

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor X (Homo sapiens (Human)) | BDBM12545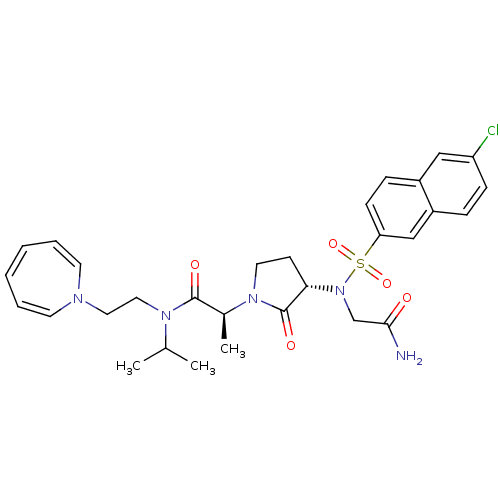 ((2S)-N-[2-(1H-azepin-1-yl)ethyl]-2-[(3S)-3-{2-[(6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12543 ((2S)-2-[(3S)-3-{2-[(6-chloronaphthalene-2-)sulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12542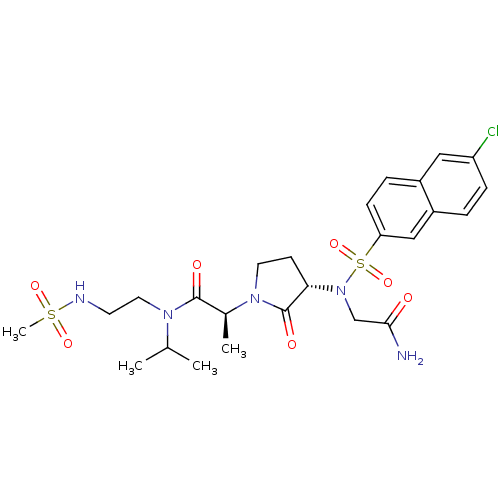 ((2S)-2-[(3S)-3-{2-[(6-chloronaphthalene-2-)sulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12541 ((2S)-N-[2-(carbamoylamino)ethyl]-2-[(3S)-3-{2-[(6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12540 ((2S)-N-(2-aminoethyl)-2-[(3S)-3-{2-[(6-chloronapht...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12539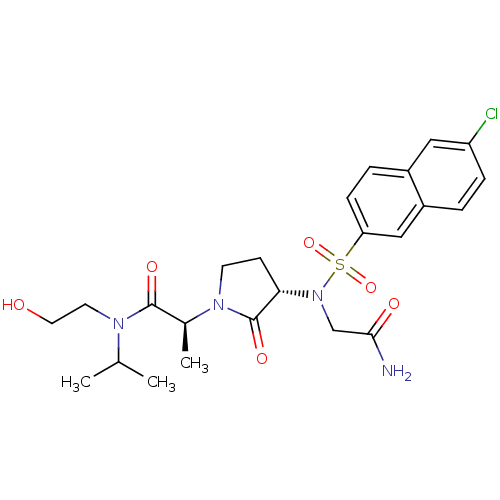 ((2S)-2-[(3S)-3-{2-[(6-chloronaphthalene-2-)sulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12528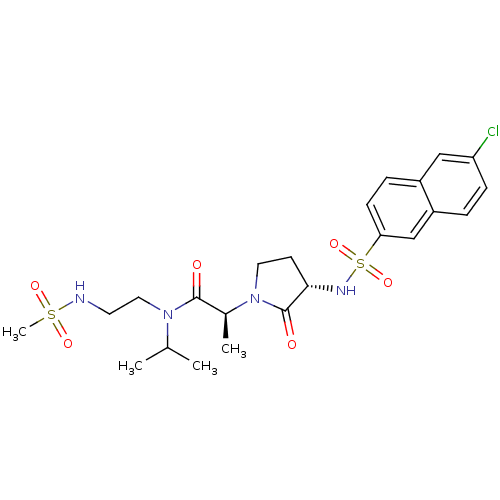 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12544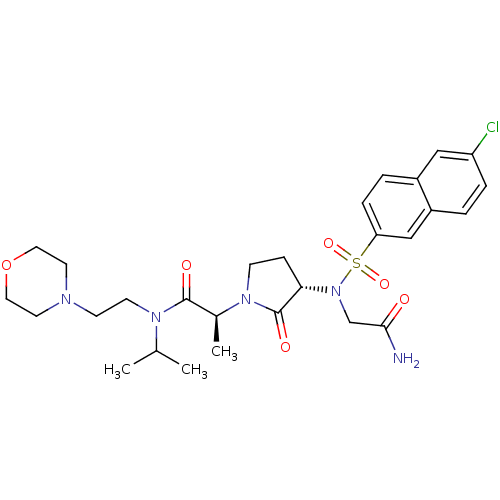 ((2S)-2-[(3S)-3-{2-[(6-chloronaphthalene-2-)sulfona...