

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255003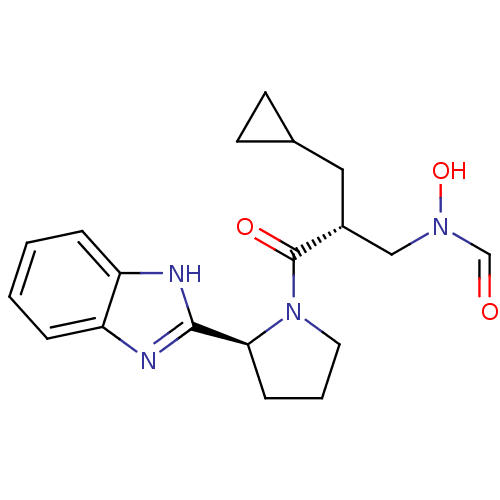 (CHEMBL519613 | N-((R)-3-((S)-2-(1H-benzo[d]imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255000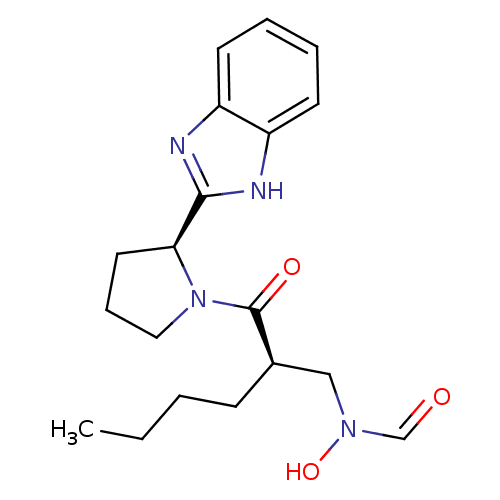 (CHEMBL443904 | N-((R)-2-((S)-2-(1H-benzo[d]imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255030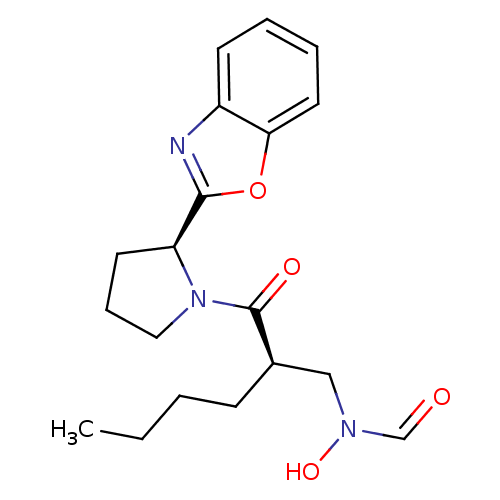 (CHEMBL506649 | N-((R)-2-((S)-2-(benzo[d]oxazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255032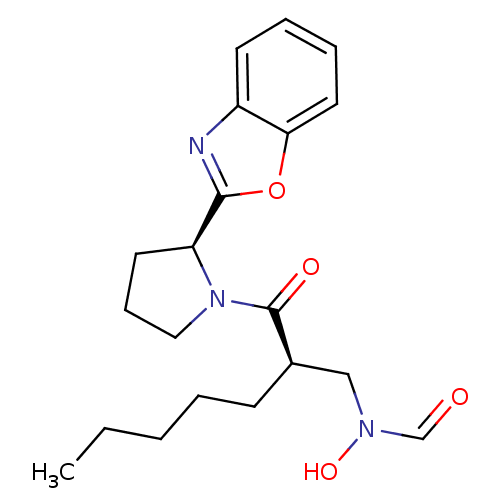 (CHEMBL465740 | N-((R)-2-((S)-2-(benzo[d]oxazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255002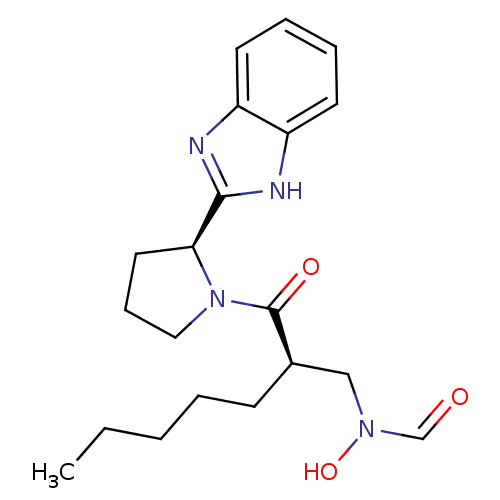 (CHEMBL482180 | N-((R)-2-((S)-2-(1H-benzo[d]imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255065 ((S)-2-((S)-2-(1H-benzo[d]imidazol-2-yl)pyrrolidine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255033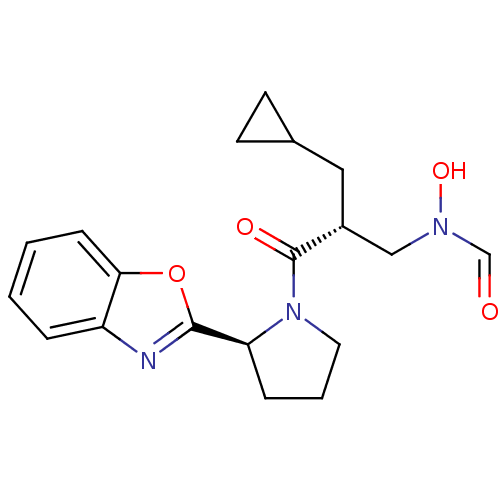 (CHEMBL463859 | N-((R)-3-((S)-2-(benzo[d]oxazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255001 (CHEMBL482179 | N-((R)-2-((S)-2-(1H-benzo[d]imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255031 (CHEMBL480220 | N-((R)-2-((S)-2-(benzo[d]oxazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255066 ((S)-2-((S)-2-(benzo[d]oxazol-2-yl)pyrrolidine-1-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255004 (CHEMBL481391 | N-((R)-3-((S)-2-(1H-benzo[d]imidazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50536185 (CHEMBL4588910 | US10870625, Compound 57) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 144 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Enzyme activity and inhibition assays were performed by monitoring the NADPH-dependent reduction of dihydrofolate catalyzed by the DHFR enzyme. The r... | US Patent US10870625 (2020) BindingDB Entry DOI: 10.7270/Q27W6G9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255034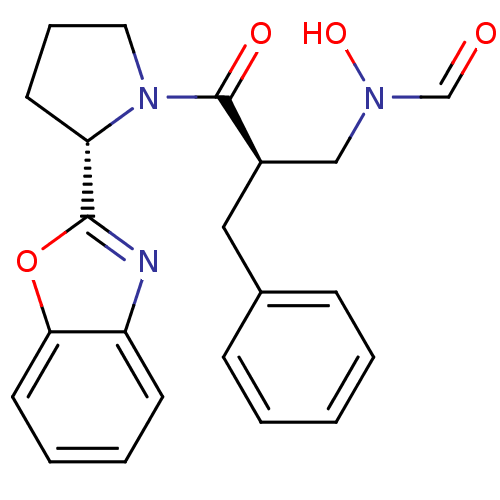 (CHEMBL518077 | N-((R)-3-((S)-2-(benzo[d]oxazol-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 161 