-7856 (2020) Article DOI: 10.1021/acs.jmedchem.0c00688 BindingDB Entry DOI: 10.7270/Q28D00T0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM225238 (BTK inhibitor, 3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human N-terminal His-tagged BTK expressed in baculovirus infected in Sf9 cells incubated for 1 hr by TR-FRET assay | Citation and Details Article DOI: 10.1016/j.bmc.2021.116223 BindingDB Entry DOI: 10.7270/Q2M04964 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 590 total ) | Next | Last >> |