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12535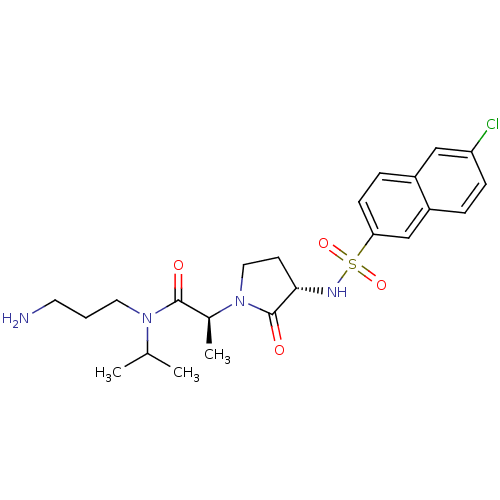 ((2S)-N-(3-aminopropyl)-2-[(3S)-3-[(6-chloronaphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12534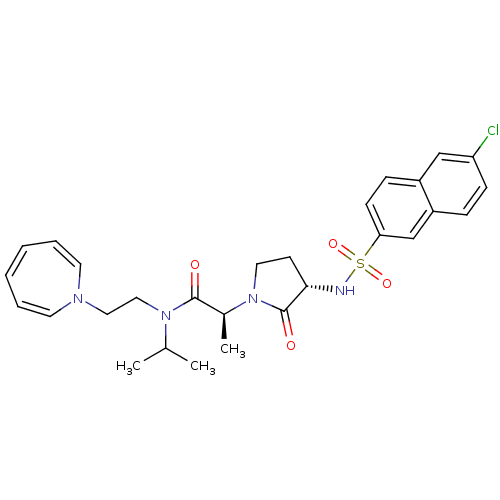 ((2S)-N-[2-(1H-azepin-1-yl)ethyl]-2-[(3S)-3-[(6-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12525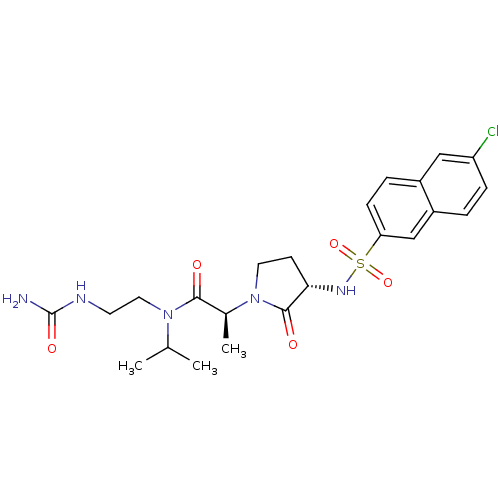 ((2S)-N-[2-(carbamoylamino)ethyl]-2-[(3S)-3-[(6-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12529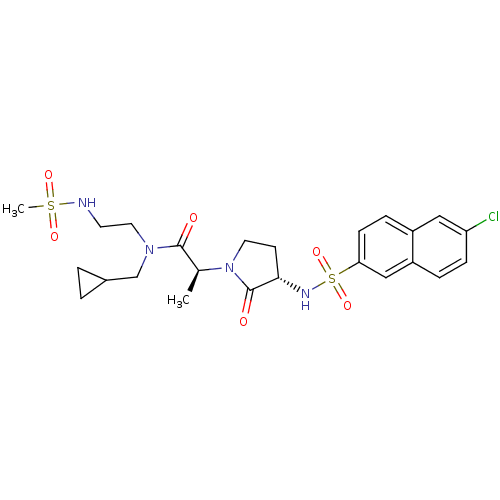 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12532 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12531 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12537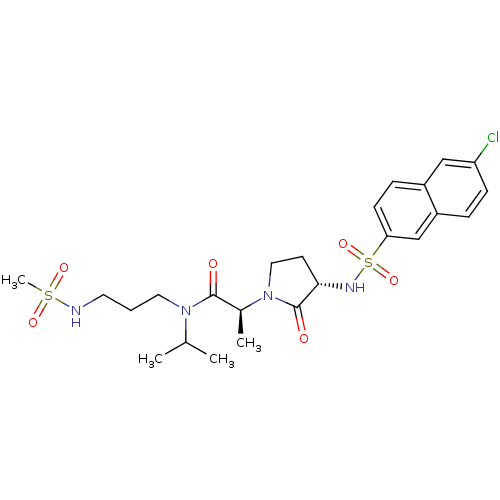 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12530 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12509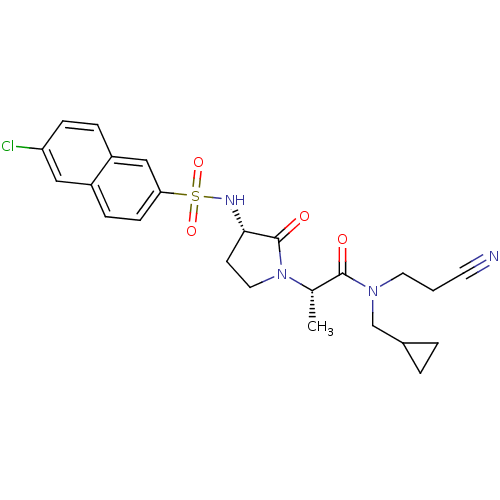 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3 | -48.6 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12522 ((2S)-N-(2-aminoethyl)-2-[(3S)-3-[(6-chloronaphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12533 