 Found 1637 hits with Last Name = 'coleman' and Initial = 'j'
Found 1637 hits with Last Name = 'coleman' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500526
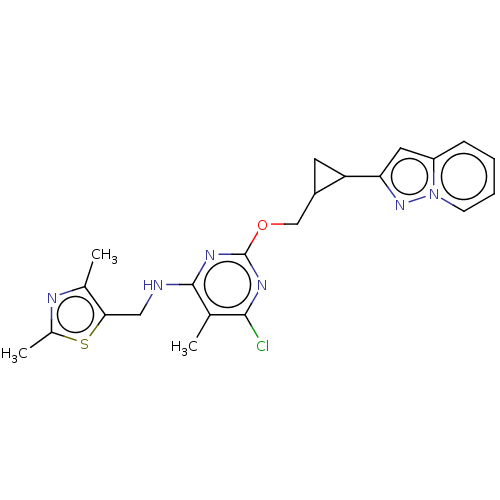
(CHEMBL3747517)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc4ccccn4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C22H23ClN6OS/c1-12-20(23)26-22(27-21(12)24-10-19-13(2)25-14(3)31-19)30-11-15-8-17(15)18-9-16-6-4-5-7-29(16)28-18/h4-7,9,15,17H,8,10-11H2,1-3H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126826
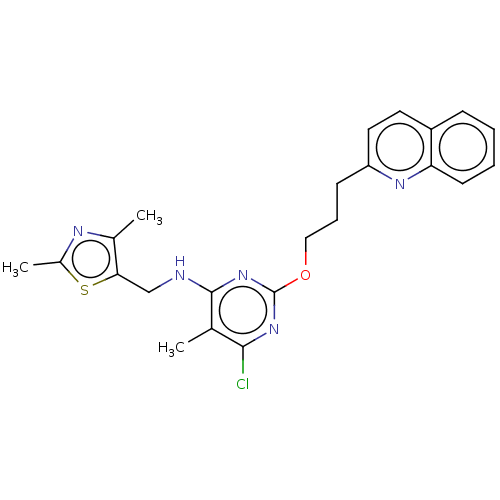
(US8785467, 1-29)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C23H24ClN5OS/c1-14-21(24)28-23(29-22(14)25-13-20-15(2)26-16(3)31-20)30-12-6-8-18-11-10-17-7-4-5-9-19(17)27-18/h4-5,7,9-11H,6,8,12-13H2,1-3H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.00820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500520

(CHEMBL3746993)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C24H24ClN5OS/c1-13-22(25)29-24(30-23(13)26-11-21-14(2)27-15(3)32-21)31-12-17-10-18(17)20-9-8-16-6-4-5-7-19(16)28-20/h4-9,17-18H,10-12H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500538

(CHEMBL3745790)Show SMILES COc1ccnc(c1)C1CC1COc1nc(Cl)c(C)c(NCc2sc(C)nc2C)n1 Show InChI InChI=1S/C21H24ClN5O2S/c1-11-19(22)26-21(27-20(11)24-9-18-12(2)25-13(3)30-18)29-10-14-7-16(14)17-8-15(28-4)5-6-23-17/h5-6,8,14,16H,7,9-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500521

(CHEMBL3746277)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cc(C)ccn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H24ClN5OS/c1-11-5-6-23-17(7-11)16-8-15(16)10-28-21-26-19(22)12(2)20(27-21)24-9-18-13(3)25-14(4)29-18/h5-7,15-16H,8-10H2,1-4H3,(H,24,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500519
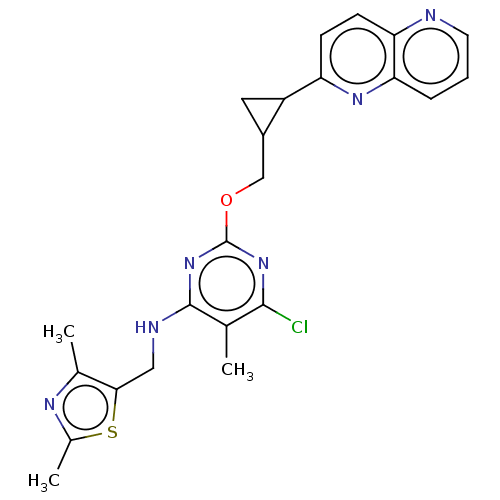
(CHEMBL3746917)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3ccc4ncccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C23H23ClN6OS/c1-12-21(24)29-23(30-22(12)26-10-20-13(2)27-14(3)32-20)31-11-15-9-16(15)17-6-7-18-19(28-17)5-4-8-25-18/h4-8,15-16H,9-11H2,1-3H3,(H,26,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126825
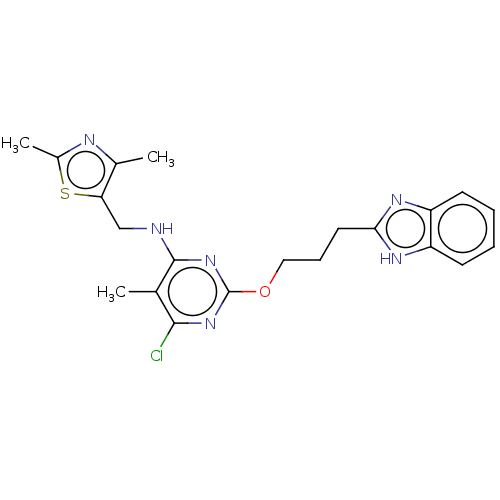
(US8785467, 1-27)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3nc4ccccc4[nH]3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H23ClN6OS/c1-12-19(22)27-21(28-20(12)23-11-17-13(2)24-14(3)30-17)29-10-6-9-18-25-15-7-4-5-8-16(15)26-18/h4-5,7-8H,6,9-11H2,1-3H3,(H,25,26)(H,23,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50500535

(CHEMBL3747450)Show SMILES Cc1nc(C)c(CNc2nc(OCC3CC3c3cn(C)cn3)nc(Cl)c2C)s1 Show InChI InChI=1S/C19H23ClN6OS/c1-10-17(20)24-19(25-18(10)21-6-16-11(2)23-12(3)28-16)27-8-13-5-14(13)15-7-26(4)9-22-15/h7,9,13-14H,5-6,8H2,1-4H3,(H,21,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50125967

