

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor kappa 1 in Guinea pig Caudate stimulated by U69,593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50291970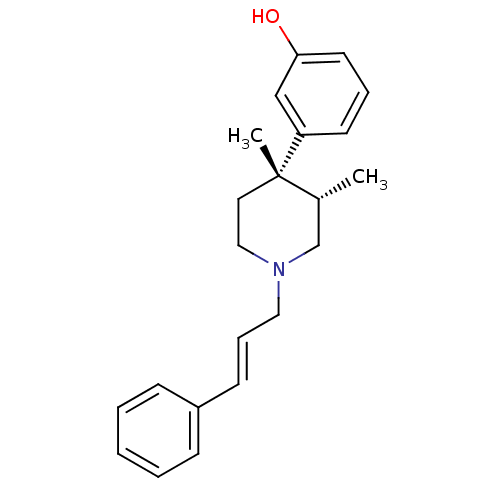 (3-[(3S,4S)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor mu 1 in Guinea pig Caudate stimulated by DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor kappa 1 in Guinea pig brain membranes using radioligand [3H]U-69593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50291970 (3-[(3S,4S)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor mu 1 in Guinea pig brain membranes using radioligand [3H]DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor mu 1 in Guinea pig Caudate stimulated by DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50291970 (3-[(3S,4S)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor kappa 1 in Guinea pig Caudate stimulated by U69,593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor mu 1 in Guinea pig brain membranes using radioligand [3H]DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50291970 (3-[(3S,4S)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor delta 1 in Guinea pig Caudate stimulated by SNC80 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor kappa 1 in Guinea pig Caudate stimulated by U69,593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102710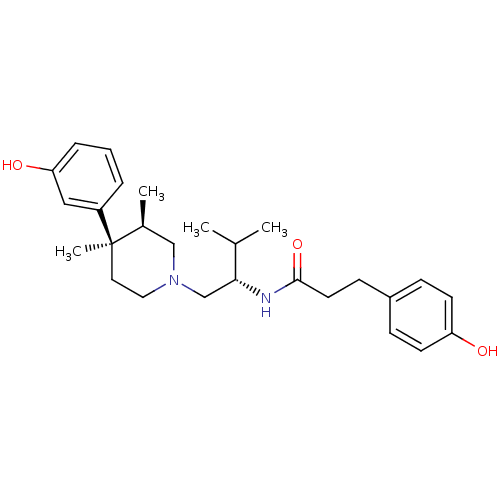 (3-(4-Hydroxy-phenyl)-N-{(S)-1-[(3R,4R)-4-(3-hydrox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor kappa 1 in Guinea pig Caudate stimulated by U69,593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor kappa 1 in Guinea pig brain membranes using radioligand [3H]U-69593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102710 (3-(4-Hydroxy-phenyl)-N-{(S)-1-[(3R,4R)-4-(3-hydrox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor kappa 1 in Guinea pig brain membranes using radioligand [3H]U-69593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50102710 (3-(4-Hydroxy-phenyl)-N-{(S)-1-[(3R,4R)-4-(3-hydrox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor mu 1 in Guinea pig Caudate stimulated by DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50291973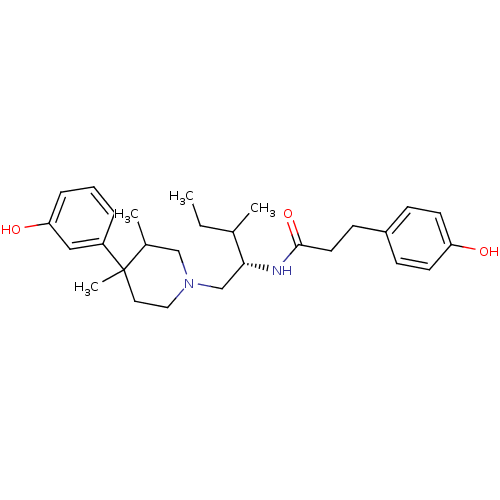 (3-(4-Hydroxy-phenyl)-N-{(S)-1-[4-(3-hydroxy-phenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor kappa 1 in Guinea pig brain membranes using radioligand [3H]U-69593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50291972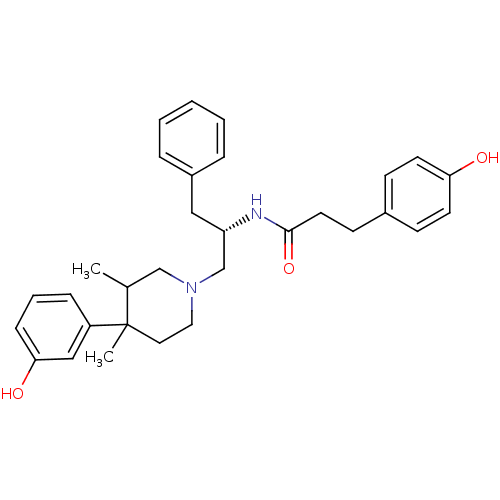 (CHEMBL148843 | N-{(S)-1-Benzyl-2-[4-(3-hydroxy-phe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor mu 1 in Guinea pig brain membranes using radioligand [3H]DAMGO. | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor delta 1 in Guinea pig Caudate stimulated by SNC80 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor mu 1 in Guinea pig Caudate stimulated by DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor delta 1 in Guinea pig Caudate stimulated by SNC80 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor delta 1 in Guinea pig brain membranes using radioligand [3H]DADLE | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor mu 1 in Guinea pig brain membranes using radioligand [3H]DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50291972 (CHEMBL148843 | N-{(S)-1-Benzyl-2-[4-(3-hydroxy-phe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor kappa 1 in Guinea pig brain membranes using radioligand [3H]U-69593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50291974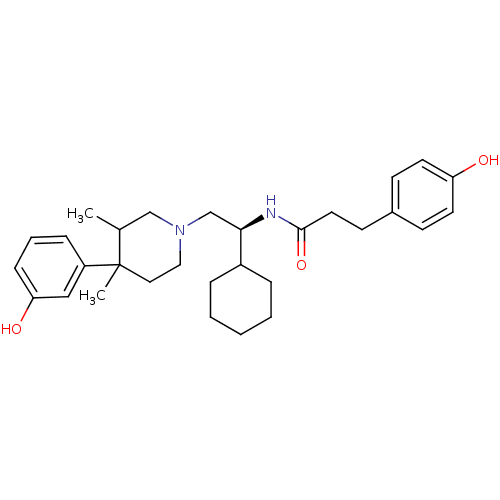 (CHEMBL359088 | N-{(S)-1-Cyclohexyl-2-[4-(3-hydroxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor kappa 1 in Guinea pig brain membranes using radioligand [3H]U-69593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50291971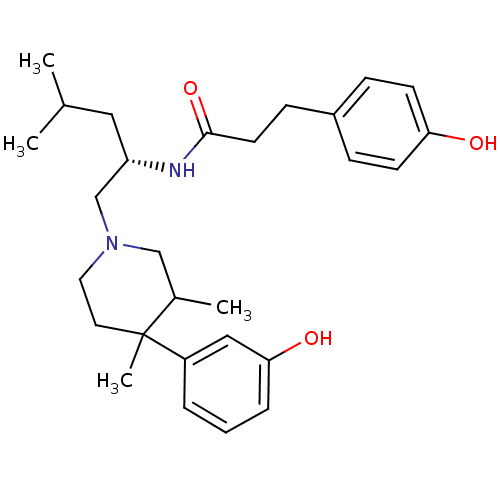 (3-(4-Hydroxy-phenyl)-N-{(S)-1-[4-(3-hydroxy-phenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor kappa 1 in Guinea pig brain membranes using radioligand [3H]U-69593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor delta 1 in Guinea pig brain membranes using radioligand [3H]DADLE | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50291970 (3-[(3S,4S)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor kappa 1 in Guinea pig brain membranes using radioligand [3H]U-69593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50291974 (CHEMBL359088 | N-{(S)-1-Cyclohexyl-2-[4-(3-hydroxy...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 234 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor mu 1 in Guinea pig brain membranes using radioligand [3H]DAMGO. | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50291970 (3-[(3S,4S)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor delta 1 in Guinea pig brain membranes using radioligand [3H]DADLE | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Rho-associated protein kinase 1/2 (Homo sapiens (Human)) | BDBM14027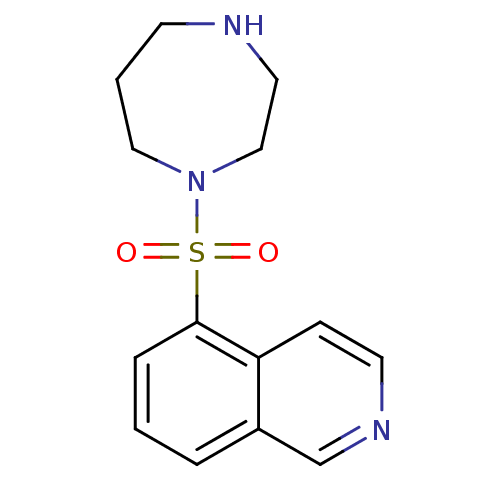 (5-(1,4-diazepan-1-ylsulfonyl)isoquinoline | 5-(1,4...