 Found 756 hits with Last Name = 'cowan' and Initial = 'd'
Found 756 hits with Last Name = 'cowan' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50086433
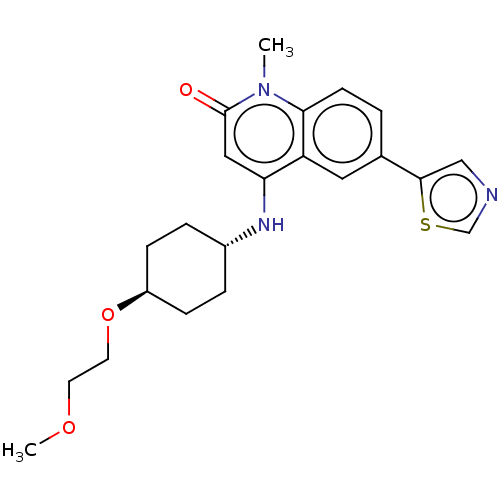
(CHEMBL3426034)Show SMILES COCCO[C@H]1CC[C@@H](CC1)Nc1cc(=O)n(C)c2ccc(cc12)-c1cncs1 |r,wU:5.4,wD:8.11,(9.09,10.61,;8.02,10,;8.02,8.46,;6.68,7.69,;6.68,6.15,;5.34,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;2.68,5.39,;4.01,6.15,;1.33,3.08,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;1.33,-2.77,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.01,1.54,;-5.4,.91,;-6.43,2.07,;-5.65,3.39,;-4.14,3.06,)| Show InChI InChI=1S/C22H27N3O3S/c1-25-20-8-3-15(21-13-23-14-29-21)11-18(20)19(12-22(25)26)24-16-4-6-17(7-5-16)28-10-9-27-2/h3,8,11-14,16-17,24H,4-7,9-10H2,1-2H3/t16-,17- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis |
J Med Chem 58: 3548-71 (2015)
Article DOI: 10.1021/jm502009h
BindingDB Entry DOI: 10.7270/Q2NV9M00 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50086438

(CHEMBL3426039)Show SMILES CO[C@H]1CC[C@@H](CC1)Nc1cc(=O)n(C)c2c(C)cc(cc12)-c1cncs1 |r,wU:2.1,wD:5.8,(6.69,7.38,;6.68,6.15,;5.35,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;2.68,5.39,;4.02,6.16,;1.34,3.08,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.39,;1.33,-1.54,;1.33,-2.77,;,-.77,;-1.33,-1.54,;-1.33,-2.77,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.02,1.54,;-5.41,.92,;-6.43,2.07,;-5.65,3.4,;-4.15,3.07,)| Show InChI InChI=1S/C21H25N3O2S/c1-13-8-14(19-11-22-12-27-19)9-17-18(10-20(25)24(2)21(13)17)23-15-4-6-16(26-3)7-5-15/h8-12,15-16,23H,4-7H2,1-3H3/t15-,16- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis |
J Med Chem 58: 3548-71 (2015)
Article DOI: 10.1021/jm502009h
BindingDB Entry DOI: 10.7270/Q2NV9M00 |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50086434
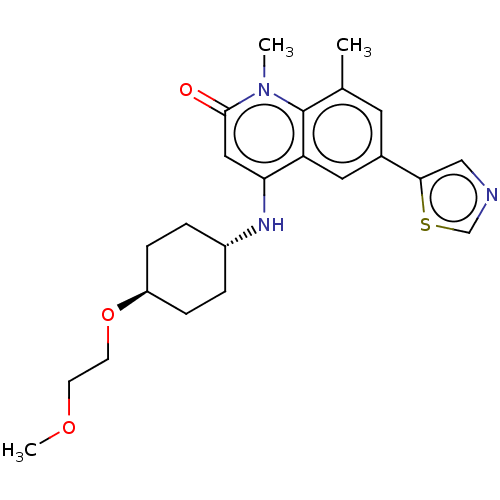
(CHEMBL3426035)Show SMILES COCCO[C@H]1CC[C@@H](CC1)Nc1cc(=O)n(C)c2c(C)cc(cc12)-c1cncs1 |r,wU:5.4,wD:8.11,(9.09,10.61,;8.02,10,;8.02,8.46,;6.68,7.69,;6.68,6.15,;5.34,5.38,;5.34,3.84,;4,3.07,;2.67,3.85,;2.68,5.39,;4.01,6.15,;1.33,3.08,;1.33,1.54,;2.66,.77,;2.66,-.77,;3.73,-1.38,;1.33,-1.54,;1.33,-2.77,;,-.77,;-1.33,-1.54,;-1.33,-2.77,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;-4.01,1.54,;-5.4,.91,;-6.43,2.07,;-5.65,3.39,;-4.14,3.06,)| Show InChI InChI=1S/C23H29N3O3S/c1-15-10-16(21-13-24-14-30-21)11-19-20(12-22(27)26(2)23(15)19)25-17-4-6-18(7-5-17)29-9-8-28-3/h10-14,17-18,25H,4-9H2,1-3H3/t17-,18- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis |
J Med Chem 58: 3548-71 (2015)
Article DOI: 10.1021/jm502009h
BindingDB Entry DOI: 10.7270/Q2NV9M00 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063920
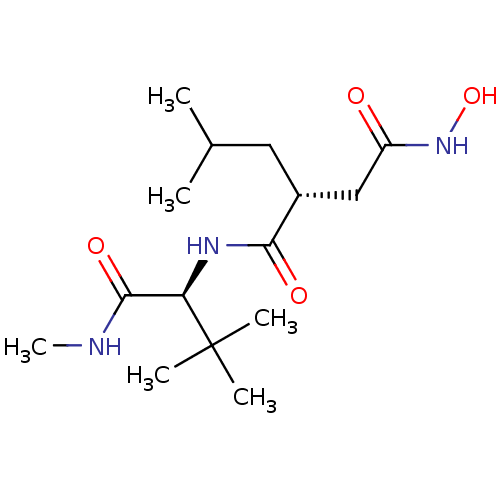
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-10(8-11(19)18-22)13(20)17-12(14(21)16-6)15(3,4)5/h9-10,12,22H,7-8H2,1-6H3,(H,16,21)(H,17,20)(H,18,19)/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063920