(CHEMBL3627846)Show SMILES Cc1ccc2c(c1)nc(CCN1C(=O)c3ccccc3C1=O)n(-c1ccc3n(C)ncc3c1)c2=O Show InChI InChI=1S/C27H21N5O3/c1-16-7-9-21-22(13-16)29-24(11-12-31-25(33)19-5-3-4-6-20(19)26(31)34)32(27(21)35)18-8-10-23-17(14-18)15-28-30(23)2/h3-10,13-15H,11-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.0250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126823
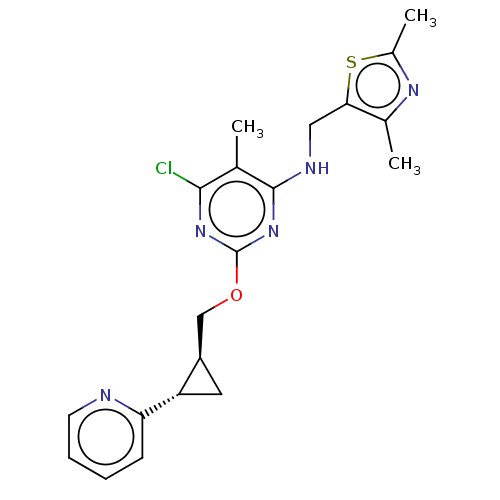
(US8785467, 1-20)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C20H22ClN5OS/c1-11-18(21)25-20(26-19(11)23-9-17-12(2)24-13(3)28-17)27-10-14-8-15(14)16-6-4-5-7-22-16/h4-7,14-15H,8-10H2,1-3H3,(H,23,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126823

(US8785467, 1-20)Show SMILES Cc1nc(C)c(CNc2nc(OC[C@H]3C[C@@H]3c3ccccn3)nc(Cl)c2C)s1 |r| Show InChI InChI=1S/C20H22ClN5OS/c1-11-18(21)25-20(26-19(11)23-9-17-12(2)24-13(3)28-17)27-10-14-8-15(14)16-6-4-5-7-22-16/h4-7,14-15H,8-10H2,1-3H3,(H,23,25,26)/t14-,15+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM126827
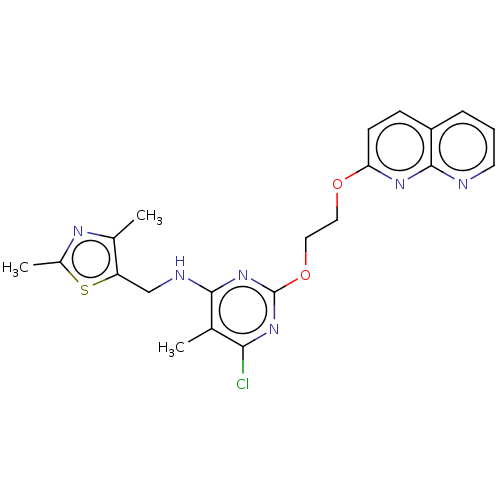
(US8785467, 1-32)Show SMILES Cc1nc(C)c(CNc2nc(OCCOc3ccc4cccnc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H21ClN6O2S/c1-12-18(22)27-21(28-19(12)24-11-16-13(2)25-14(3)31-16)30-10-9-29-17-7-6-15-5-4-8-23-20(15)26-17/h4-8H,9-11H2,1-3H3,(H,24,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50124648
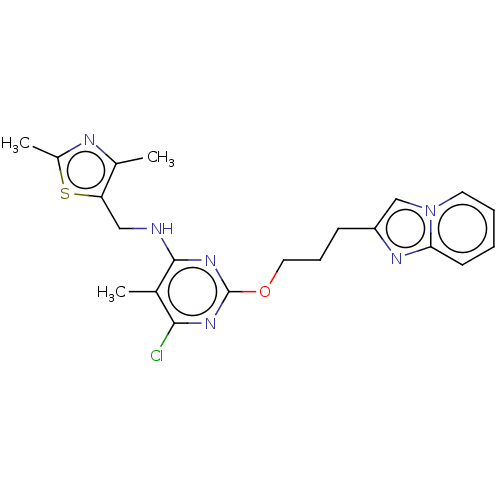
(CHEMBL3622901)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3cn4ccccc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H23ClN6OS/c1-13-19(22)26-21(27-20(13)23-11-17-14(2)24-15(3)30-17)29-10-6-7-16-12-28-9-5-4-8-18(28)25-16/h4-5,8-9,12H,6-7,10-11H2,1-3H3,(H,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50485494
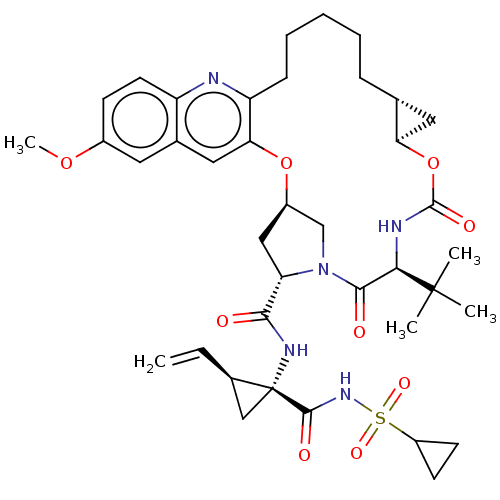
(CHEMBL2063089)Show SMILES [H][C@@]12C[C@@]1([H])OC(=O)N[C@H](C(=O)N1C[C@@]([H])(C[C@H]1C(=O)N[C@@]1(C[C@@]1([H])C=C)C(=O)NS(=O)(=O)C1CC1)Oc1cc3cc(OC)ccc3nc1CCCCC2)C(C)(C)C |r| Show InChI InChI=1S/C39H51N5O9S/c1-6-24-20-39(24,36(47)43-54(49,50)27-13-14-27)42-34(45)30-19-26-21-44(30)35(46)33(38(2,3)4)41-37(48)53-31-17-22(31)10-8-7-9-11-29-32(52-26)18-23-16-25(51-5)12-15-28(23)40-29/h6,12,15-16,18,22,24,26-27,30-31,33H,1,7-11,13-14,17,19-21H2,2-5H3,(H,41,48)(H,42,45)(H,43,47)/t22-,24-,26-,30+,31-,33-,39-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... |
ACS Med Chem Lett 3: 332-6 (2012)
Article DOI: 10.1021/ml300017p
BindingDB Entry DOI: 10.7270/Q2KH0R6V |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50124649

