

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50417287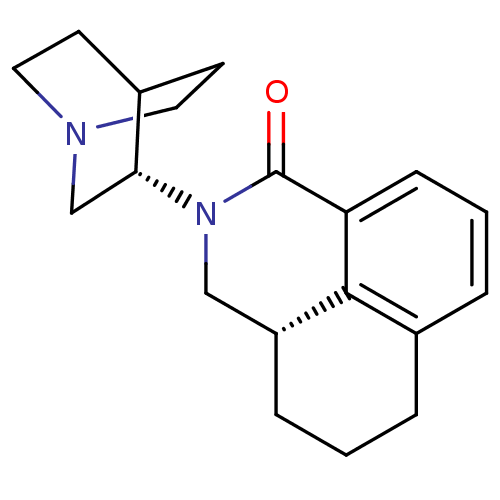 (Aloxi | Aurothioglucose | PALONOSETRON | PALONOSET...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-4/beta-2 (Homo sapiens (Human)) | BDBM50049750 ((S)-3-(azetidin-2-ylmethoxy)pyridine | 3-((S)-1-Az...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Critical Therapeutics, Inc. Curated by ChEMBL | Assay Description Agonist activity at alpha4beta2 nAChR (unknown origin) | J Med Chem 57: 3966-83 (2014) Article DOI: 10.1021/jm5004599 BindingDB Entry DOI: 10.7270/Q2CV4K8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50099273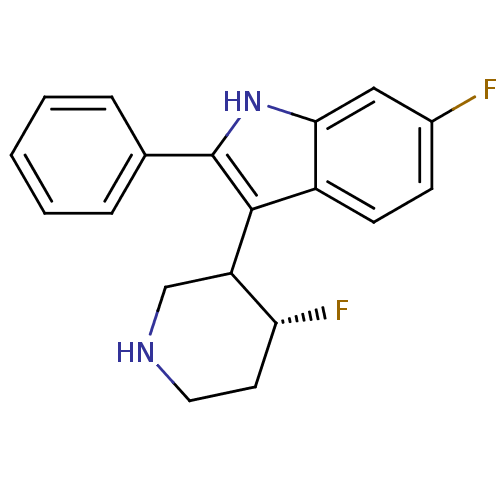 (6-Fluoro-3-(4-fluoro-piperidin-3-yl)-2-phenyl-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50477491 (CHEMBL398034) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 3504-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.093 BindingDB Entry DOI: 10.7270/Q29P34FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50095027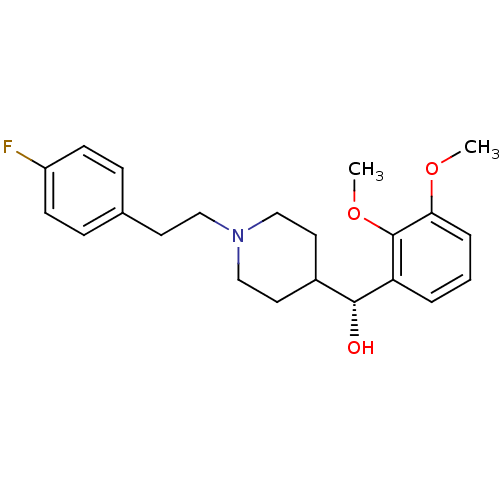 ((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50477488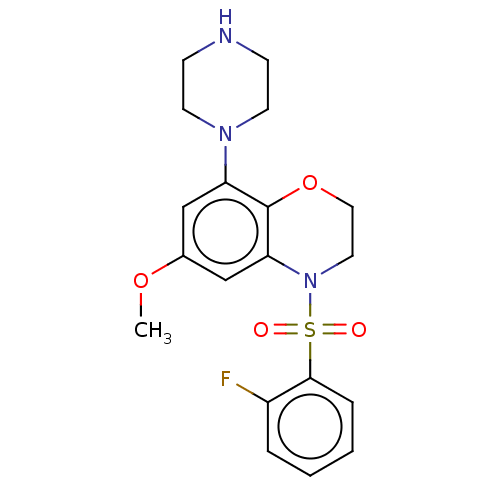 (CHEMBL394690) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 3504-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.093 BindingDB Entry DOI: 10.7270/Q29P34FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000523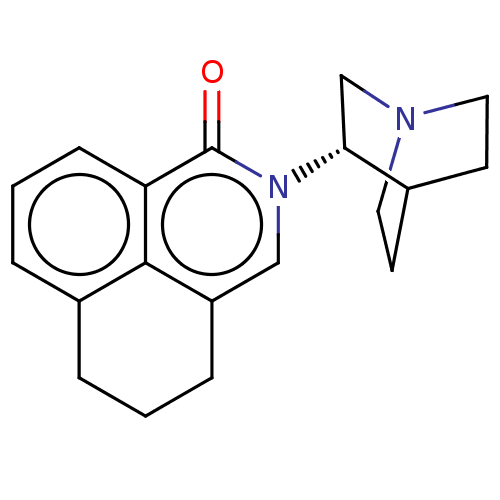 (CHEMBL544784) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000453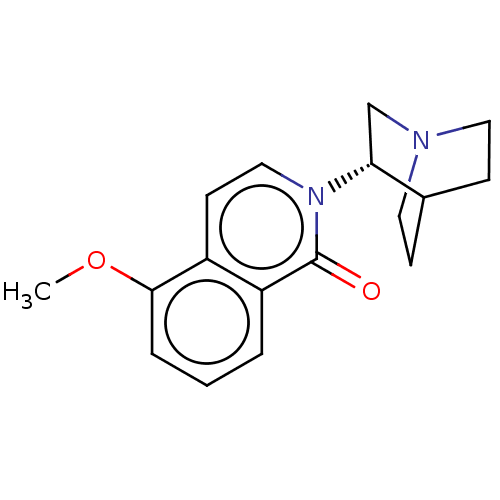 (CHEMBL540055) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108690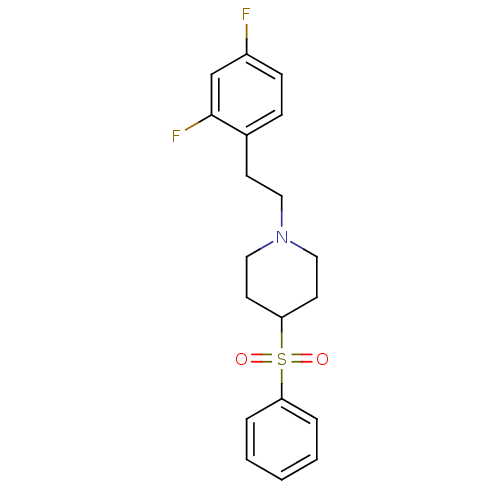 (1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000471 (CHEMBL542900) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000457 (CHEMBL542904) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000450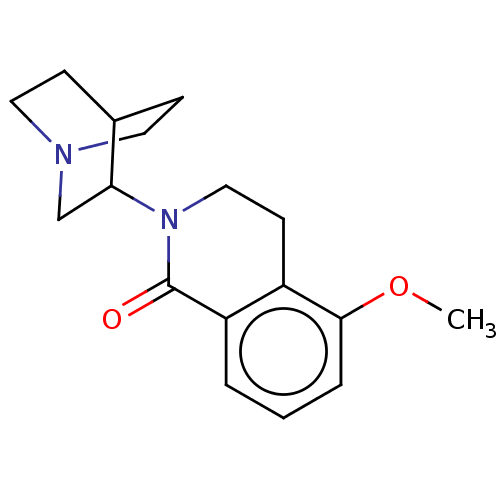 (CHEMBL542669) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108689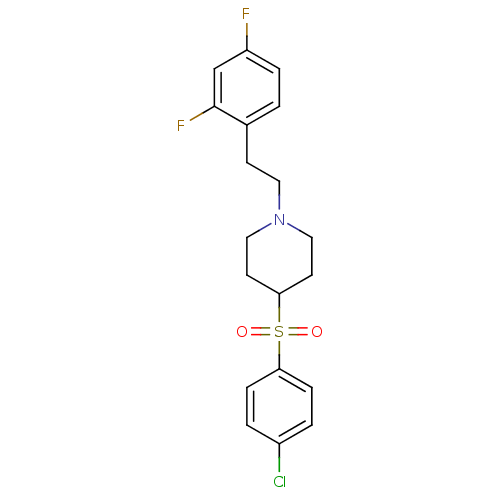 (4-(4-Chloro-benzenesulfonyl)-1-[2-(2,4-difluoro-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50056419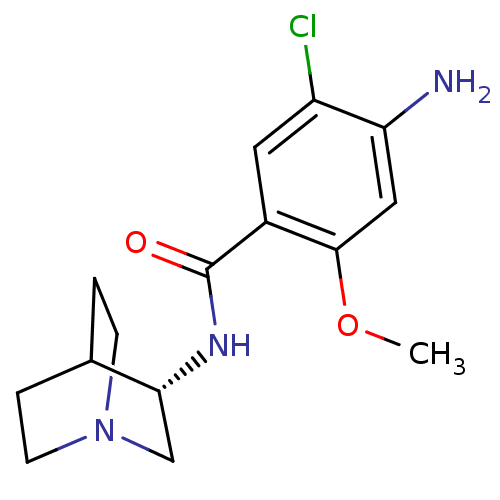 (4-Amino-N-(1-aza-bicyclo[2.2.2]oct-3-yl)-5-chloro-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50547978 (CHEMBL4758147) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of of human recombinant His6-tagged B-Raf V600E mutant expressed in baculovirus infected cells co-expresing CDC37 in presence of [gamma33P... | Citation and Details Article DOI: 10.1021/acsmedchemlett.0c00344 BindingDB Entry DOI: 10.7270/Q24Q7ZMD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235631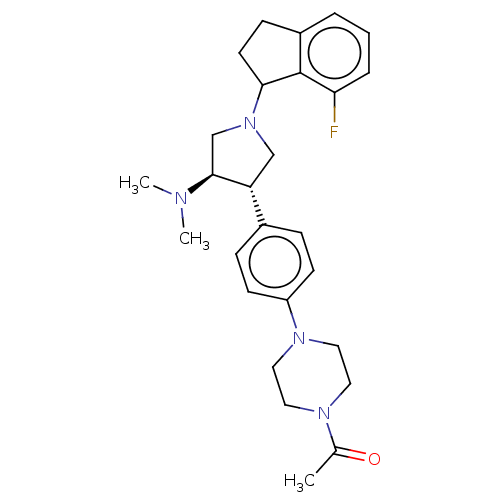 (CHEMBL4060827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50372966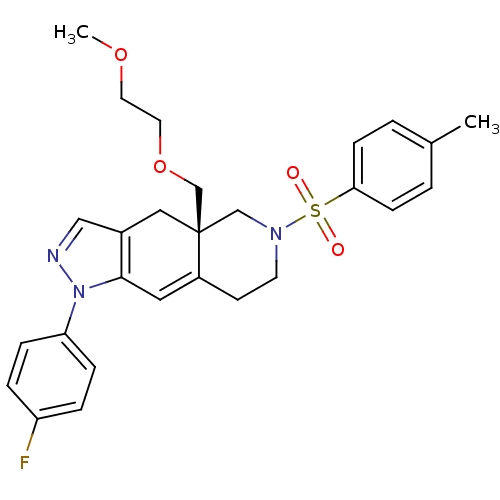 (CHEMBL270668) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant GR | Bioorg Med Chem Lett 18: 