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12536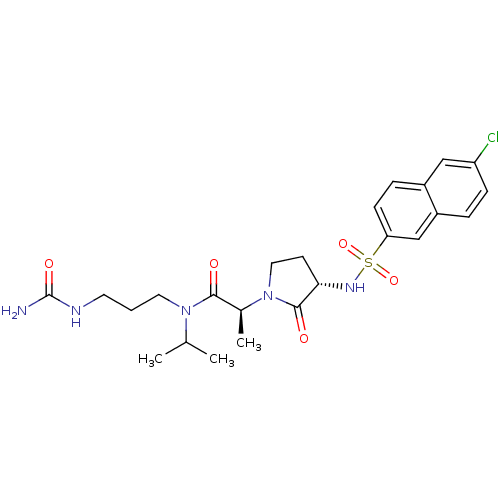 ((2S)-N-[3-(carbamoylamino)propyl]-2-[(3S)-3-[(6-ch...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12506 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5 | -47.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12538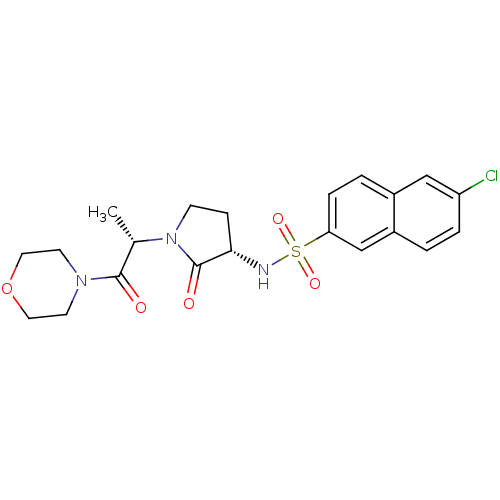 (6-chloro-N-[(3S)-1-[(2S)-1-(morpholin-4-yl)-1-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12519 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12517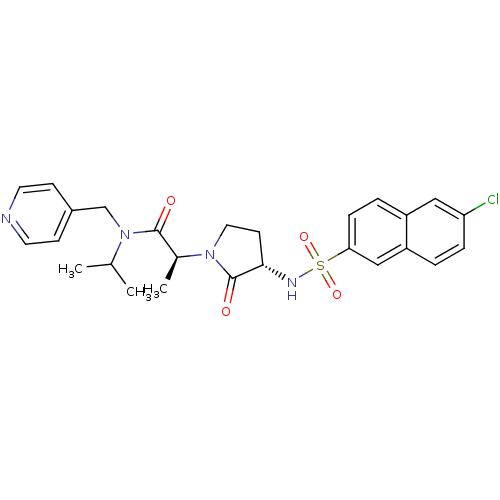 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12520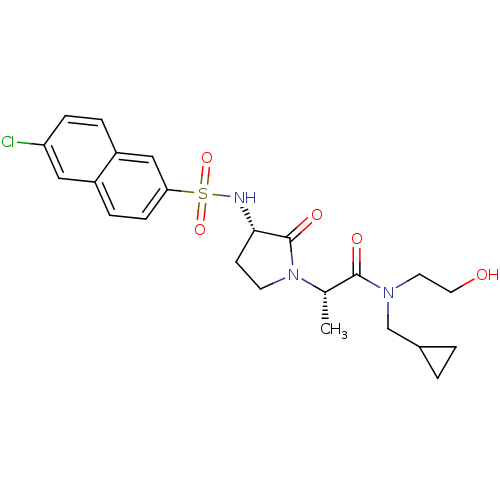 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12526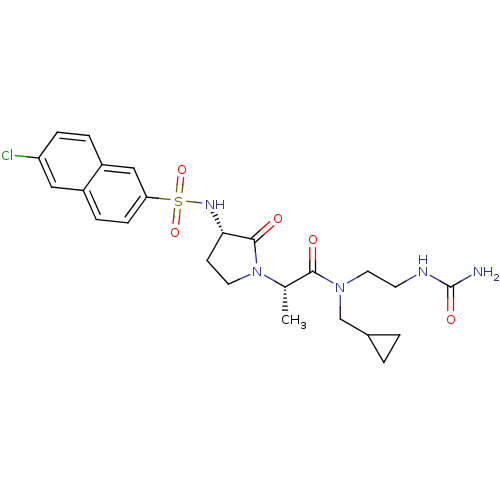 ((2S)-N-[2-(carbamoylamino)ethyl]-2-[(3S)-3-[(6-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12516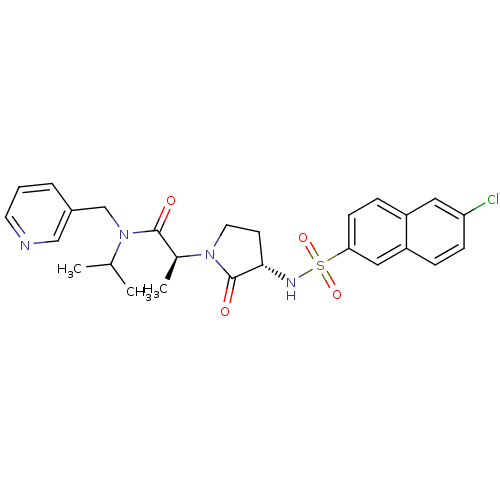 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12515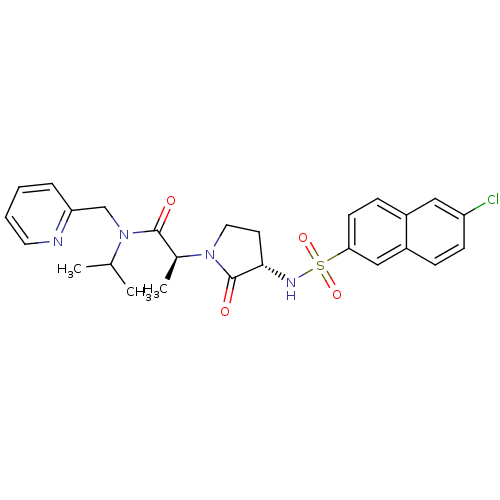 