(CHEMBL3622902)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3nc4ccccc4s3)nc(Cl)c2C)s1 Show InChI InChI=1S/C21H22ClN5OS2/c1-12-19(22)26-21(27-20(12)23-11-17-13(2)24-14(3)29-17)28-10-6-9-18-25-15-7-4-5-8-16(15)30-18/h4-5,7-8H,6,9-11H2,1-3H3,(H,23,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50108620
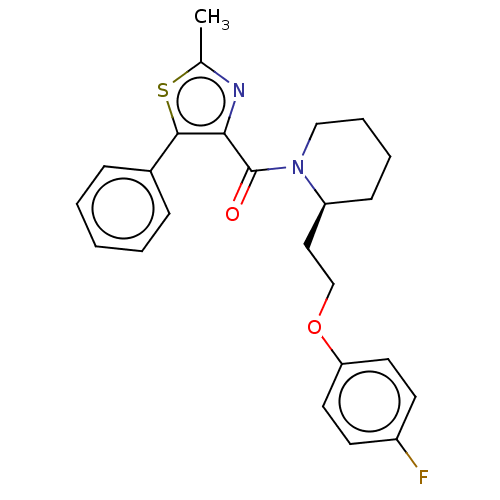
(CHEMBL3597953)Show SMILES Cc1nc(C(=O)N2CCCC[C@H]2CCOc2ccc(F)cc2)c(s1)-c1ccccc1 |r| Show InChI InChI=1S/C24H25FN2O2S/c1-17-26-22(23(30-17)18-7-3-2-4-8-18)24(28)27-15-6-5-9-20(27)14-16-29-21-12-10-19(25)11-13-21/h2-4,7-8,10-13,20H,5-6,9,14-16H2,1H3/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50485491
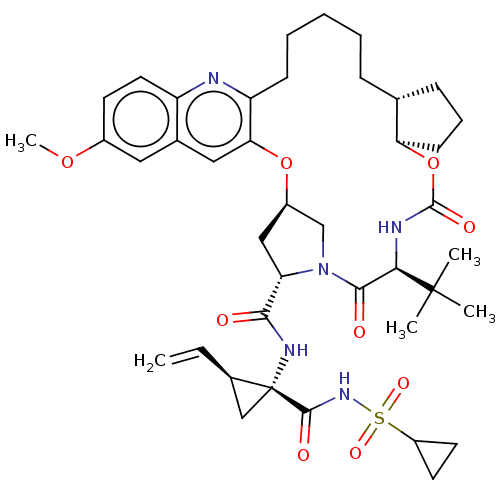
(CHEMBL2063088)Show SMILES [H][C@@]12C[C@H](N(C1)C(=O)[C@@H](NC(=O)O[C@]1([H])CCC[C@@]1([H])CCCCCc1nc3ccc(OC)cc3cc1O2)C(C)(C)C)C(=O)N[C@@]1(C[C@H]1C=C)C(=O)NS(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C41H55N5O9S/c1-6-26-22-41(26,38(49)45-56(51,52)29-16-17-29)44-36(47)32-21-28-23-46(32)37(48)35(40(2,3)4)43-39(50)55-33-14-10-12-24(33)11-8-7-9-13-31-34(54-28)20-25-19-27(53-5)15-18-30(25)42-31/h6,15,18-20,24,26,28-29,32-33,35H,1,7-14,16-17,21-23H2,2-5H3,(H,43,50)(H,44,47)(H,45,49)/t24-,26-,28-,32+,33-,35-,41-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... |
ACS Med Chem Lett 3: 332-6 (2012)
Article DOI: 10.1021/ml300017p
BindingDB Entry DOI: 10.7270/Q2KH0R6V |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50444605
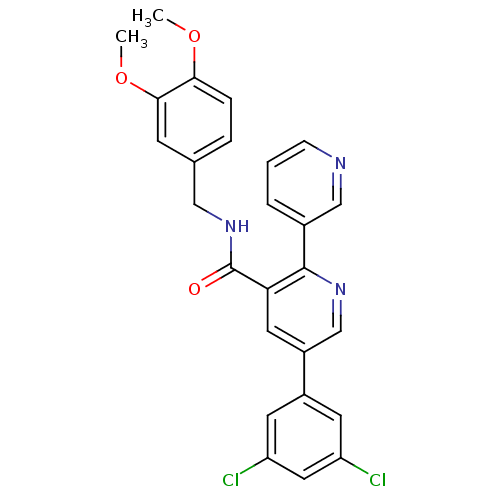
(CHEMBL3099899)Show SMILES COc1ccc(CNC(=O)c2cc(cnc2-c2cccnc2)-c2cc(Cl)cc(Cl)c2)cc1OC Show InChI InChI=1S/C26H21Cl2N3O3/c1-33-23-6-5-16(8-24(23)34-2)13-31-26(32)22-11-19(18-9-20(27)12-21(28)10-18)15-30-25(22)17-4-3-7-29-14-17/h3-12,14-15H,13H2,1-2H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]SB-674042 from human orexin-2 receptor after 60 mins by scintillation counting analysis |
Bioorg Med Chem Lett 23: 6620-4 (2013)
Article DOI: 10.1016/j.bmcl.2013.10.045
BindingDB Entry DOI: 10.7270/Q2WH2RFJ |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135600
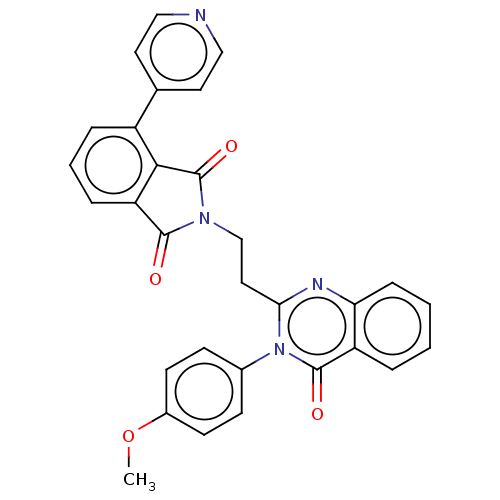
(US8846000, 1-16)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(c3C2=O)-c2ccncc2)nc2ccccc2c1=O Show InChI InChI=1S/C30H22N4O4/c1-38-21-11-9-20(10-12-21)34-26(32-25-8-3-2-5-23(25)29(34)36)15-18-33-28(35)24-7-4-6-22(27(24)30(33)37)19-13-16-31-17-14-19/h2-14,16-17H,15,18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104700
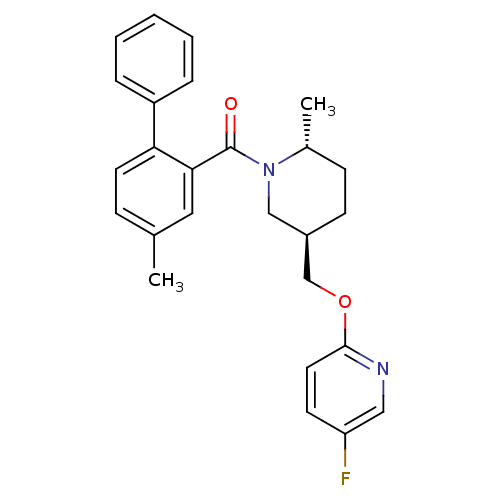
(US8569311, 1-10)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ccccc1 |r| Show InChI InChI=1S/C26H27FN2O2/c1-18-8-12-23(21-6-4-3-5-7-21)24(14-18)26(30)29-16-20(10-9-19(29)2)17-31-25-13-11-22(27)15-28-25/h3-8,11-15,19-20H,9-10,16-17H2,1-2H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50374443
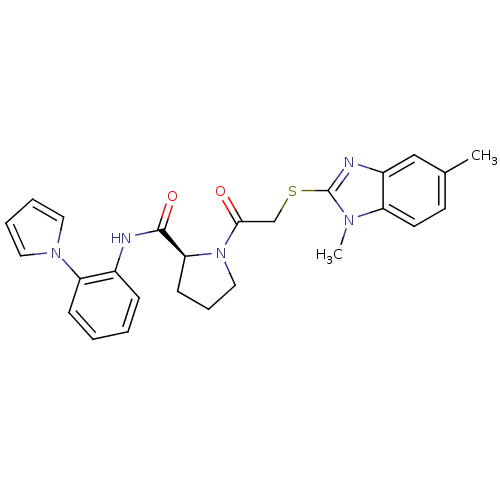
(CHEMBL272715)Show SMILES Cc1ccc2n(C)c(SCC(=O)N3CCC[C@H]3C(=O)Nc3ccccc3-n3cccc3)nc2c1 Show InChI InChI=1S/C26H27N5O2S/c1-18-11-12-21-20(16-18)28-26(29(21)2)34-17-24(32)31-15-7-10-23(31)25(33)27-19-8-3-4-9-22(19)30-13-5-6-14-30/h3-6,8-9,11-14,16,23H,7,10,15,17H2,1-2H3,(H,27,33)/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
Bioorg Med Chem Lett 18: 1425-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.001
BindingDB Entry DOI: 10.7270/Q2N87BM4 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50374442
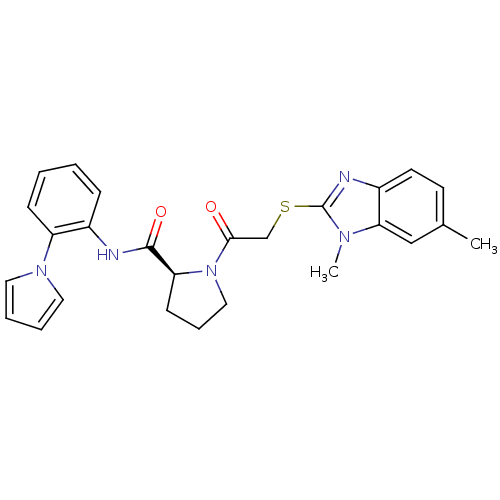
(CHEMBL255763)Show SMILES Cc1ccc2nc(SCC(=O)N3CCC[C@H]3C(=O)Nc3ccccc3-n3cccc3)n(C)c2c1 Show InChI InChI=1S/C26H27N5O2S/c1-18-11-12-20-23(16-18)29(2)26(28-20)34-17-24(32)31-15-7-10-22(31)25(33)27-19-8-3-4-9-21(19)30-13-5-6-14-30/h3-6,8-9,11-14,16,22H,7,10,15,17H2,1-2H3,(H,27,33)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
Bioorg Med Chem Lett 18: 1425-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.001
BindingDB Entry DOI: 10.7270/Q2N87BM4 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50148575
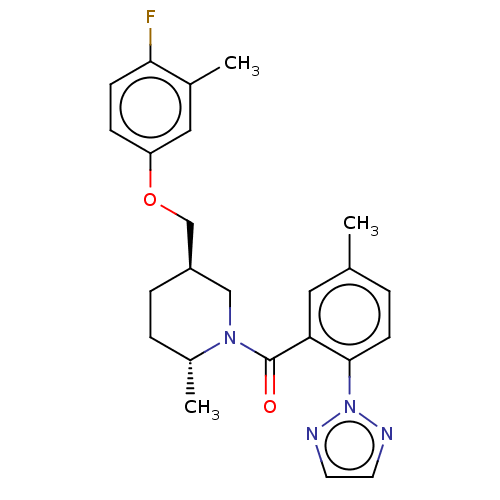
(CHEMBL3770503)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)c(C)c2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C24H27FN4O2/c1-16-4-9-23(29-26-10-11-27-29)21(12-16)24(30)28-14-19(6-5-18(28)3)15-31-20-7-8-22(25)17(2)13-20/h4,7-13,18-19H,5-6,14-15H2,1-3H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM120778
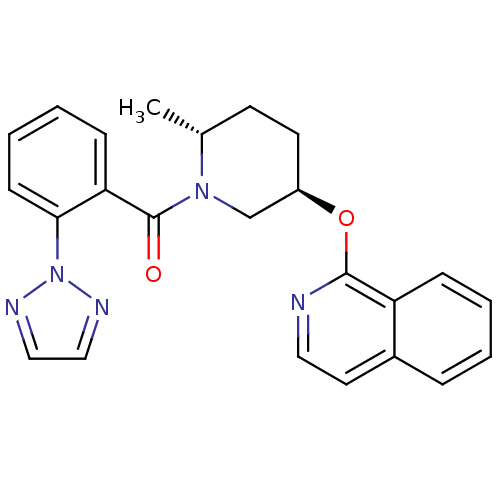
(US8710076, F-2)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1nccc2ccccc12 |r| Show InChI InChI=1S/C24H23N5O2/c1-17-10-11-19(31-23-20-7-3-2-6-18(20)12-13-25-23)16-28(17)24(30)21-8-4-5-9-22(21)29-26-14-15-27-29/h2-9,12-15,17,19H,10-11,16H2,1H3/t17-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Compound potency can be assessed by a radioligand binding assay (described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430) in which ... |
US Patent US8710076 (2014)
BindingDB Entry DOI: 10.7270/Q2QN65DD |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM50124650
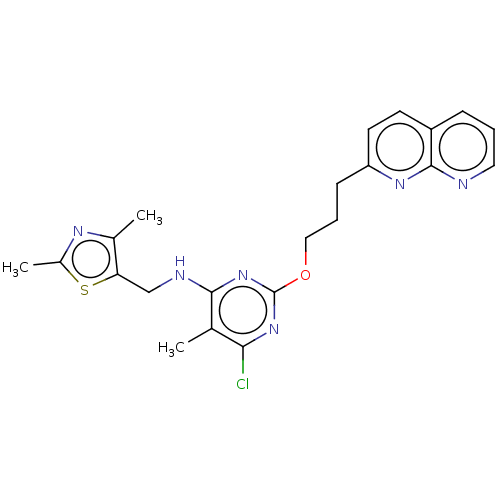
(CHEMBL3622903)Show SMILES Cc1nc(C)c(CNc2nc(OCCCc3ccc4cccnc4n3)nc(Cl)c2C)s1 Show InChI InChI=1S/C22H23ClN6OS/c1-13-19(23)28-22(29-20(13)25-12-18-14(2)26-15(3)31-18)30-11-5-7-17-9-8-16-6-4-10-24-21(16)27-17/h4,6,8-10H,5,7,11-12H2,1-3H3,(H,25,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assay |
J Med Chem 58: 7888-94 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00983
BindingDB Entry DOI: 10.7270/Q26Q202C |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepacivirus C) | BDBM50485492
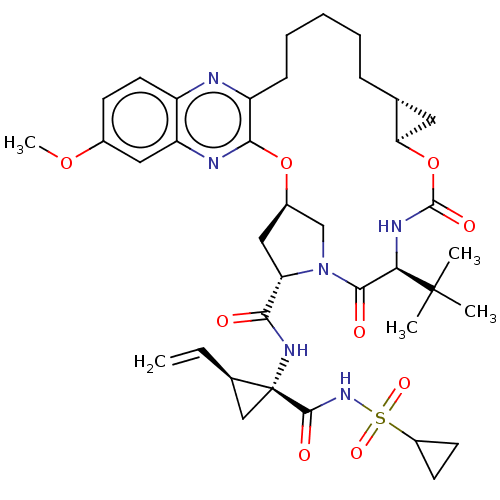
(Grazoprevir | Grazoprevir monohydrate | MK-5172 | ...)Show SMILES [H][C@@]12C[C@@]1([H])OC(=O)N[C@H](C(=O)N1C[C@@]([H])(C[C@H]1C(=O)N[C@@]1(C[C@H]1C=C)C(=O)NS(=O)(=O)C1CC1)Oc1nc3cc(OC)ccc3nc1CCCCC2)C(C)(C)C |r| Show InChI InChI=1S/C38H50N6O9S/c1-6-22-19-38(22,35(47)43-54(49,50)25-13-14-25)42-32(45)29-18-24-20-44(29)34(46)31(37(2,3)4)41-36(48)53-30-16-21(30)10-8-7-9-11-27-33(52-24)40-28-17-23(51-5)12-15-26(28)39-27/h6,12,15,17,21-22,24-25,29-31H,1,7-11,13-14,16,18-20H2,2-5H3,(H,41,48)(H,42,45)(H,43,47)/t21-,22-,24-,29+,30-,31-,38-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Hepatitis C virus (isolate BK) genotype 1b NS3/4a protease D168V mutant expressed in Escherichia coli by time-resolved fluorescence ana... |
ACS Med Chem Lett 3: 332-6 (2012)
Article DOI: 10.1021/ml300017p
BindingDB Entry DOI: 10.7270/Q2KH0R6V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM135614