((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-10(8-11(19)18-22)13(20)17-12(14(21)16-6)15(3,4)5/h9-10,12,22H,7-8H2,1-6H3,(H,16,21)(H,17,20)(H,18,19)/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103099
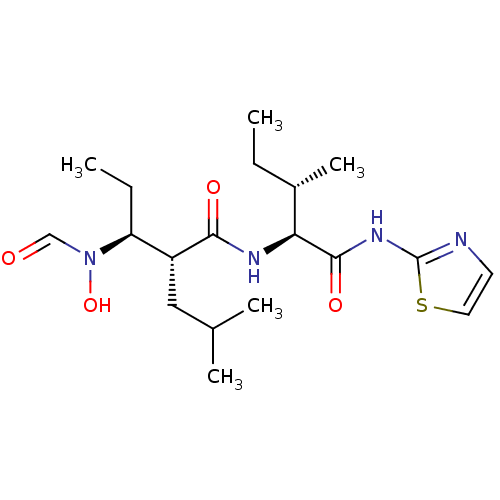
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
ADP-ribosyl cyclase/cyclic ADP-ribose hydrolase 1
(Homo sapiens (Human)) | BDBM50086425
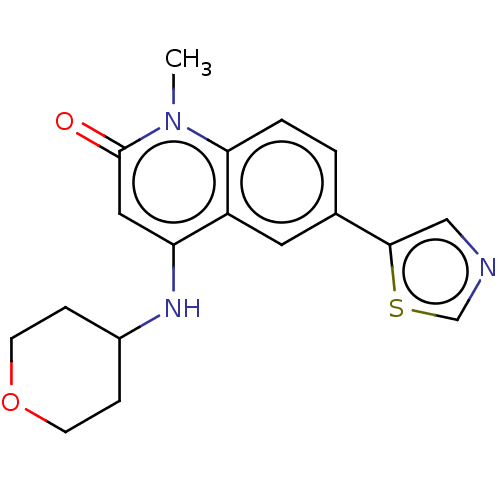
(CHEMBL3426030)Show InChI InChI=1S/C18H19N3O2S/c1-21-16-3-2-12(17-10-19-11-24-17)8-14(16)15(9-18(21)22)20-13-4-6-23-7-5-13/h2-3,8-11,13,20H,4-7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of wild type fully glycosylated human recombinant CD38-catalyzed NAD hydrolysis |
J Med Chem 58: 3548-71 (2015)
Article DOI: 10.1021/jm502009h
BindingDB Entry DOI: 10.7270/Q2NV9M00 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103097
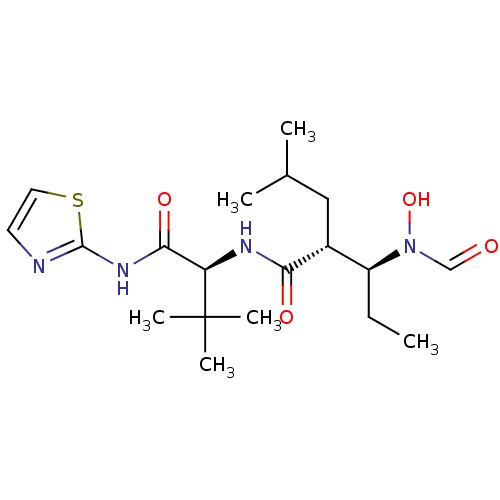
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C19H32N4O4S/c1-7-14(23(27)11-24)13(10-12(2)3)16(25)21-15(19(4,5)6)17(26)22-18-20-8-9-28-18/h8-9,11-15,27H,7,10H2,1-6H3,(H,21,25)(H,20,22,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103097