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12511 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12505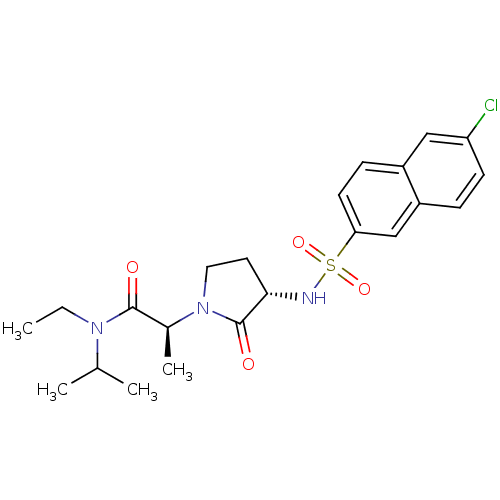 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | -45.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12524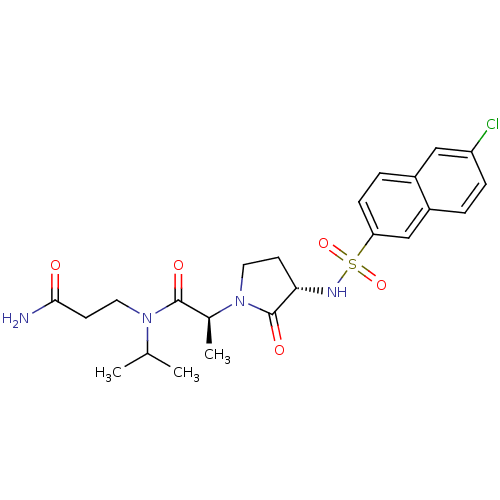 ((2S)-N-(2-carbamoylethyl)-2-[(3S)-3-[(6-chloronaph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12518 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12510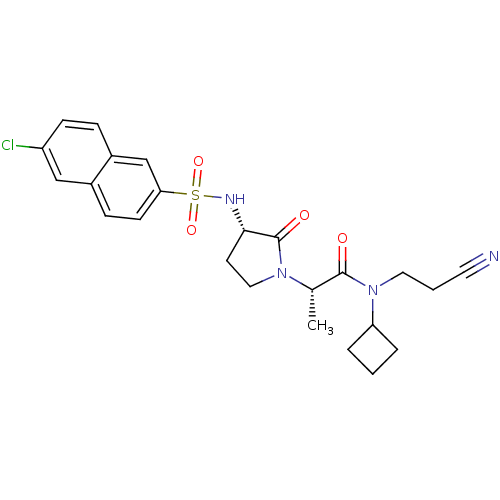 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | -44.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12523 ((2S)-N-(2-aminoethyl)-2-[(3S)-3-[(6-chloronaphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12504 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 22 | -43.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12502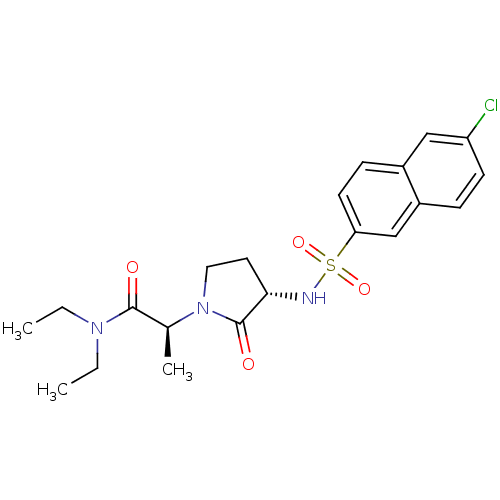 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 24 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12514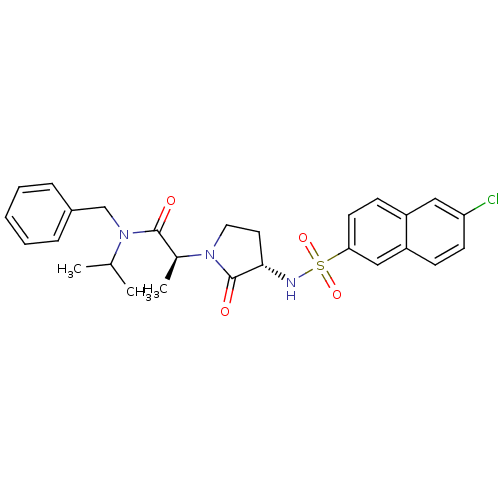 ((2S)-N-benzyl-2-[(3S)-3-[(6-chloronaphthalene-2-)s...