(US8846000, D-7)Show SMILES COc1ccc(cc1)-n1c(CCN2C(=O)c3cccc(OC(C)C)c3C2=O)nc2ccccc2c1=O Show InChI InChI=1S/C28H25N3O5/c1-17(2)36-23-10-6-8-21-25(23)28(34)30(26(21)32)16-15-24-29-22-9-5-4-7-20(22)27(33)31(24)18-11-13-19(35-3)14-12-18/h4-14,17H,15-16H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) |
Bioorg Med Chem Lett 25: 4893-8 (2015)
Article DOI: 10.1016/j.bmcl.2015.05.080
BindingDB Entry DOI: 10.7270/Q2RJ4M9Z |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318697
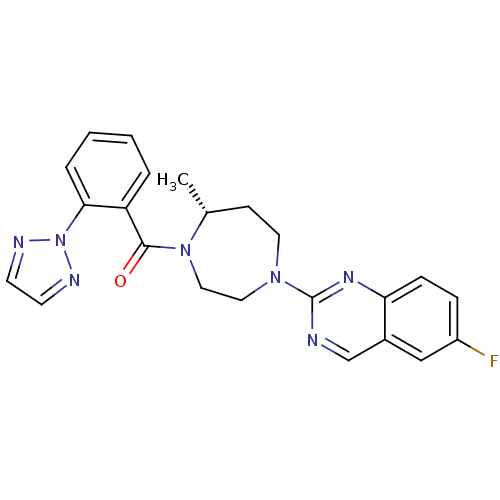
(6-Fluoro-2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2cc(F)ccc2n1 |r| Show InChI InChI=1S/C23H22FN7O/c1-16-8-11-29(23-25-15-17-14-18(24)6-7-20(17)28-23)12-13-30(16)22(32)19-4-2-3-5-21(19)31-26-9-10-27-31/h2-7,9-10,14-16H,8,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084394
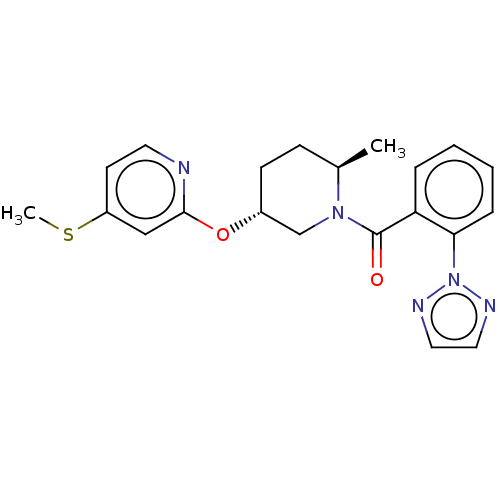
(CHEMBL3426145)Show SMILES CSc1ccnc(O[C@@H]2CC[C@@H](C)N(C2)C(=O)c2ccccc2-n2nccn2)c1 |r| Show InChI InChI=1S/C21H23N5O2S/c1-15-7-8-16(28-20-13-17(29-2)9-10-22-20)14-25(15)21(27)18-5-3-4-6-19(18)26-23-11-12-24-26/h3-6,9-13,15-16H,7-8,14H2,1-2H3/t15-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50084384
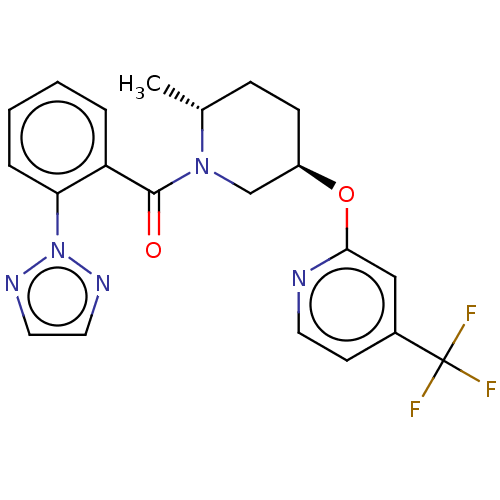
(CHEMBL3426135)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1cc(ccn1)C(F)(F)F |r| Show InChI InChI=1S/C21H20F3N5O2/c1-14-6-7-16(31-19-12-15(8-9-25-19)21(22,23)24)13-28(14)20(30)17-4-2-3-5-18(17)29-26-10-11-27-29/h2-5,8-12,14,16H,6-7,13H2,1H3/t14-,16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]-(S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R ex... |
Bioorg Med Chem Lett 25: 2488-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.066
BindingDB Entry DOI: 10.7270/Q28S4RN5 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50314676
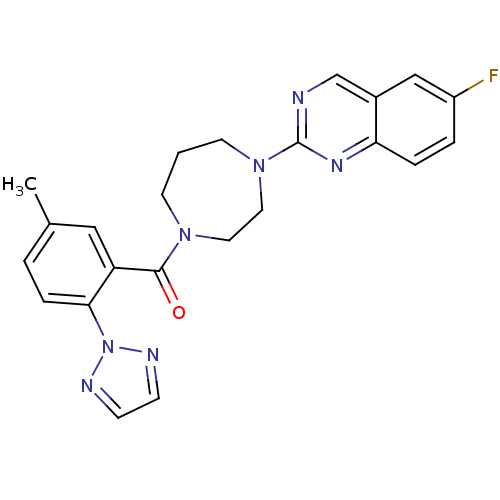
((4-(6-fluoroquinazolin-2-yl)-1,4-diazepan-1-yl)(5-...)Show SMILES Cc1ccc(c(c1)C(=O)N1CCCN(CC1)c1ncc2cc(F)ccc2n1)-n1nccn1 Show InChI InChI=1S/C23H22FN7O/c1-16-3-6-21(31-26-7-8-27-31)19(13-16)22(32)29-9-2-10-30(12-11-29)23-25-15-17-14-18(24)4-5-20(17)28-23/h3-8,13-15H,2,9-12H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2 receptor by radioligand displacement assay |
Bioorg Med Chem Lett 20: 2311-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.01.138
BindingDB Entry DOI: 10.7270/Q2MK6D26 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50374441
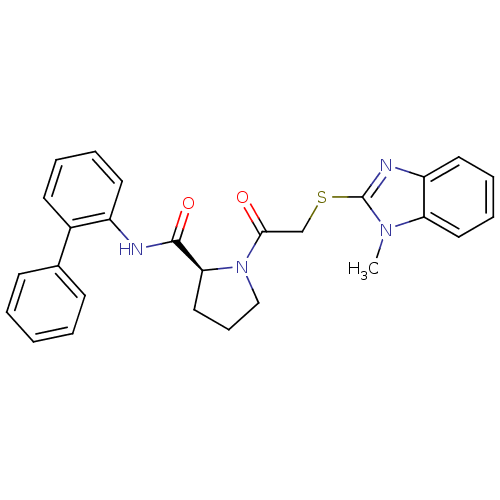
(CHEMBL429848)Show SMILES Cn1c(SCC(=O)N2CCC[C@H]2C(=O)Nc2ccccc2-c2ccccc2)nc2ccccc12 Show InChI InChI=1S/C27H26N4O2S/c1-30-23-15-8-7-14-22(23)29-27(30)34-18-25(32)31-17-9-16-24(31)26(33)28-21-13-6-5-12-20(21)19-10-3-2-4-11-19/h2-8,10-15,24H,9,16-18H2,1H3,(H,28,33)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
Bioorg Med Chem Lett 18: 1425-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.001
BindingDB Entry DOI: 10.7270/Q2N87BM4 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318697