1312-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.027 BindingDB Entry DOI: 10.7270/Q22Z16CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235630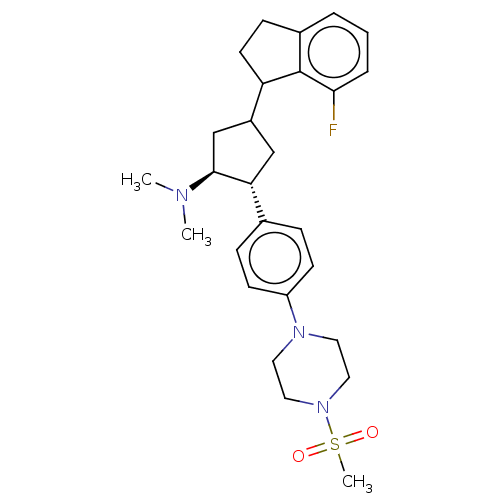 (CHEMBL4093096) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description Inhibition of OG(488) labeled probe binding to GST-tagged EED (unknown origin) after 1 hr by LanthaScreen TR-FRET assay | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50095027 ((2,3-Dimethoxy-phenyl)-{1-[2-(4-fluoro-phenyl)-eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Mus musculus (Mouse)) | BDBM50002184 (CHEMBL415330 | H-Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-A...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-diprenorphine from mouse KOR expressed in HEK293 cell membranes by radioligand binding assay relative to control | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00158 BindingDB Entry DOI: 10.7270/Q21G0R4N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM82505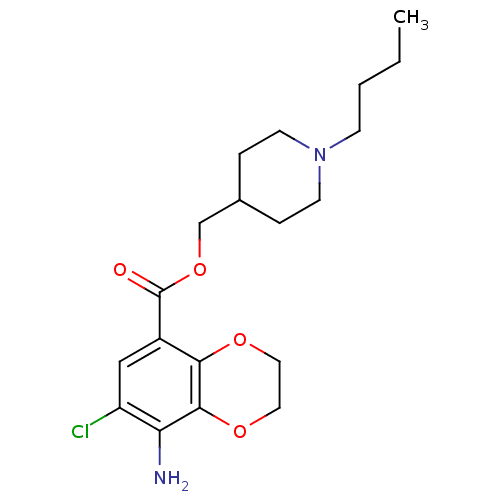 (CAS_121881 | NSC_121881 | SB204070) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by PDSP Ki Database | Neuropharmacology 36: 671-9 (1997) Article DOI: 10.1016/s0028-3908(97)00039-7 BindingDB Entry DOI: 10.7270/Q2RN36D0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50477473 (CHEMBL246918) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 3504-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.093 BindingDB Entry DOI: 