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C19H32N4O4S/c1-7-14(23(27)11-24)13(10-12(2)3)16(25)21-15(19(4,5)6)17(26)22-18-20-8-9-28-18/h8-9,11-15,27H,7,10H2,1-6H3,(H,21,25)(H,20,22,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103096
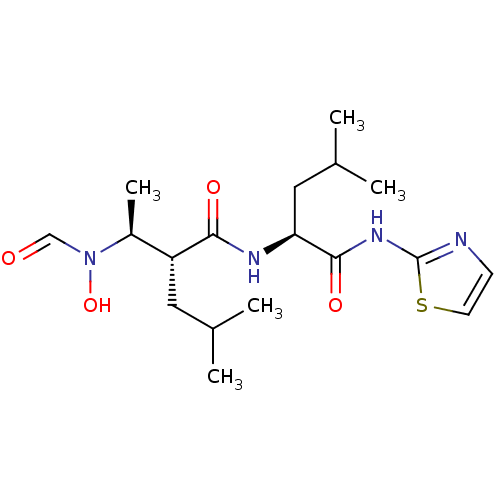
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-11(2)8-14(13(5)22(26)10-23)16(24)20-15(9-12(3)4)17(25)21-18-19-6-7-27-18/h6-7,10-15,26H,8-9H2,1-5H3,(H,20,24)(H,19,21,25)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103102
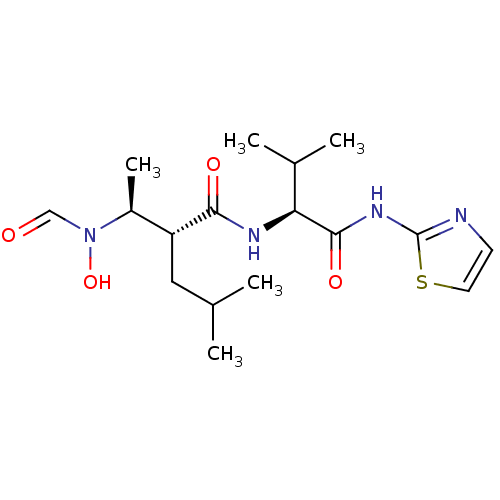
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@@H](C(C)C)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H28N4O4S/c1-10(2)8-13(12(5)21(25)9-22)15(23)19-14(11(3)4)16(24)20-17-18-6-7-26-17/h6-7,9-14,25H,8H2,1-5H3,(H,19,23)(H,18,20,24)/t12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103092
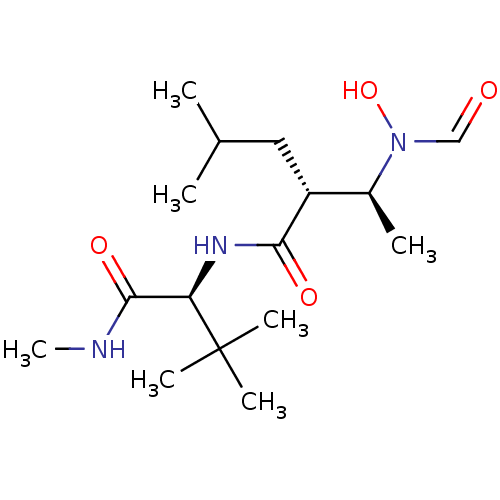
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-10(2)8-12(11(3)19(23)9-20)14(21)18-13(15(22)17-7)16(4,5)6/h9-13,23H,8H2,1-7H3,(H,17,22)(H,18,21)/t11-,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103093
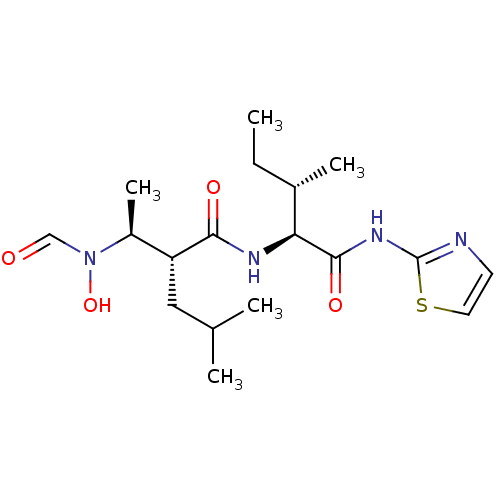
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-6-12(4)15(17(25)21-18-19-7-8-27-18)20-16(24)14(9-11(2)3)13(5)22(26)10-23/h7-8,10-15,26H,6,9H2,1-5H3,(H,20,24)(H,19,21,25)/t12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103098
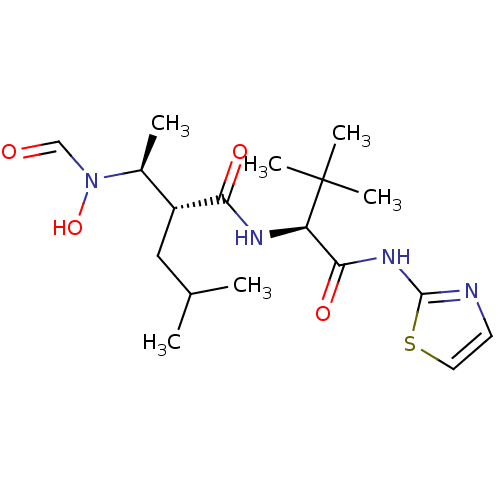
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C Show InChI InChI=1S/C18H30N4O4S/c1-11(2)9-13(12(3)22(26)10-23)15(24)20-14(18(4,5)6)16(25)21-17-19-7-8-27-17/h7-8,10-14,26H,9H2,1-6H3,(H,20,24)(H,19,21,25)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103092

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(C)(C)C Show InChI InChI=1S/C16H31N3O4/c1-10(2)8-12(11(3)19(23)9-20)14(21)18-13(15(22)17-7)16(4,5)6/h9-13,23H,8H2,1-7H3,(H,17,22)(H,18,21)/t11-,12+,13+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103099

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50029954
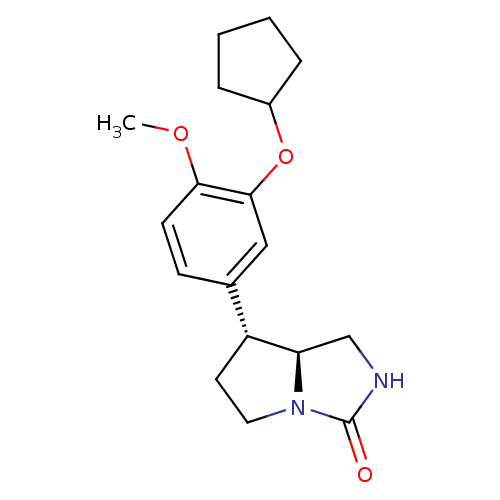
((7R,7aS)-7-(3-Cyclopentyloxy-4-methoxy-phenyl)-hex...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@H]1CCN2[C@@H]1CNC2=O Show InChI InChI=1S/C18H24N2O3/c1-22-16-7-6-12(10-17(16)23-13-4-2-3-5-13)14-8-9-20-15(14)11-19-18(20)21/h6-7,10,13-15H,2-5,8-9,11H2,1H3,(H,19,21)/t14-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 4B |
J Med Chem 38: 4848-54 (1996)
BindingDB Entry DOI: 10.7270/Q2DZ079X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103102