(6-Fluoro-2-{(5R)-5-Methyl-4-[5-methyl-2-(2H-1,2,3-...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2cc(F)ccc2n1 |r| Show InChI InChI=1S/C23H22FN7O/c1-16-8-11-29(23-25-15-17-14-18(24)6-7-20(17)28-23)12-13-30(16)22(32)19-4-2-3-5-21(19)31-26-9-10-27-31/h2-7,9-10,14-16H,8,11-13H2,1H3/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-2 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM104689
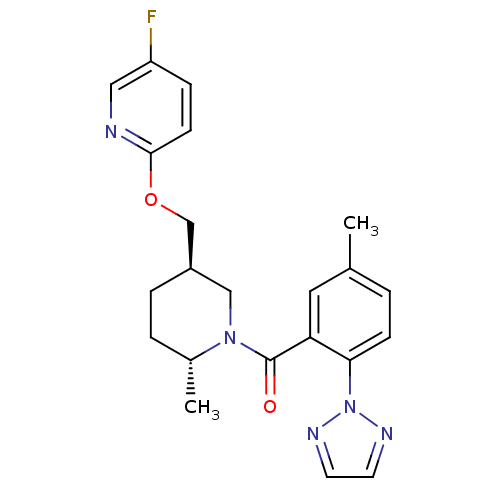
(US8569311, A-9)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H24FN5O2/c1-15-3-7-20(28-25-9-10-26-28)19(11-15)22(29)27-13-17(5-4-16(27)2)14-30-21-8-6-18(23)12-24-21/h3,6-12,16-17H,4-5,13-14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to human OX2 receptor in cell membrane by in vitro radioligand binding assay |
Bioorg Med Chem Lett 24: 1784-9 (2014)
Article DOI: 10.1016/j.bmcl.2014.02.026
BindingDB Entry DOI: 10.7270/Q2RV0Q6F |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012606
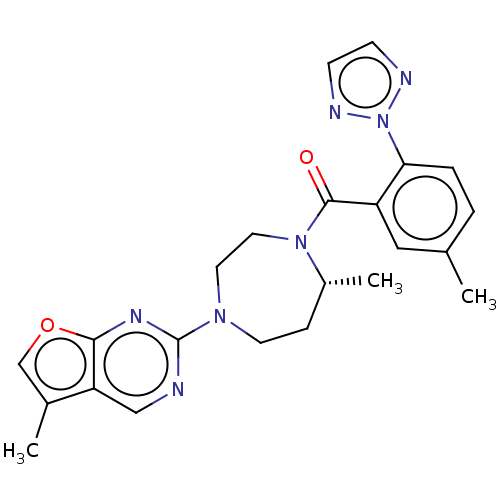
(CHEMBL3260826)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2c(C)coc2n1 |r| Show InChI InChI=1S/C23H25N7O2/c1-15-4-5-20(30-25-7-8-26-30)18(12-15)22(31)29-11-10-28(9-6-17(29)3)23-24-13-19-16(2)14-32-21(19)27-23/h4-5,7-8,12-14,17H,6,9-11H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50012608
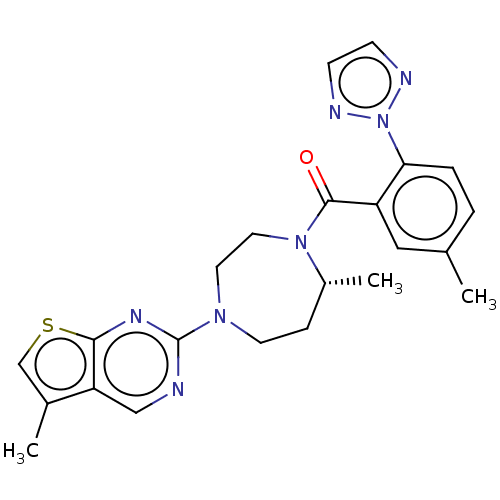
(CHEMBL3260828)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2c(C)csc2n1 |r| Show InChI InChI=1S/C23H25N7OS/c1-15-4-5-20(30-25-7-8-26-30)18(12-15)22(31)29-11-10-28(9-6-17(29)3)23-24-13-19-16(2)14-32-21(19)27-23/h4-5,7-8,12-14,17H,6,9-11H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-2 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50374444
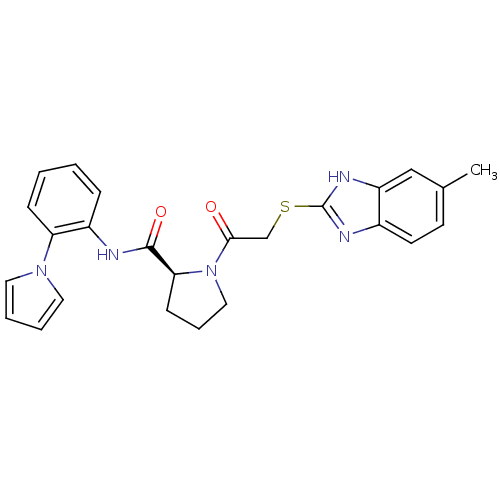
(CHEMBL401653)Show SMILES Cc1ccc2nc(SCC(=O)N3CCC[C@H]3C(=O)Nc3ccccc3-n3cccc3)[nH]c2c1 Show InChI InChI=1S/C25H25N5O2S/c1-17-10-11-18-20(15-17)28-25(27-18)33-16-23(31)30-14-6-9-22(30)24(32)26-19-7-2-3-8-21(19)29-12-4-5-13-29/h2-5,7-8,10-13,15,22H,6,9,14,16H2,1H3,(H,26,32)(H,27,28)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
Bioorg Med Chem Lett 18: 1425-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.001
BindingDB Entry DOI: 10.7270/Q2N87BM4 |
More data for this
Ligand-Target Pair | |
cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A
(Homo sapiens (Human)) | BDBM147134
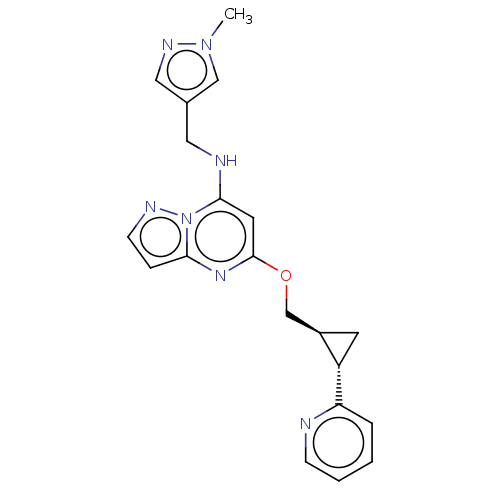
(US8957077, J-5)Show SMILES Cn1cc(CNc2cc(OC[C@H]3C[C@@H]3c3ccccn3)nc3ccnn23)cn1 |r| Show InChI InChI=1S/C20H21N7O/c1-26-12-14(11-24-26)10-22-19-9-20(25-18-5-7-23-27(18)19)28-13-15-8-16(15)17-4-2-3-6-21-17/h2-7,9,11-12,15-16,22H,8,10,13H2,1H3/t15-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of PDE10A (unknown origin) by IMAP assay |
Bioorg Med Chem Lett 26: 126-32 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.013
BindingDB Entry DOI: 10.7270/Q2NP27FW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104689