10.7270/Q29P34FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000475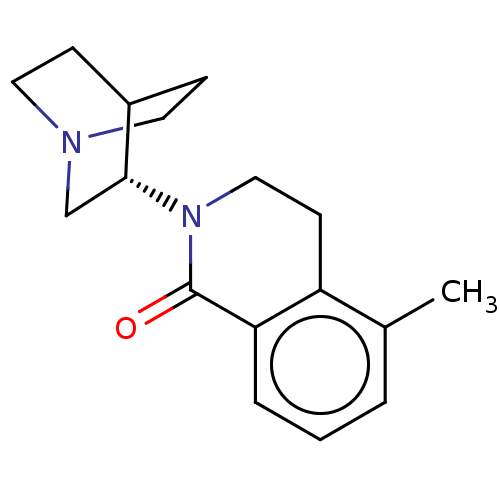 (CHEMBL555068) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000460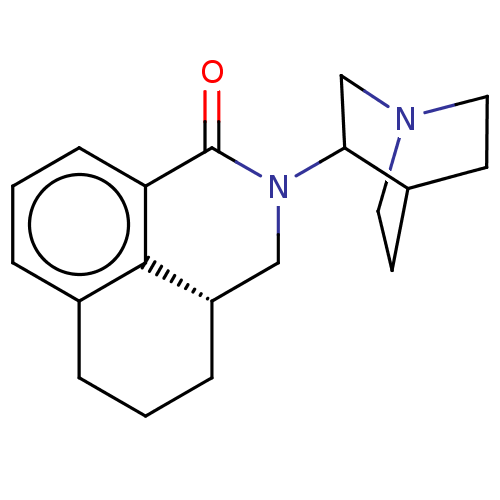 (CHEMBL540057) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108690 (1-(2,4-difluorophenethyl)-4-(phenylsulfonyl)piperi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228318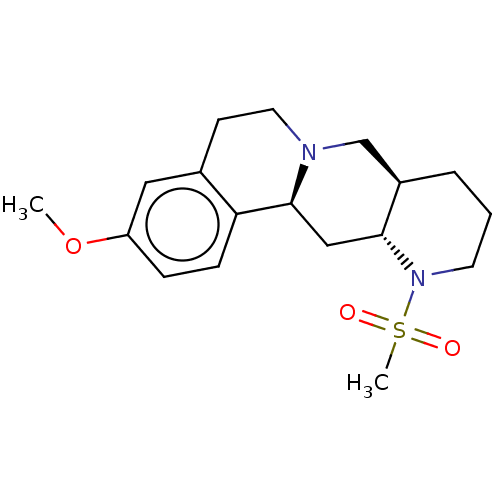 (CHEMBL279807) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 32: 2034-6 (1989) BindingDB Entry DOI: 10.7270/Q2WH2S78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50228318 (CHEMBL279807) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane | J Med Chem 34: 705-17 (1991) BindingDB Entry DOI: 10.7270/Q2MW2KBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108688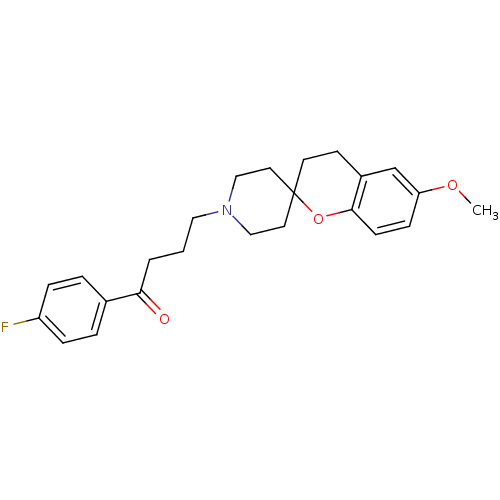 (1-(4-fluorophenyl)-4-[6-methoxyspiro[3,4-dihydro-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108688 (1-(4-fluorophenyl)-4-[6-methoxyspiro[3,4-dihydro-2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (RAT) | BDBM82505 (CAS_121881 | NSC_121881 | SB204070) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Bioscience Curated by PDSP Ki Database | Neuropharmacology 36: 671-9 (1997) Article DOI: 10.1016/s0028-3908(97)00039-7 BindingDB Entry DOI: 10.7270/Q2RN36D0 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50418229 (CHEMBL1760646) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]SR141716A