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University Curated by ChEMBL | Assay Description Inhibition of ROCK (unknown origin) | Eur J Med Chem 180: 449-456 (2019) Article DOI: 10.1016/j.ejmech.2019.06.089 BindingDB Entry DOI: 10.7270/Q2C82DN1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50102710 (3-(4-Hydroxy-phenyl)-N-{(S)-1-[(3R,4R)-4-(3-hydrox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 393 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor mu 1 in Guinea pig brain membranes using radioligand [3H]DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50291971 (3-(4-Hydroxy-phenyl)-N-{(S)-1-[4-(3-hydroxy-phenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor mu 1 in Guinea pig brain membranes using radioligand [3H]DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50291973 (3-(4-Hydroxy-phenyl)-N-{(S)-1-[4-(3-hydroxy-phenyl...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 421 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor mu 1 in Guinea pig brain membranes using radioligand [3H]DAMGO. | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50102710 (3-(4-Hydroxy-phenyl)-N-{(S)-1-[(3R,4R)-4-(3-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor delta 1 in Guinea pig Caudate stimulated by SNC80 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight protein-tyrosine phosphatase A (Mycobacterium tuberculosis) | BDBM50341977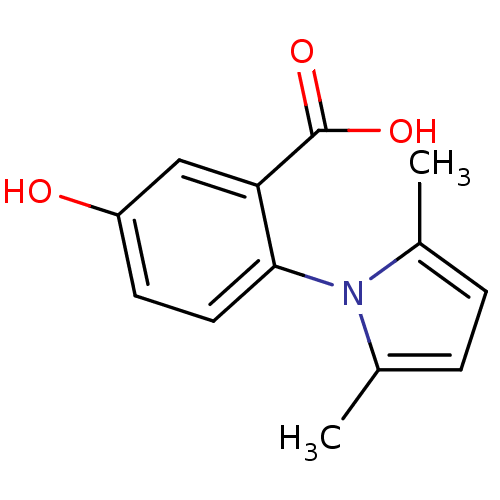 (2-(2,5-dimethyl-1H-pyrrol-1-yl)-5-hydroxybenzoic a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Global Alliance for TB Drug Development (TB Alliance) Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis PtpA | J Med Chem 56: 7755-60 (2013) Article DOI: 10.1021/jm400381v BindingDB Entry DOI: 10.7270/Q2000514 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50102710 (3-(4-Hydroxy-phenyl)-N-{(S)-1-[(3R,4R)-4-(3-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | >5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor delta 1 in Guinea pig brain membranes using radioligand [3H]DADLE | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50036920 (2-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description In vitro binding affinity was measured on serotonergic 5-hydroxytryptamine 1A receptor by displacement of [3H]- tetralin | J Med Chem 37: 2552-63 (1994) BindingDB