(US8569311, A-9)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C22H24FN5O2/c1-15-3-7-20(28-25-9-10-26-28)19(11-15)22(29)27-13-17(5-4-16(27)2)14-30-21-8-6-18(23)12-24-21/h3,6-12,16-17H,4-5,13-14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50060938
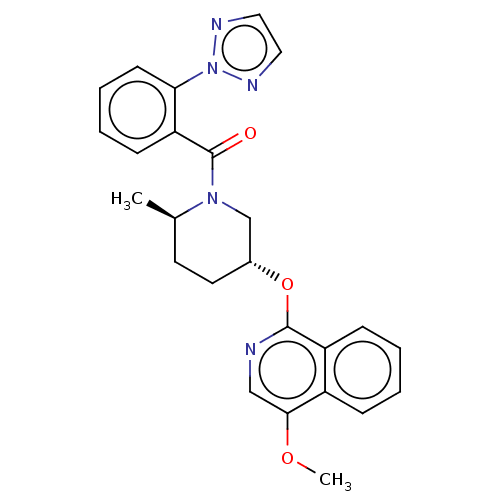
(CHEMBL3394847)Show SMILES COc1cnc(O[C@@H]2CC[C@@H](C)N(C2)C(=O)c2ccccc2-n2nccn2)c2ccccc12 |r| Show InChI InChI=1S/C25H25N5O3/c1-17-11-12-18(33-24-20-8-4-3-7-19(20)23(32-2)15-26-24)16-29(17)25(31)21-9-5-6-10-22(21)30-27-13-14-28-30/h3-10,13-15,17-18H,11-12,16H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Rattus norvegicus (Rat)) | BDBM104697
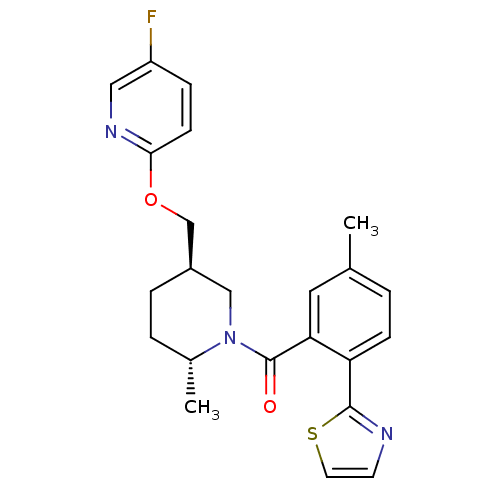
(US8569311, 1-1)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1nccs1 |r| Show InChI InChI=1S/C23H24FN3O2S/c1-15-3-7-19(22-25-9-10-30-22)20(11-15)23(28)27-13-17(5-4-16(27)2)14-29-21-8-6-18(24)12-26-21/h3,6-12,16-17H,4-5,13-14H2,1-2H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merch Sharp & Dohme Corp.
US Patent
| Assay Description
Radioligand binding assay described in Bergman et. al. Bioorg. Med. Chem. Lett. 2008, 18, 1425-1430. |
US Patent US8569311 (2013)
BindingDB Entry DOI: 10.7270/Q2TT4PM6 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318698