from human CB1 receptor expressed in CHO cells | Bioorg Med Chem Lett 21: 2034-9 (2011) Article DOI: 10.1016/j.bmcl.2011.02.019 BindingDB Entry DOI: 10.7270/Q27082PM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50477479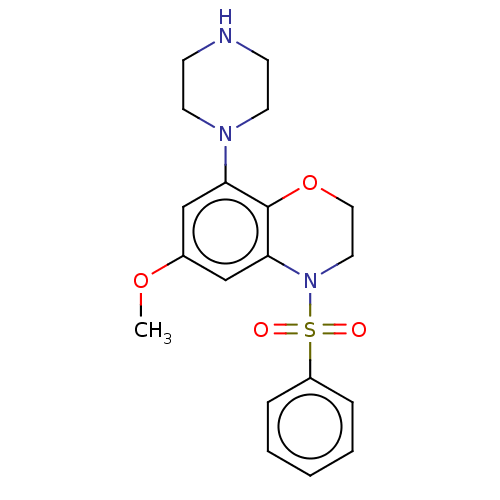 (CHEMBL246706) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 3504-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.093 BindingDB Entry DOI: 10.7270/Q29P34FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50477475 (CHEMBL246499) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 3504-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.093 BindingDB Entry DOI: 10.7270/Q29P34FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50477489 (CHEMBL246705) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 3504-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.093 BindingDB Entry DOI: 10.7270/Q29P34FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50036756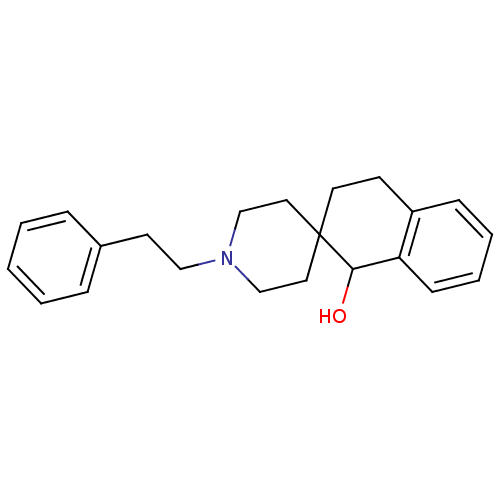 (1'-phenethylspiro[1,2,3,4-tetrahydronaphthalene-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50319623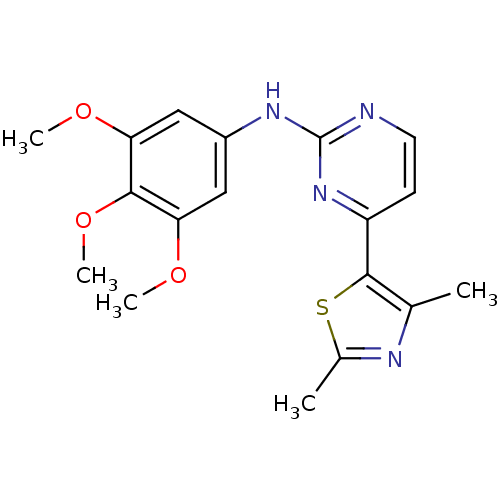 (4-(2,4-Dimethylthiazol-5-yl)-N-(3,4,5-trimethoxyph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cyclacel Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant Aurora A after 30 mins | J Med Chem 53: 4367-78 (2010) Article DOI: 10.1021/jm901913s BindingDB Entry DOI: 