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 24 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12513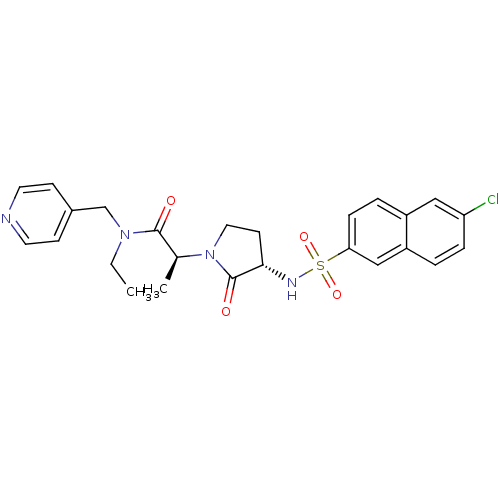 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 27 | -43.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12501 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 56 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12508 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 57 | -41.3 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12512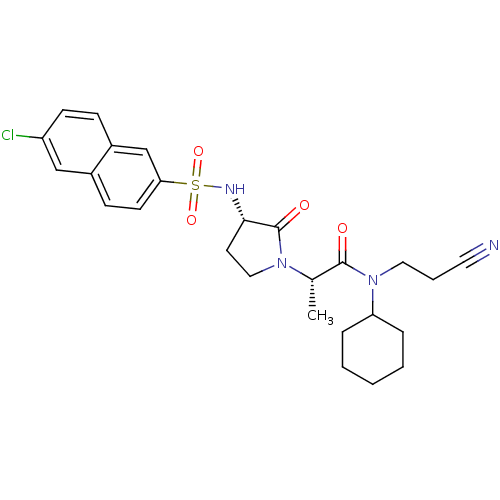 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 73 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12507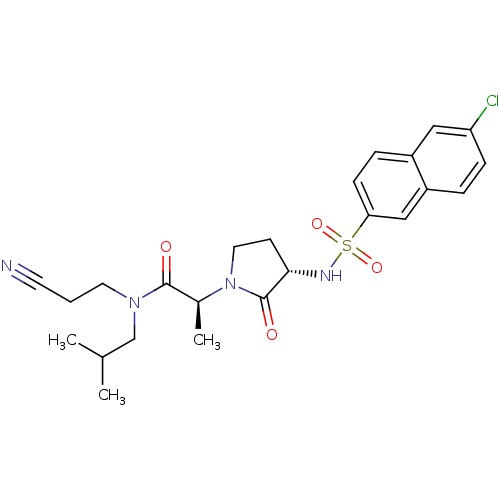 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 136 | -39.2 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12499 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 346 | -36.9 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12503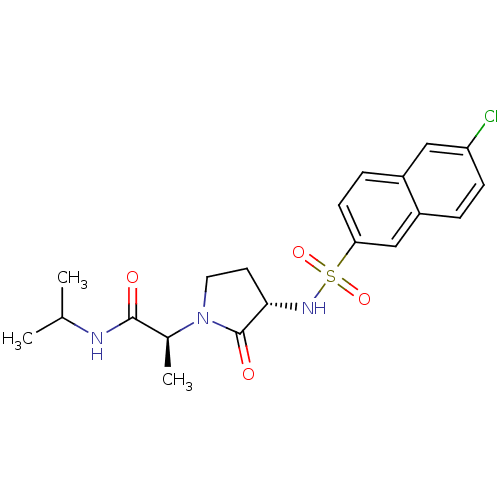 