(6,7-Fluoro-2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1cnc2cc(F)c(F)cc2n1 |r| Show InChI InChI=1S/C23H21F2N7O/c1-15-6-9-30(22-14-26-19-12-17(24)18(25)13-20(19)29-22)10-11-31(15)23(33)16-4-2-3-5-21(16)32-27-7-8-28-32/h2-5,7-8,12-15H,6,9-11H2,1H3/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50318699
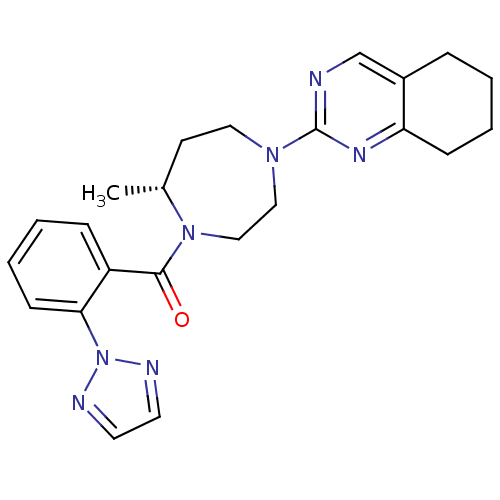
(2-{(5R)-5-Methyl-4-[2-(2H-1,2,3-triazol-2-yl)benzo...)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1ncc2CCCCc2n1 |r| Show InChI InChI=1S/C23H27N7O/c1-17-10-13-28(23-24-16-18-6-2-4-8-20(18)27-23)14-15-29(17)22(31)19-7-3-5-9-21(19)30-25-11-12-26-30/h3,5,7,9,11-12,16-17H,2,4,6,8,10,13-15H2,1H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
J Med Chem 53: 5320-32 (2010)
Article DOI: 10.1021/jm100541c
BindingDB Entry DOI: 10.7270/Q29K4C61 |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM120778