10.7270/Q2930T9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000469 (CHEMBL542903) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50000455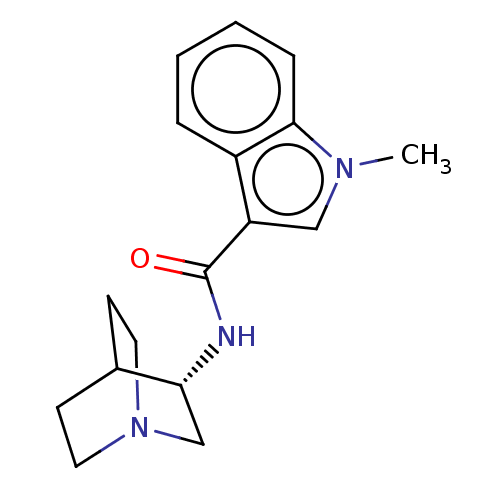 (CHEMBL2093898) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity against 5-hydroxytryptamine 3 (5-HT3) receptor in rat brain cortical membranes using radioligand [3H]quipazine | J Med Chem 36: 2645-57 (1993) Checked by Author BindingDB Entry DOI: 10.7270/Q2GM88T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human glucocorticoid receptor expressed in recombinant baculovirus | Bioorg Med Chem Lett 17: 5704-8 (2007) Article DOI: 10.1016/j.bmcl.2007.07.055 BindingDB Entry DOI: 10.7270/Q2FX7B8B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant GR | Bioorg Med Chem Lett 18: 1312-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.027 BindingDB Entry DOI: 10.7270/Q22Z16CP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108701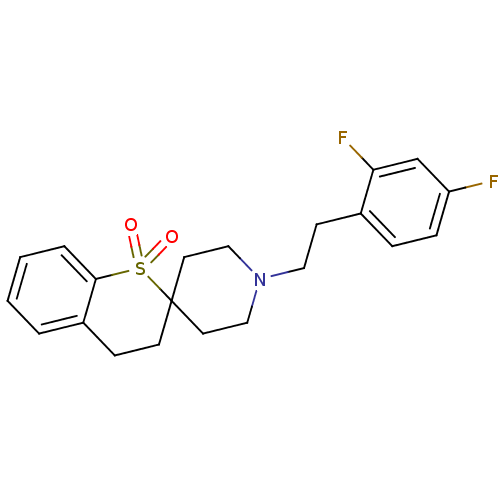 (1'-[2-(2,4-Difluorophenyl)ethyl]-3,4-dihydrospiro[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108699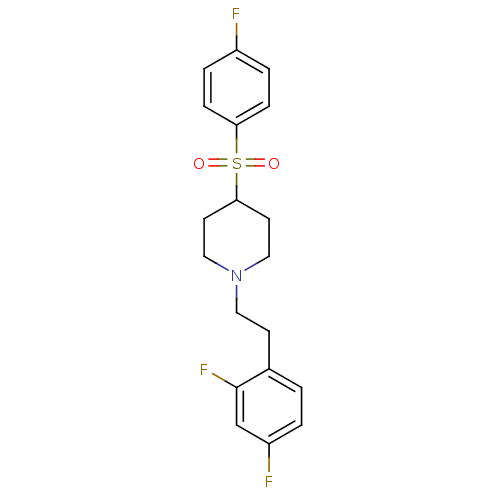 (1-(2,4-difluorophenethyl)-4-(4-fluorophenylsulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polycomb protein EED (Homo sapiens (Human)) | BDBM50235643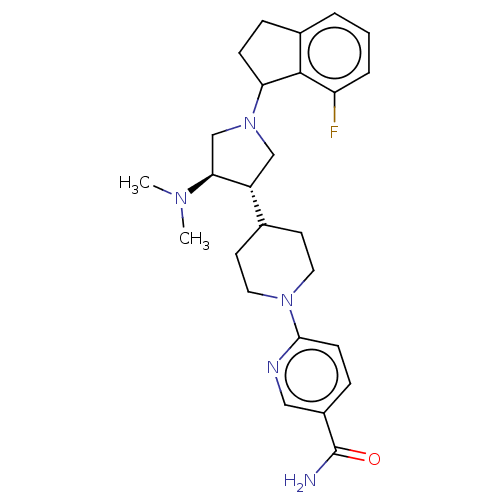 (CHEMBL4076017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. Curated by ChEMBL | Assay Description In vitro displacement of [3H]-LY 278584 from rat cerebral cortex 