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255063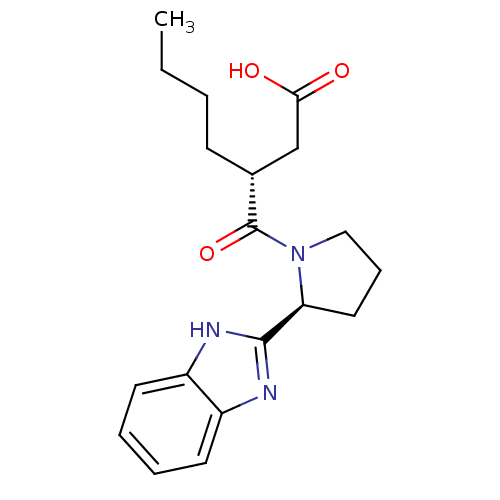 ((R)-3-((S)-2-(1H-benzo[d]imidazol-2-yl)pyrrolidine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 202 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM476664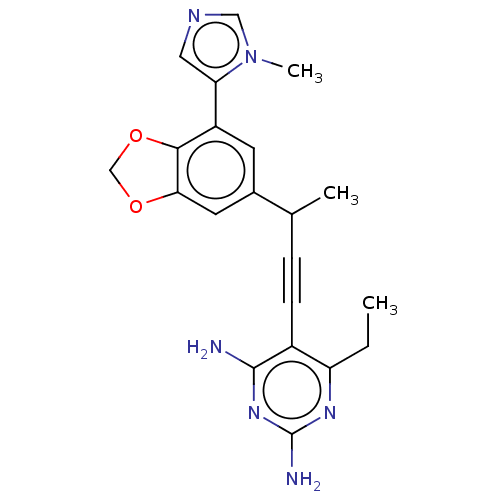 (US10870625, Compound 54) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Enzyme activity and inhibition assays were performed by monitoring the NADPH-dependent reduction of dihydrofolate catalyzed by the DHFR enzyme. The r... | US Patent US10870625 (2020) BindingDB Entry DOI: 10.7270/Q27W6G9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM476672 (US10870625, Compound 59) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 688 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Enzyme activity and inhibition assays were performed by monitoring the NADPH-dependent reduction of dihydrofolate catalyzed by the DHFR enzyme. The r... | US Patent US10870625 (2020) BindingDB Entry DOI: 10.7270/Q27W6G9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Mycobacterium tuberculosis) | BDBM50255064 ((R)-3-((S)-2-(benzo[d]oxazol-2-yl)pyrrolidine-1-ca...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 803 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Tropical Diseases Curated by ChEMBL | Assay Description Inhibition of Mycobacterium tuberculosis peptide deformylase expressed in Escherichia coli M15(pREp4) by microplate assay | Bioorg Med Chem Lett 18: 6568-72 (2008) Article DOI: 10.1016/j.bmcl.2008.10.040 BindingDB Entry DOI: 10.7270/Q2VX0GCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50190621 (CHEMBL3827532 | US10870625, Compound 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | US Patent | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Enzyme activity and inhibition assays were performed by monitoring the NADPH-dependent reduction of dihydrofolate catalyzed by the DHFR enzyme. The r... | US Patent US10870625 (2020) BindingDB Entry DOI: 10.7270/Q27W6G9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM476671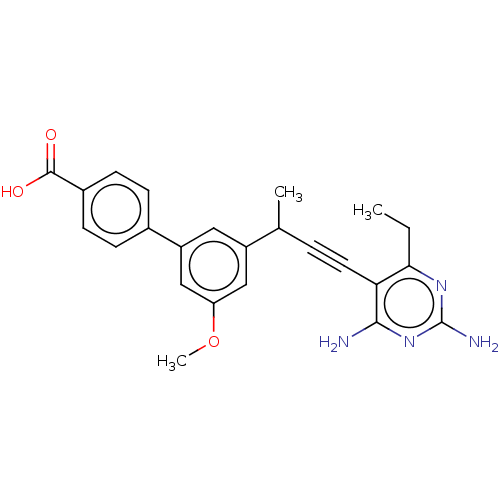 (US10870625, Compound 58) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Enzyme activity and inhibition assays were performed by monitoring the NADPH-dependent reduction of dihydrofolate catalyzed by the DHFR enzyme. The r... | US Patent US10870625 (2020) BindingDB Entry DOI: 10.7270/Q27W6G9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM476668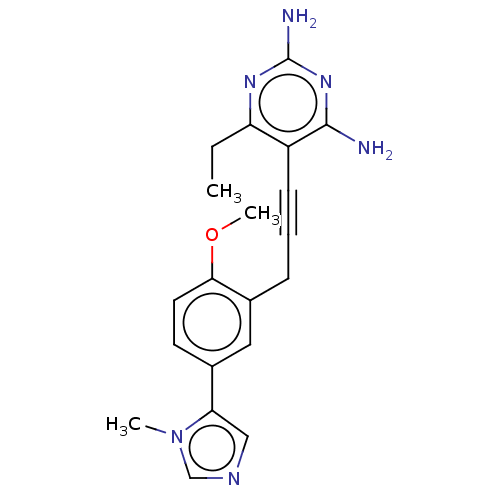 (US10870625, Compound 56) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Enzyme activity and inhibition assays were performed by monitoring the NADPH-dependent reduction of dihydrofolate catalyzed by the DHFR enzyme. The r... | US Patent US10870625 (2020) BindingDB Entry DOI: 10.7270/Q27W6G9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50190619 (CHEMBL3827086 | US10870625, Compound 14) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Enzyme activity and inhibition assays were performed by monitoring the NADPH-dependent reduction of dihydrofolate catalyzed by the DHFR enzyme. The r... | US Patent US10870625 (2020) BindingDB Entry DOI: 10.7270/Q27W6G9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM476663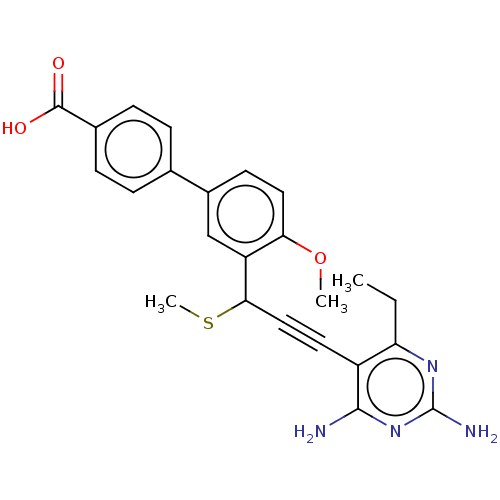 (US10870625, Compound 29) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Enzyme activity and inhibition assays were performed by monitoring the NADPH-dependent reduction of dihydrofolate catalyzed by the DHFR enzyme. The r... | US Patent US10870625 (2020) BindingDB Entry DOI: 10.7270/Q27W6G9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50330327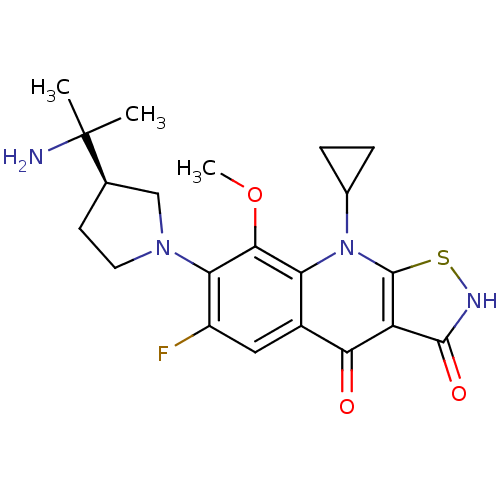 ((R)-7-(3-(2-aminopropan-2-yl)pyrrolidin-1-yl)-9-cy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of DNA supercoiling activity of wild type Mycobacterium tuberculosis DNA gyrase A | Antimicrob Agents Chemother 54: 3478-80 (2010) Article DOI: 10.1128/AAC.00287-10 BindingDB Entry DOI: 10.7270/Q2959HS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50536190 (CHEMBL4483336 | US10870625, Compound 55) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Enzyme activity