(US8710076, F-2)Show SMILES C[C@@H]1CC[C@H](CN1C(=O)c1ccccc1-n1nccn1)Oc1nccc2ccccc12 |r| Show InChI InChI=1S/C24H23N5O2/c1-17-10-11-19(31-23-20-7-3-2-6-18(20)12-13-25-23)16-28(17)24(30)21-8-4-5-9-22(21)29-26-14-15-27-29/h2-9,12-15,17,19H,10-11,16H2,1H3/t17-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50148575

(CHEMBL3770503)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)c(C)c2)CN1C(=O)c1cc(C)ccc1-n1nccn1 |r| Show InChI InChI=1S/C24H27FN4O2/c1-16-4-9-23(29-26-10-11-27-29)21(12-16)24(30)28-14-19(6-5-18(28)3)15-31-20-7-8-22(25)17(2)13-20/h4,7-13,18-19H,5-6,14-15H2,1-3H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX1R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM104692
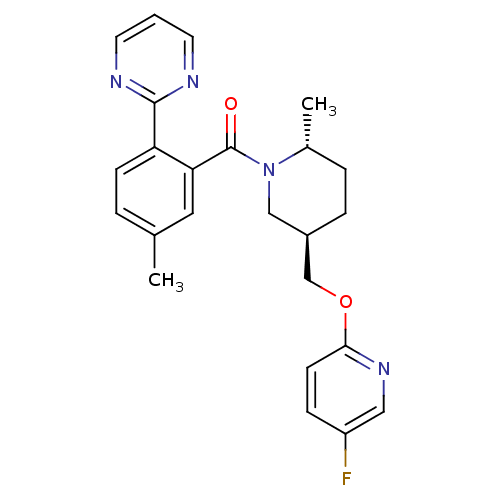
(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity to OX2R (unknown origin) |
J Med Chem 59: 504-30 (2016)
Article DOI: 10.1021/acs.jmedchem.5b00832
BindingDB Entry DOI: 10.7270/Q29C709W |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM50374448
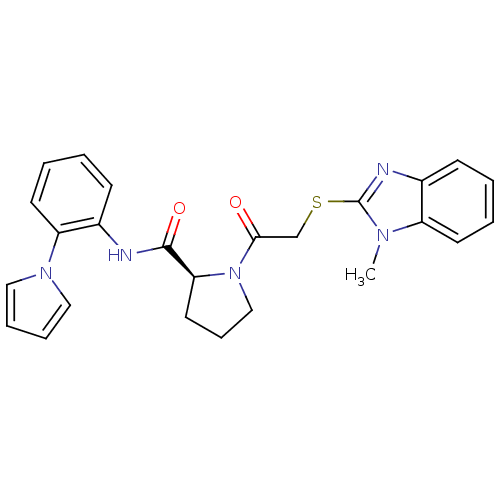
(CHEMBL429130)Show SMILES Cn1c(SCC(=O)N2CCC[C@H]2C(=O)Nc2ccccc2-n2cccc2)nc2ccccc12 Show InChI InChI=1S/C25H25N5O2S/c1-28-20-11-4-2-9-18(20)27-25(28)33-17-23(31)30-16-8-13-22(30)24(32)26-19-10-3-5-12-21(19)29-14-6-7-15-29/h2-7,9-12,14-15,22H,8,13,16-17H2,1H3,(H,26,32)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H](S)-N-(2-(1H-pyrrol-1-yl)phenyl)-1-(2-(1-methyl-1H-benzo[d]imidazol-2-ylthio)acetyl)pyrrolidine-2-carboxamide from human OX2R exp... |
Bioorg Med Chem Lett 18: 1425-30 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.001
BindingDB Entry DOI: 10.7270/Q2N87BM4 |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012608

(CHEMBL3260828)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1cc(C)ccc1-n1nccn1)c1ncc2c(C)csc2n1 |r| Show InChI InChI=1S/C23H25N7OS/c1-15-4-5-20(30-25-7-8-26-30)18(12-15)22(31)29-11-10-28(9-6-17(29)3)23-24-13-19-16(2)14-32-21(19)27-23/h4-5,7-8,12-14,17H,6,9-11H2,1-3H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |
Orexin receptor type 2
(Homo sapiens (Human)) | BDBM104692

(US8569311, E-5)Show SMILES C[C@@H]1CC[C@@H](COc2ccc(F)cn2)CN1C(=O)c1cc(C)ccc1-c1ncccn1 |r| Show InChI InChI=1S/C24H25FN4O2/c1-16-4-8-20(23-26-10-3-11-27-23)21(12-16)24(30)29-14-18(6-5-17(29)2)15-31-22-9-7-19(25)13-28-22/h3-4,7-13,17-18H,5-6,14-15H2,1-2H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human OX2R by radioligand displacement assay |
Bioorg Med Chem Lett 25: 444-50 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.056
BindingDB Entry DOI: 10.7270/Q2KD20MV |
More data for this
Ligand-Target Pair | |
Orexin/Hypocretin receptor type 1
(Homo sapiens (Human)) | BDBM50012602
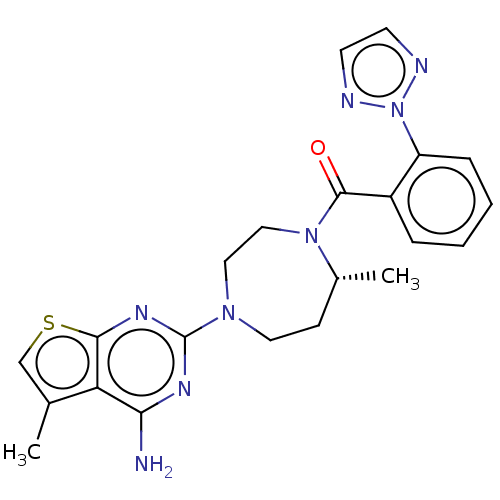
(CHEMBL3260836)Show SMILES C[C@@H]1CCN(CCN1C(=O)c1ccccc1-n1nccn1)c1nc(N)c2c(C)csc2n1 |r| Show InChI InChI=1S/C22H24N8OS/c1-14-13-32-20-18(14)19(23)26-22(27-20)28-10-7-15(2)29(12-11-28)21(31)16-5-3-4-6-17(16)30-24-8-9-25-30/h3-6,8-9,13,15H,7,10-12H2,1-2H3,(H2,23,26,27)/t15-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]radioligand from human orexin-1 receptor expressed in CHO cells after 3 hrs by scintillation counting analysis |
Bioorg Med Chem Lett 24: 2079-85 (2014)
Article DOI: 10.1016/j.bmcl.2014.03.052
BindingDB Entry DOI: 10.7270/Q2MW2JPH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data