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@@H](C(C)C)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H28N4O4S/c1-10(2)8-13(12(5)21(25)9-22)15(23)19-14(11(3)4)16(24)20-17-18-6-7-26-17/h6-7,9-14,25H,8H2,1-5H3,(H,19,23)(H,18,20,24)/t12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103098

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C Show InChI InChI=1S/C18H30N4O4S/c1-11(2)9-13(12(3)22(26)10-23)15(24)20-14(18(4,5)6)16(25)21-17-19-7-8-27-17/h7-8,10-14,26H,9H2,1-6H3,(H,20,24)(H,19,21,25)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103097

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C19H32N4O4S/c1-7-14(23(27)11-24)13(10-12(2)3)16(25)21-15(19(4,5)6)17(26)22-18-20-8-9-28-18/h8-9,11-15,27H,7,10H2,1-6H3,(H,21,25)(H,20,22,26)/t13-,14+,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103093

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-6-12(4)15(17(25)21-18-19-7-8-27-18)20-16(24)14(9-11(2)3)13(5)22(26)10-23/h7-8,10-15,26H,6,9H2,1-5H3,(H,20,24)(H,19,21,25)/t12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103095
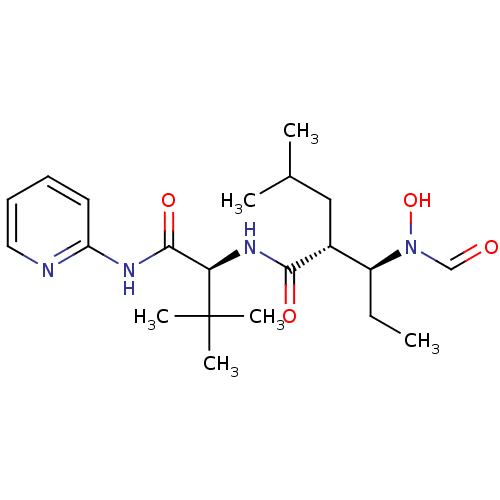
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1ccccn1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C21H34N4O4/c1-7-16(25(29)13-26)15(12-14(2)3)19(27)24-18(21(4,5)6)20(28)23-17-10-8-9-11-22-17/h8-11,13-16,18,29H,7,12H2,1-6H3,(H,24,27)(H,22,23,28)/t15-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285059
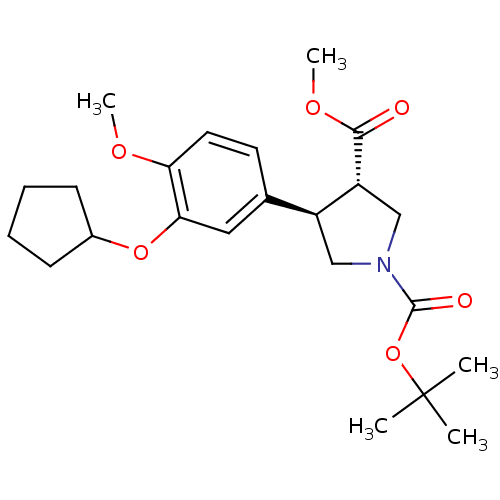
(1-(tert-butyl) 3-methyl 4-(3-cyclopentyloxy-4-meth...)Show SMILES COC(=O)[C@@H]1CN(C[C@H]1c1ccc(OC)c(OC2CCCC2)c1)C(=O)OC(C)(C)C Show InChI InChI=1S/C23H33NO6/c1-23(2,3)30-22(26)24-13-17(18(14-24)21(25)28-5)15-10-11-19(27-4)20(12-15)29-16-8-6-7-9-16/h10-12,16-18H,6-9,13-14H2,1-5H3/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470745