and inhibition assays were performed by monitoring the NADPH-dependent reduction of dihydrofolate catalyzed by the DHFR enzyme. The r... | US Patent US10870625 (2020) BindingDB Entry DOI: 10.7270/Q27W6G9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50131428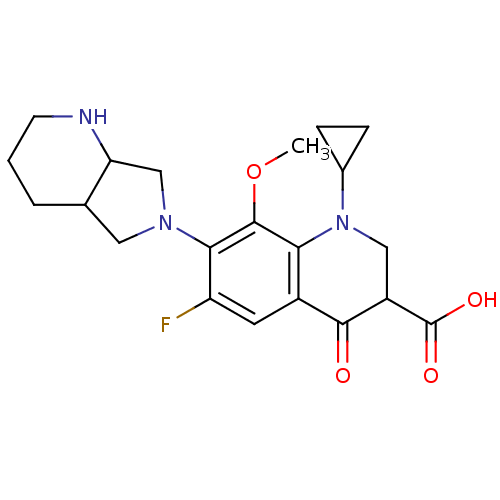 (1-Cyclopropyl-6-fluoro-8-methoxy-7-(1S,7aS)-octahy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of DNA supercoiling activity of wild type Mycobacterium tuberculosis DNA gyrase A | Antimicrob Agents Chemother 54: 3478-80 (2010) Article DOI: 10.1128/AAC.00287-10 BindingDB Entry DOI: 10.7270/Q2959HS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA gyrase subunit A (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50117914 (1-Cyclopropyl-1,4-dihydro-6-fluoro-8-methoxy-7-(3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of DNA supercoiling activity of wild type Mycobacterium tuberculosis DNA gyrase A | Antimicrob Agents Chemother 54: 3478-80 (2010) Article DOI: 10.1128/AAC.00287-10 BindingDB Entry DOI: 10.7270/Q2959HS2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA gyrase subunit A (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50131445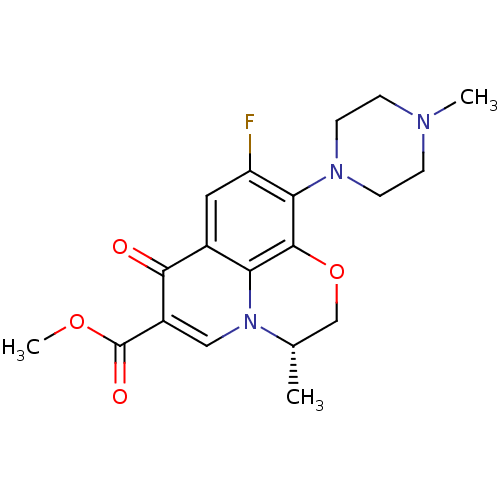 ((3S)-9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Achillion Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of DNA supercoiling activity of wild type Mycobacterium tuberculosis DNA gyrase A | Antimicrob Agents Chemother 54: 3478-80 (2010) Article DOI: 10.1128/AAC.00287-10 BindingDB Entry DOI: 10.7270/Q2959HS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18069 (5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB US Patent | n/a | n/a | 9.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
UNIVERSITY OF CONNECTICUT US Patent | Assay Description Enzyme activity and inhibition assays were performed by monitoring the NADPH-dependent reduction of dihydrofolate catalyzed by the DHFR enzyme. The r... | US Patent US10870625 (2020) BindingDB Entry DOI: 10.7270/Q27W6G9V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||