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.72E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 7.8 | 25 |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12521 ((2S)-N-(2-aminoethyl)-2-[(3S)-3-[(6-chloronaphthal...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM12527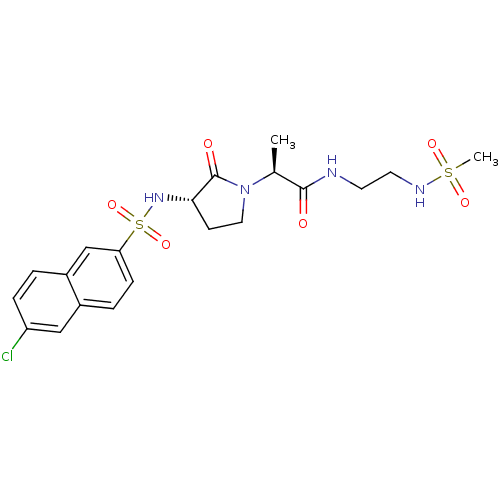 ((2S)-2-[(3S)-3-[(6-chloronaphthalene-2-)sulfonamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline | Assay Description The inhibitory effect of test compound for human fXa was determined by using the chromogenic substrate. The hydrolysis rates of chromogenic substrate... | Bioorg Med Chem Lett 16: 5953-7 (2006) Article DOI: 10.1016/j.bmcl.2006.09.001 BindingDB Entry DOI: 10.7270/Q2CF9NB8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM408717 ( N-[(3-fluoro-4-methoxypyridin-2-yl)methyl]-3-(met...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BG2SXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM528099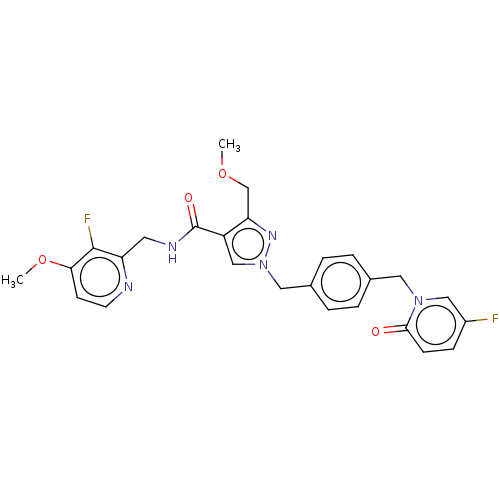 (1-({4-[(5-fluoro-2-oxopyridin-1-yl)methyl]phenyl}m...