(CHEMBL286840)Show SMILES COc1ccc(cc1OC1CCCC1)C1CCN(C1)C(=O)OC(C)(C)C Show InChI InChI=1S/C21H31NO4/c1-21(2,3)26-20(23)22-12-11-16(14-22)15-9-10-18(24-4)19(13-15)25-17-7-5-6-8-17/h9-10,13,16-17H,5-8,11-12,14H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50048248
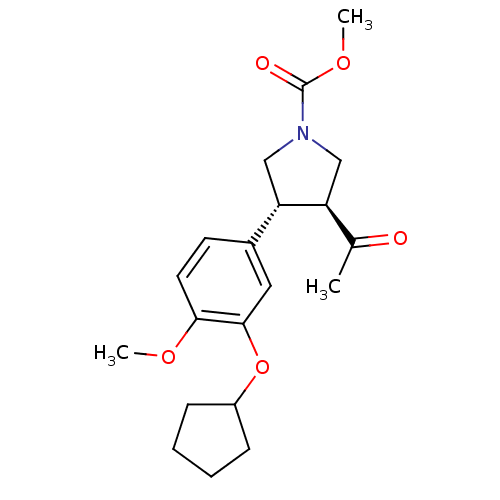
((3S,4R)-3-Acetyl-4-(3-cyclopentyloxy-4-methoxy-phe...)Show SMILES COC(=O)N1C[C@H]([C@@H](C1)c1ccc(OC)c(OC2CCCC2)c1)C(C)=O Show InChI InChI=1S/C20H27NO5/c1-13(22)16-11-21(20(23)25-3)12-17(16)14-8-9-18(24-2)19(10-14)26-15-6-4-5-7-15/h8-10,15-17H,4-7,11-12H2,1-3H3/t16-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103101
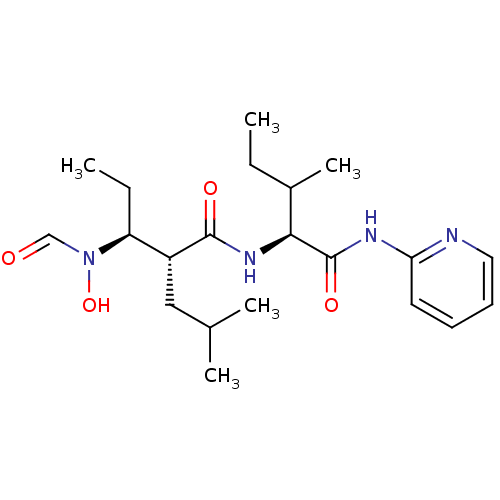
((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1ccccn1 Show InChI InChI=1S/C21H34N4O4/c1-6-15(5)19(21(28)23-18-10-8-9-11-22-18)24-20(27)16(12-14(3)4)17(7-2)25(29)13-26/h8-11,13-17,19,29H,6-7,12H2,1-5H3,(H,24,27)(H,22,23,28)/t15?,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103100
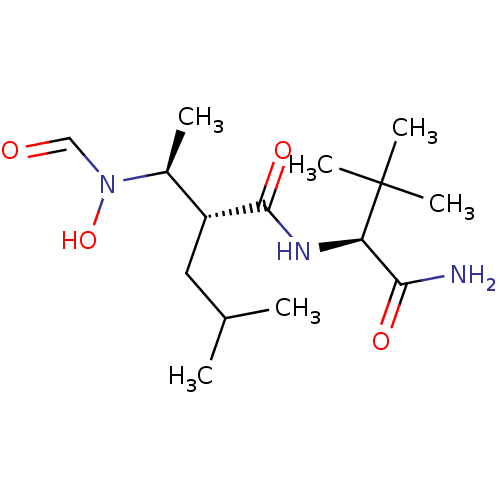
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(N)=O)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-11(10(3)18(22)8-19)14(21)17-12(13(16)20)15(4,5)6/h8-12,22H,7H2,1-6H3,(H2,16,20)(H,17,21)/t10-,11+,12+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470752
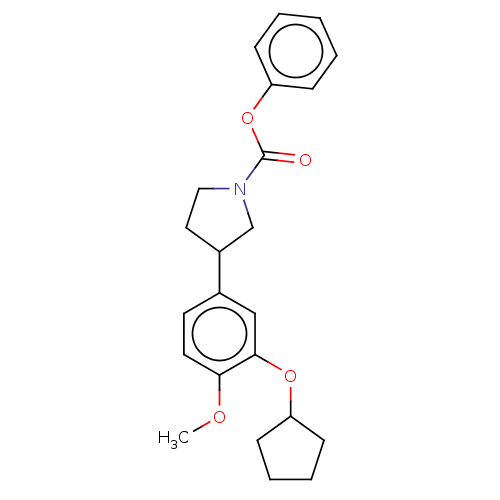
(CHEMBL38328)Show SMILES COc1ccc(cc1OC1CCCC1)C1CCN(C1)C(=O)Oc1ccccc1 Show InChI InChI=1S/C23H27NO4/c1-26-21-12-11-17(15-22(21)27-19-9-5-6-10-19)18-13-14-24(16-18)23(25)28-20-7-3-2-4-8-20/h2-4,7-8,11-12,15,18-19H,5-6,9-10,13-14,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50103098

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@H](C(=O)Nc1nccs1)C(C)(C)C Show InChI InChI=1S/C18H30N4O4S/c1-11(2)9-13(12(3)22(26)10-23)15(24)20-14(18(4,5)6)16(25)21-17-19-7-8-27-17/h7-8,10-14,26H,9H2,1-6H3,(H,20,24)(H,19,21,25)/t12-,13+,14+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-9 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103102

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@@H](C(C)C)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H28N4O4S/c1-10(2)8-13(12(5)21(25)9-22)15(23)19-14(11(3)4)16(24)20-17-18-6-7-26-17/h6-7,9-14,25H,8H2,1-5H3,(H,19,23)(H,18,20,24)/t12-,13+,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50103101

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1ccccn1 Show InChI InChI=1S/C21H34N4O4/c1-6-15(5)19(21(28)23-18-10-8-9-11-22-18)24-20(27)16(12-14(3)4)17(7-2)25(29)13-26/h8-11,13-17,19,29H,6-7,12H2,1-5H3,(H,24,27)(H,22,23,28)/t15?,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 (MMP3) |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285045
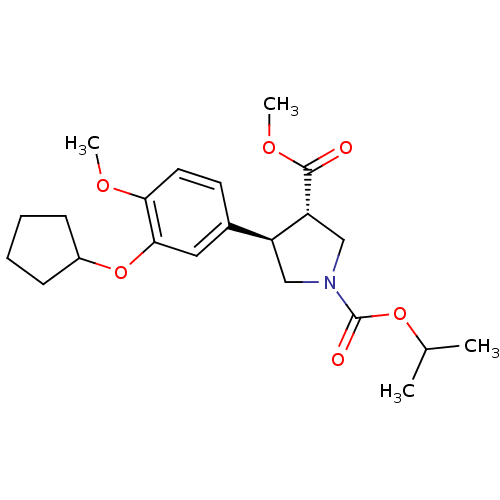
(1-isopropyl 3-methyl 4-(3-cyclopentyloxy-4-methoxy...)Show SMILES COC(=O)[C@@H]1CN(C[C@H]1c1ccc(OC)c(OC2CCCC2)c1)C(=O)OC(C)C Show InChI InChI=1S/C22H31NO6/c1-14(2)28-22(25)23-12-17(18(13-23)21(24)27-4)15-9-10-19(26-3)20(11-15)29-16-7-5-6-8-16/h9-11,14,16-18H,5-8,12-13H2,1-4H3/t17-,18+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103093