5-hydroxytryptamine 3 receptor | Bioorg Med Chem Lett 27: 1576-1583 (2017) Article DOI: 10.1016/j.bmcl.2017.02.030 BindingDB Entry DOI: 10.7270/Q22F7QQG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465]/Alpha-2B adrenergic receptor/Alpha-2C adrenergic receptor (RAT-NEONATAL RAT-Rattus norvegicus (rat)) | BDBM50229080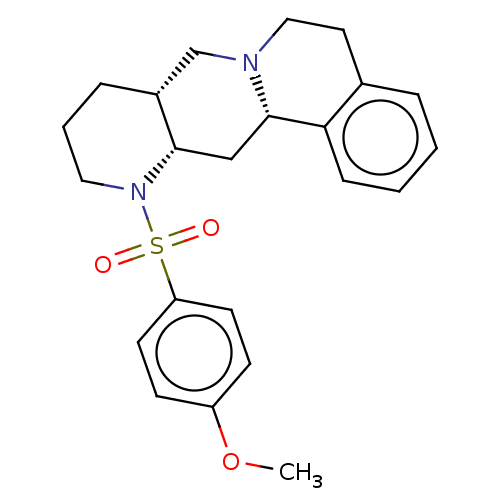 (CHEMBL349665) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.457 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Syntex Research Curated by ChEMBL | Assay Description Binding affinity to alpha-2 adrenergic receptor determined by measurement of [3H]yohimbine displacement from rat cortical membrane | J Med Chem 34: 705-17 (1991) BindingDB Entry DOI: 10.7270/Q2MW2KBF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 2 (Homo sapiens (Human)) | BDBM217096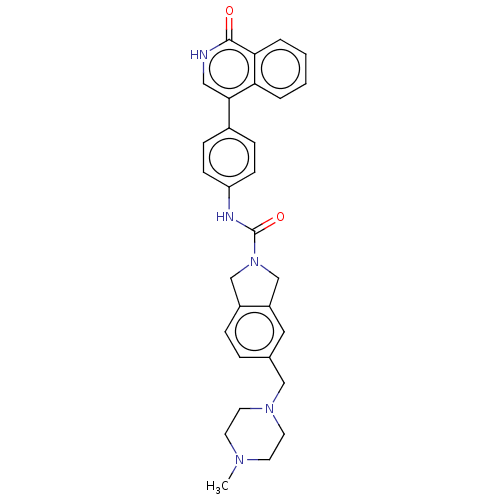 (US9302989, 391) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description HTRF assay: This assay used the CisBio HTRF KinEASE kit (kit 62ST2PEZ) and the kinase reaction containing 0.2 uM biotinylated substrate peptide (S2, ... | US Patent US9302989 (2016) BindingDB Entry DOI: 10.7270/Q2HH6HX1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50372975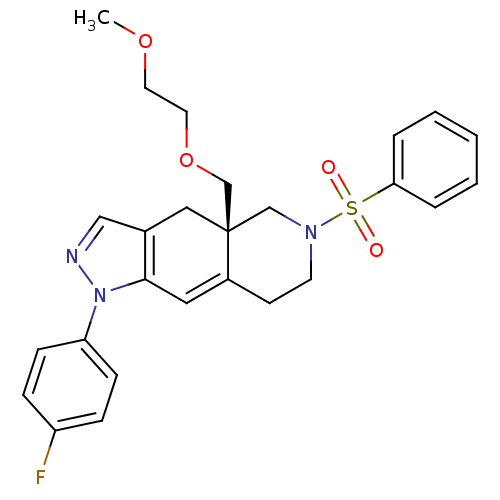 (CHEMBL270667) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant GR | Bioorg Med Chem Lett 18: 1312-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.027 BindingDB Entry DOI: 