Entry DOI: 10.7270/Q2G15ZWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50036920 (2-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description In vitro binding affinity was measured on serotonergic 5-hydroxytryptamine 1A receptor by displacement of [3H]- tetralin | J Med Chem 37: 2552-63 (1994) BindingDB Entry DOI: 10.7270/Q2G15ZWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Protein farnesyltransferase subunit beta (Homo sapiens (Human)) | BDBM50177232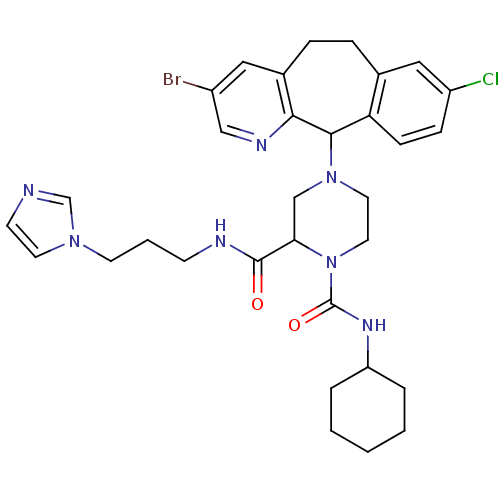 (4-(3-bromo-8-chloro-6,11-dihydro-5H-benzo[5,6]cycl...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Princ Curated by ChEMBL | Assay Description Inhibitory activity against Farnesyltransferase quantified by modified SPA assay with improved sensitivity | Bioorg Med Chem Lett 16: 507-11 (2005) Article DOI: 10.1016/j.bmcl.2005.10.070 BindingDB Entry DOI: 10.7270/Q2M0450S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 [23-292,A23S,F278E] (Homo sapiens (Human)) | BDBM32522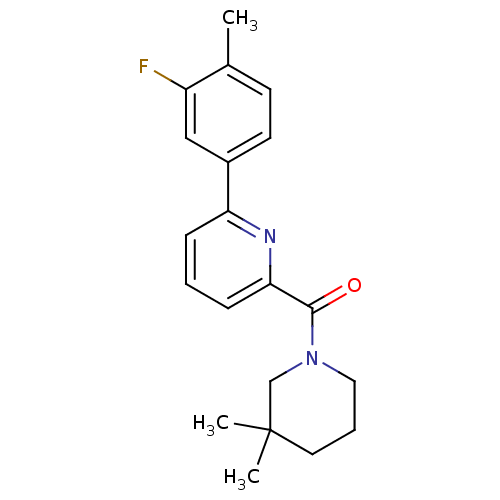 (pyridine amide, 30) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 6.5 | 23 |
Bristol-Myers Squibb Company | Assay Description 11beta-HSD1 microsomes isolated from HEK 293 cells over-expressing human 11beta-HSD1 were incubated with the substrate cortisone and cofactor NADPH a... | Bioorg Med Chem Lett 18: 3168-72 (2008) Article DOI: 10.1016/j.bmcl.2008.04.069 BindingDB Entry DOI: 10.7270/Q2P26WGT | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495899 (CHEMBL3115256) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495912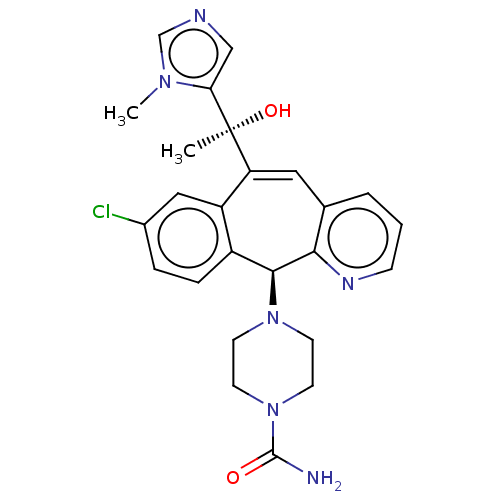 (CHEMBL3115255) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50005417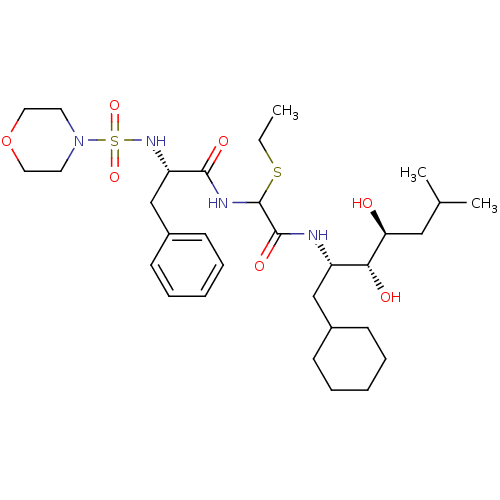 (CHEMBL266334 | N-[(1-Cyclohexylmethyl-2,3-dihydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against renin monkey plasma | J Med Chem 36: 3809-20 (1994) BindingDB Entry DOI: 10.7270/Q24T6HFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50036918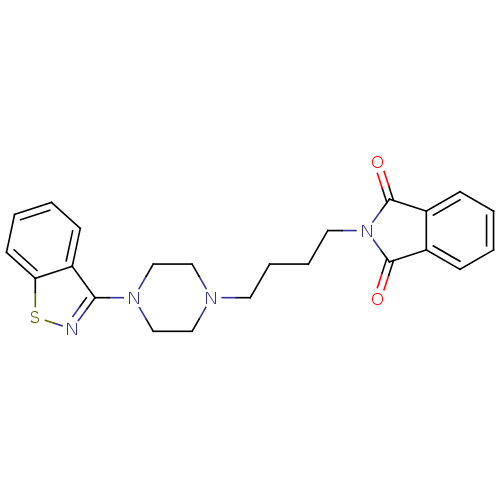 (2-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description In vitro binding affinity was measured on serotonergic 5-hydroxytryptamine 1A receptor by displacement of [3H]- tetralin | J Med Chem 37: 2552-63 (1994) BindingDB Entry DOI: 10.7270/Q2G15ZWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495906 (CHEMBL3115258) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50042819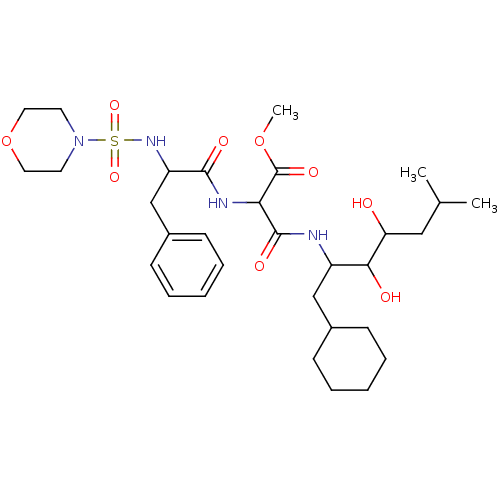 (CHEMBL339340 | N-(1-Cyclohexylmethyl-2,3-dihydroxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against renin monkey plasma | J Med Chem 36: 3809-20 (1994) BindingDB Entry DOI: 10.7270/Q24T6HFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495911 (CHEMBL601202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mitogen-activated protein kinase 1 (Mus musculus (Mouse)) | BDBM203329 (US9242981, 61) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Activated ERK2 activity was also determined in the IMAP assay format using the procedure outlined above. 1 μl of 25× compound was added to 14 &#... | US Patent US9242981 (2016) BindingDB Entry DOI: 10.7270/Q2GM864G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM50495918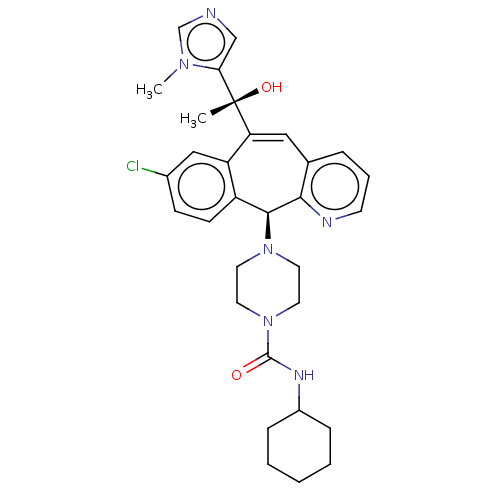 (CHEMBL3115265) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of FTase (unknown origin) assessed as transfer of [H3]farnesyl from [H3]farnesyl pyrophosphate to trichloroacetic acid-precipitable HaRas-... | Bioorg Med Chem Lett 24: 1228-31 (2014) Article DOI: 10.1016/j.bmcl.2013.12.046 BindingDB Entry DOI: 10.7270/Q26H4MCJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50042815 (CHEMBL332228 | N-[2-(2-Amino-thiazol-4-yl)-1-(1-cy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company Curated by ChEMBL | Assay Description Inhibitory concentration against renin monkey plasma | J Med Chem 36: 3809-20 (1994) BindingDB Entry DOI: 10.7270/Q24T6HFF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Rattus norvegicus (rat)) | BDBM50036928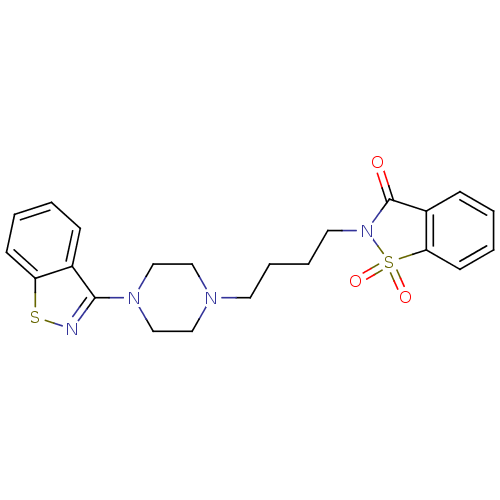 (2-[4-(4-Benzo[d]isothiazol-3-yl-piperazin-1-yl)-bu...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Burroughs Wellcome Company Curated by ChEMBL | Assay Description In vitro binding affinity was measured on serotonergic 5-hydroxytryptamine 1A receptor by displacement of [3H]- tetralin | J Med Chem 37: 2552-63 (1994) BindingDB Entry DOI: 10.7270/Q2G15ZWF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Focal adhesion kinase 1 (Homo sapiens (Human)) | BDBM50246239 (CHEMBL487229 | N-methyl-N-(2-((2-(1-oxoisoindolin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of GST-tagged FAK (unknown origin) assessed as inhibition of poly-Glu-Tyr phosphorylation | Bioorg Med Chem Lett 18: 6071-7 (2008) Article DOI: 10.1016/j.bmcl.2008.10.030 BindingDB Entry DOI: 10.7270/Q2C8295H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2434 total ) | Next | Last >> |