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-6-12(4)15(17(25)21-18-19-7-8-27-18)20-16(24)14(9-11(2)3)13(5)22(26)10-23/h7-8,10-15,26H,6,9H2,1-5H3,(H,20,24)(H,19,21,25)/t12-,13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103099

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50103099

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-6-13(5)16(18(26)22-19-20-8-9-28-19)21-17(25)14(10-12(3)4)15(7-2)23(27)11-24/h8-9,11-16,27H,6-7,10H2,1-5H3,(H,21,25)(H,20,22,26)/t13-,14+,15-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloprotease-3 (MMP3) |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470756
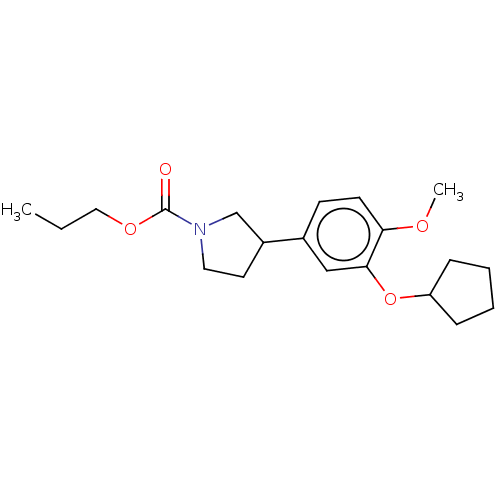
(CHEMBL37453)Show InChI InChI=1S/C20H29NO4/c1-3-12-24-20(22)21-11-10-16(14-21)15-8-9-18(23-2)19(13-15)25-17-6-4-5-7-17/h8-9,13,16-17H,3-7,10-12,14H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103095

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CC[C@@H]([C@@H](CC(C)C)C(=O)N[C@H](C(=O)Nc1ccccn1)C(C)(C)C)N(O)C=O Show InChI InChI=1S/C21H34N4O4/c1-7-16(25(29)13-26)15(12-14(2)3)19(27)24-18(21(4,5)6)20(28)23-17-10-8-9-11-22-17/h8-11,13-16,18,29H,7,12H2,1-6H3,(H,24,27)(H,22,23,28)/t15-,16+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285042
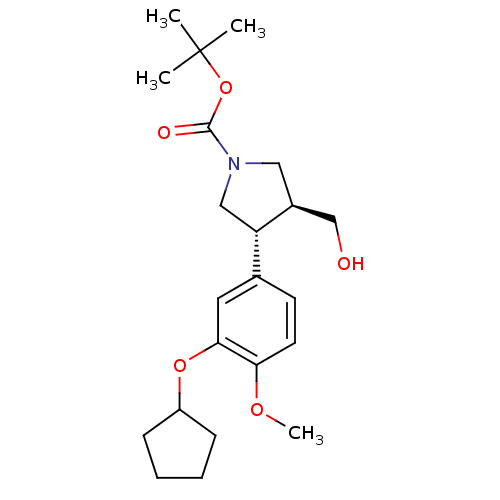
(CHEMBL302632 | tert-butyl 3-(3-cyclopentyloxy-4-me...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@@H]1CN(C[C@H]1CO)C(=O)OC(C)(C)C Show InChI InChI=1S/C22H33NO5/c1-22(2,3)28-21(25)23-12-16(14-24)18(13-23)15-9-10-19(26-4)20(11-15)27-17-7-5-6-8-17/h9-11,16-18,24H,5-8,12-14H2,1-4H3/t16-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285038
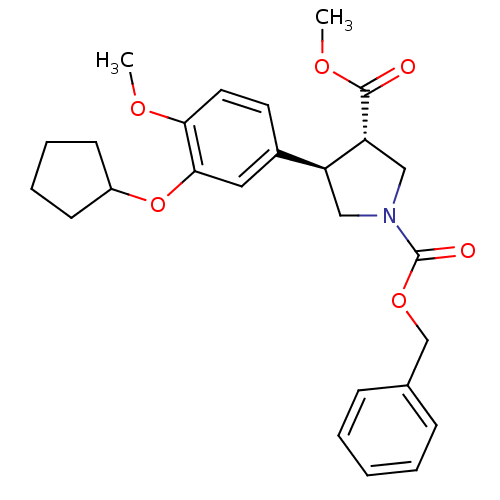
(1-benzyl 3-methyl 4-(3-cyclopentyloxy-4-methoxyphe...)Show SMILES COC(=O)[C@@H]1CN(C[C@H]1c1ccc(OC)c(OC2CCCC2)c1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H31NO6/c1-30-23-13-12-19(14-24(23)33-20-10-6-7-11-20)21-15-27(16-22(21)25(28)31-2)26(29)32-17-18-8-4-3-5-9-18/h3-5,8-9,12-14,20-22H,6-7,10-11,15-17H2,1-2H3/t21-,22+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50103101

((2R,3S)-3-(Formyl-hydroxy-amino)-2-isobutyl-pentan...)Show SMILES CCC(C)[C@H](NC(=O)[C@H](CC(C)C)[C@H](CC)N(O)C=O)C(=O)Nc1ccccn1 Show InChI InChI=1S/C21H34N4O4/c1-6-15(5)19(21(28)23-18-10-8-9-11-22-18)24-20(27)16(12-14(3)4)17(7-2)25(29)13-26/h8-11,13-17,19,29H,6-7,12H2,1-5H3,(H,24,27)(H,22,23,28)/t15?,16-,17+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloproteinase-1 |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103096