10.7270/Q22Z16CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50372977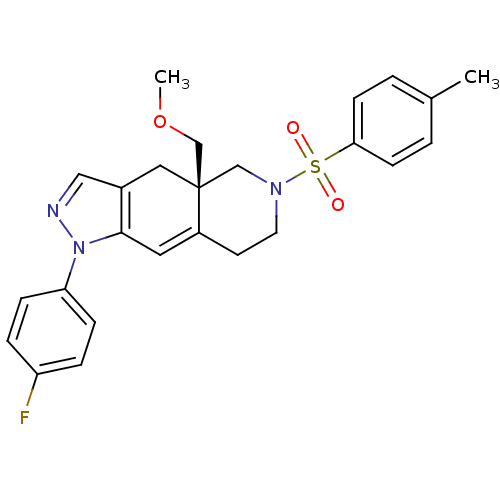 (CHEMBL255369) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics Curated by ChEMBL | Assay Description Displacement of [3H]dexamethasone from human recombinant GR | Bioorg Med Chem Lett 18: 1312-7 (2008) Article DOI: 10.1016/j.bmcl.2008.01.027 BindingDB Entry DOI: 10.7270/Q22Z16CP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50108696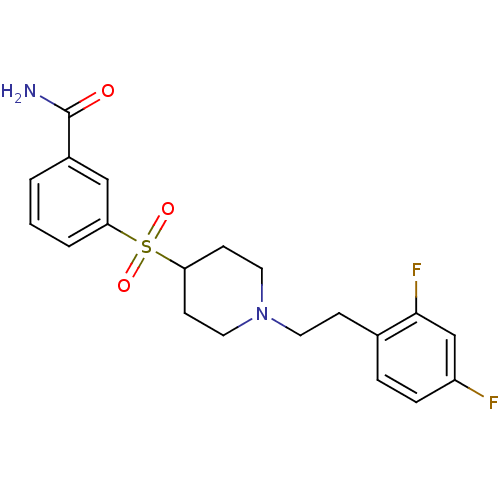 (3-[[1-(2-(2,4-Difluorophenyl)ethyl)-4-piperidinyl]...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to human 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50477477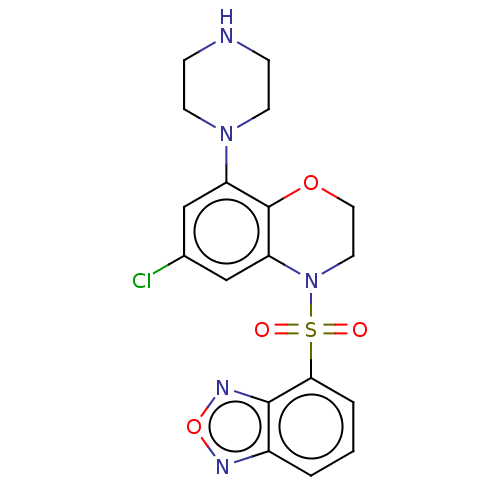 (CHEMBL246291) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human recombinant 5HT6 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 3504-7 (2007) Article DOI: 10.1016/j.bmcl.2006.12.093 BindingDB Entry DOI: 10.7270/Q29P34FX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50108705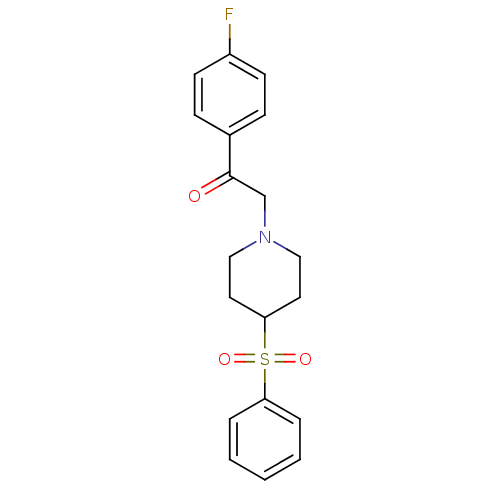 (1-(4-Fluorophenyl)-2-[4-(phenylsulfonyl)-1-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp and Dohme Curated by ChEMBL | Assay Description Binding affinity for displacement of [3H]ketanserin to rat 5-hydroxytryptamine 2A receptor stably expressed in CHO cells | J Med Chem 45: 492-503 (2002) BindingDB Entry DOI: 10.7270/Q2KH0P27 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5662 total ) | Next | Last >> |