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Plasma kallikrein inhibitory activity in vitro was determined using standard published methods (see e.g. Johansen et al., Int. J. Tiss. Reac. 1986, 8... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BG2SXX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50379670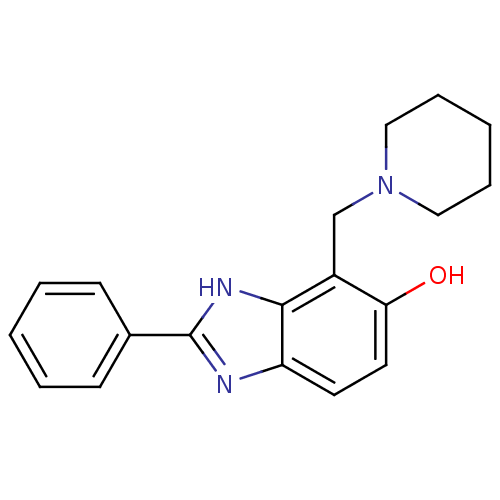 (CHEMBL2013195) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-Ro256981 from human NR2B receptor | Bioorg Med Chem Lett 22: 2620-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.108 BindingDB Entry DOI: 10.7270/Q2KD1ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamate receptor ionotropic, NMDA 2B (Homo sapiens (Human)) | BDBM50379671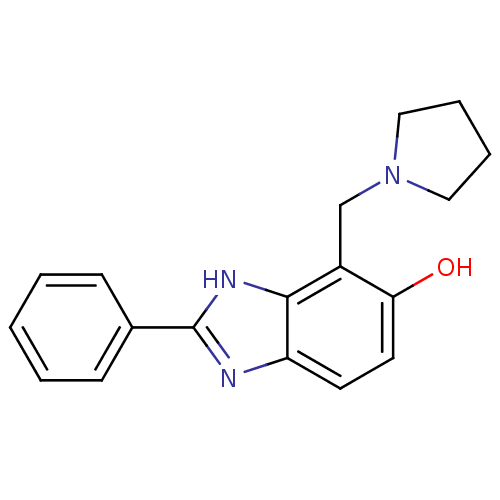 (CHEMBL2013197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline R&D Curated by ChEMBL | Assay Description Displacement of [3H]-Ro256981 from human NR2B receptor | Bioorg Med Chem Lett 22: 2620-3 (2012) Article DOI: 10.1016/j.bmcl.2012.01.108 BindingDB Entry DOI: 10.7270/Q2KD1ZX3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 195 total ) | Next | Last >> |