((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)[C@H](C)N(O)C=O)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H30N4O4S/c1-11(2)8-14(13(5)22(26)10-23)16(24)20-15(9-12(3)4)17(25)21-18-19-6-7-27-18/h6-7,10-15,26H,8-9H2,1-5H3,(H,20,24)(H,19,21,25)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470750
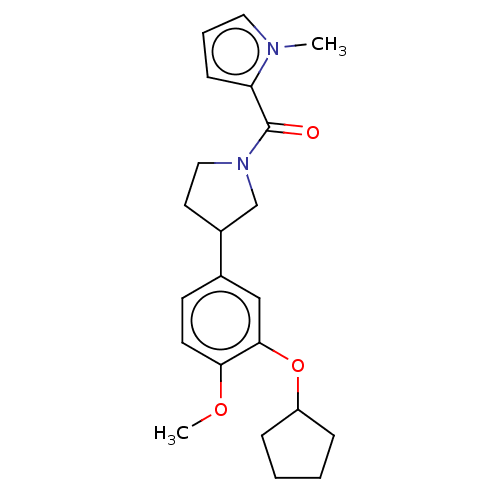
(CHEMBL286291)Show InChI InChI=1S/C22H28N2O3/c1-23-12-5-8-19(23)22(25)24-13-11-17(15-24)16-9-10-20(26-2)21(14-16)27-18-6-3-4-7-18/h5,8-10,12,14,17-18H,3-4,6-7,11,13,15H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470731
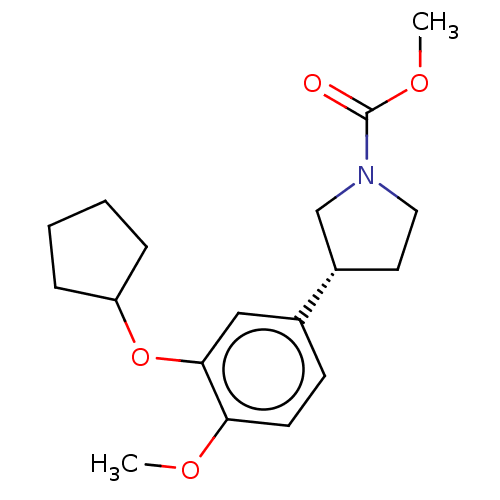
(CHEMBL146946)Show SMILES COC(=O)N1CC[C@@H](C1)c1ccc(OC)c(OC2CCCC2)c1 Show InChI InChI=1S/C18H25NO4/c1-21-16-8-7-13(11-17(16)23-15-5-3-4-6-15)14-9-10-19(12-14)18(20)22-2/h7-8,11,14-15H,3-6,9-10,12H2,1-2H3/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470736
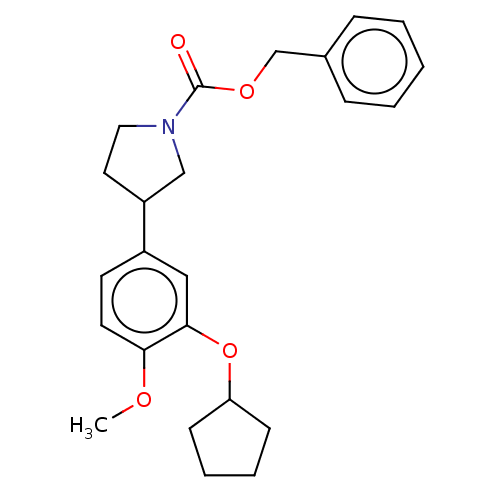
(CHEMBL37029)Show SMILES COc1ccc(cc1OC1CCCC1)C1CCN(C1)C(=O)OCc1ccccc1 Show InChI InChI=1S/C24H29NO4/c1-27-22-12-11-19(15-23(22)29-21-9-5-6-10-21)20-13-14-25(16-20)24(26)28-17-18-7-3-2-4-8-18/h2-4,7-8,11-12,15,20-21H,5-6,9-10,13-14,16-17H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50285050
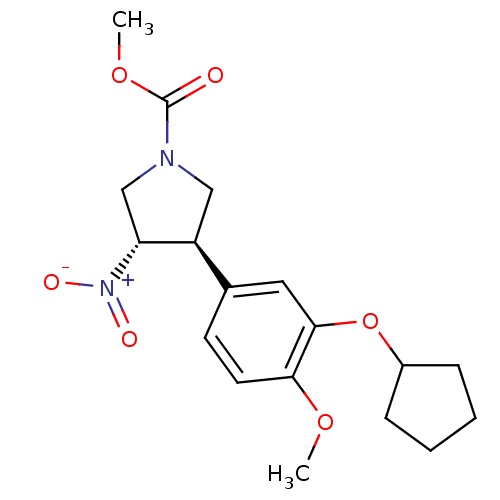
(CHEMBL59386 | methyl 3-(3-cyclopentyloxy-4-methoxy...)Show SMILES COC(=O)N1C[C@H]([C@@H](C1)[N+]([O-])=O)c1ccc(OC)c(OC2CCCC2)c1 Show InChI InChI=1S/C18H24N2O6/c1-24-16-8-7-12(9-17(16)26-13-5-3-4-6-13)14-10-19(18(21)25-2)11-15(14)20(22)23/h7-9,13-15H,3-6,10-11H2,1-2H3/t14-,15+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit the catalytic activity of human Phosphodiesterase 4B (PDE IVB) |
Bioorg Med Chem Lett 5: 1977-1982 (1995)
Article DOI: 10.1016/0960-894X(95)00336-R
BindingDB Entry DOI: 10.7270/Q2PN95MX |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50029955
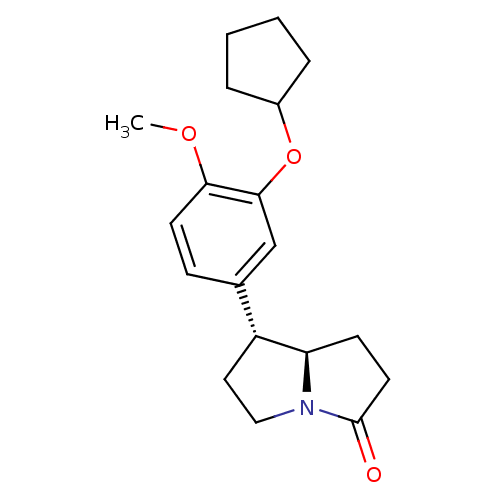
((7R,7aR)-7-(3-Cyclopentyloxy-4-methoxy-phenyl)-hex...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@H]1CCN2[C@@H]1CCC2=O Show InChI InChI=1S/C19H25NO3/c1-22-17-8-6-13(12-18(17)23-14-4-2-3-5-14)15-10-11-20-16(15)7-9-19(20)21/h6,8,12,14-16H,2-5,7,9-11H2,1H3/t15-,16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 4B |
J Med Chem 38: 4848-54 (1996)
BindingDB Entry DOI: 10.7270/Q2DZ079X |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50103094
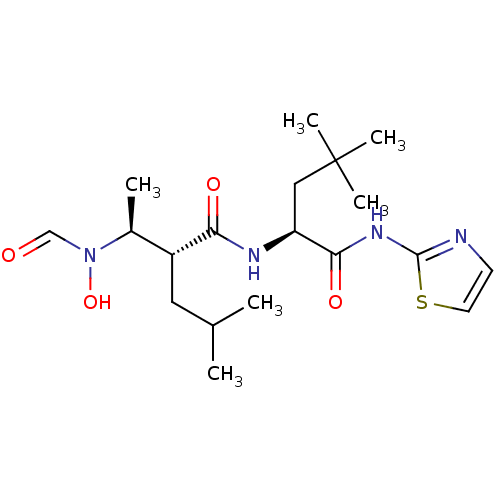
((R)-2-[(S)-1-(Formyl-hydroxy-amino)-ethyl]-4-methy...)Show SMILES CC(C)C[C@H]([C@H](C)N(O)C=O)C(=O)N[C@@H](CC(C)(C)C)C(=O)Nc1nccs1 Show InChI InChI=1S/C19H32N4O4S/c1-12(2)9-14(13(3)23(27)11-24)16(25)21-15(10-19(4,5)6)17(26)22-18-20-7-8-28-18/h7-8,11-15,27H,9-10H2,1-6H3,(H,21,25)(H,20,22,26)/t13-,14+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development
Curated by ChEMBL
| Assay Description
Inhibitionof Tumor necrosis factor alpha converting enzyme |
Bioorg Med Chem Lett 11: 2147-51 (2001)
BindingDB Entry DOI: 10.7270/Q2QZ298V |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470740

(CHEMBL264851)Show InChI InChI=1S/C20H29NO4/c1-14(2)24-20(22)21-11-10-16(13-21)15-8-9-18(23-3)19(12-15)25-17-6-4-5-7-17/h8-9,12,14,16-17H,4-7,10-11,13H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50470754
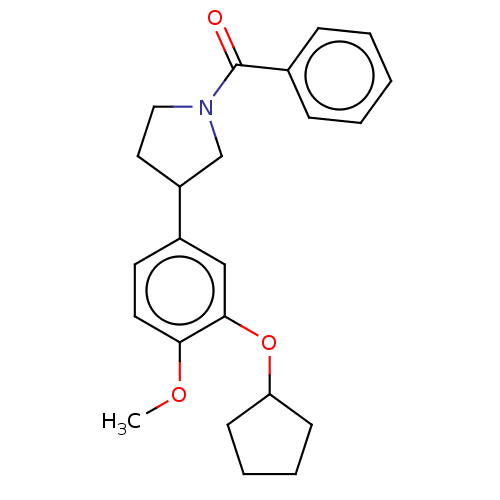
(CHEMBL288928)Show InChI InChI=1S/C23H27NO3/c1-26-21-12-11-18(15-22(21)27-20-9-5-6-10-20)19-13-14-24(16-19)23(25)17-7-3-2-4-8-17/h2-4,7-8,11-12,15,19-20H,5-6,9-10,13-14,16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of cAMP hydrolysis by the human phosphodiesterase 4B enzyme |
J Med Chem 38: 1505-10 (1995)
Article DOI: 10.1021/jm00009a011
BindingDB Entry DOI: 10.7270/Q2833VRG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4B
(Homo sapiens (Human)) | BDBM50029951
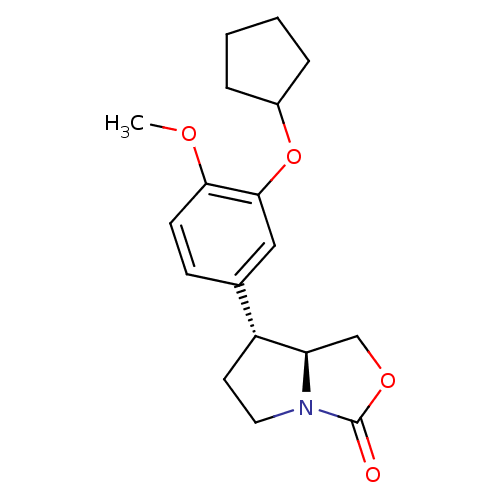
((7R,7aS)-7-(3-Cyclopentyloxy-4-methoxy-phenyl)-tet...)Show SMILES COc1ccc(cc1OC1CCCC1)[C@H]1CCN2[C@@H]1COC2=O Show InChI InChI=1S/C18H23NO4/c1-21-16-7-6-12(10-17(16)23-13-4-2-3-5-13)14-8-9-19-15(14)11-22-18(19)20/h6-7,10,13-15H,2-5,8-9,11H2,1H3/t14-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Wellcome Research
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 4B |
J Med Chem 38: 4848-54 (1996)
BindingDB Entry DOI: 10.7270/Q2DZ079X |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data