

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474629 (CHEMBL415006) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474628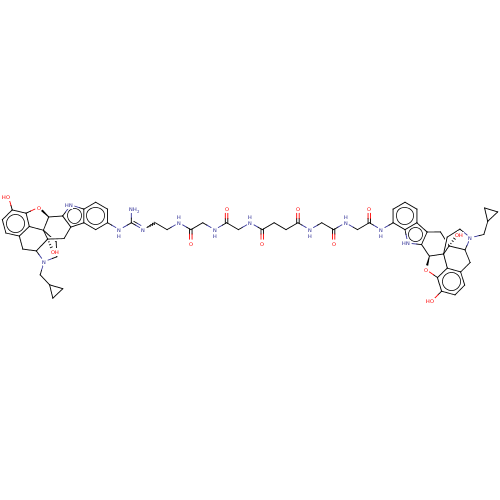 (CHEMBL386810) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474624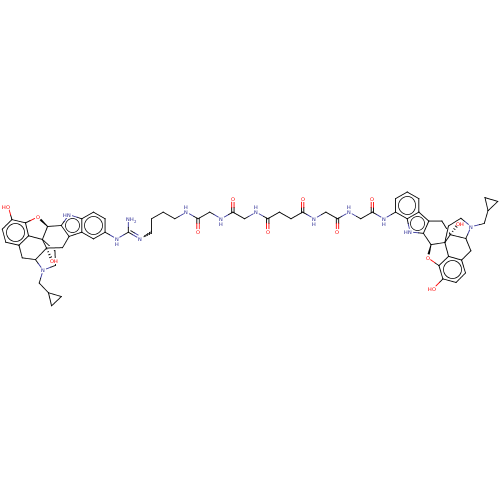 (CHEMBL409172) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand singly expressed with delta or kappa receptor | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474624 (CHEMBL409172) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474626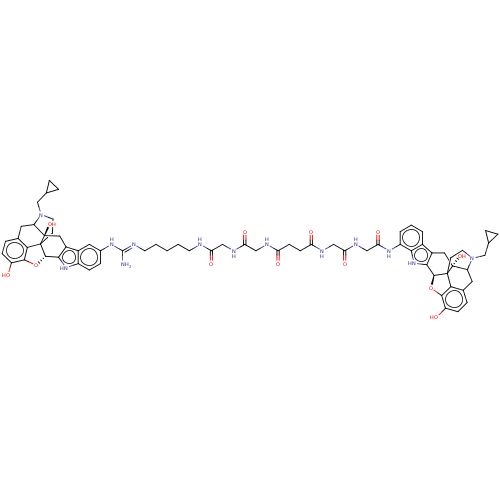 (CHEMBL414603) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand singly expressed with delta or kappa receptor | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474626 (CHEMBL414603) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474625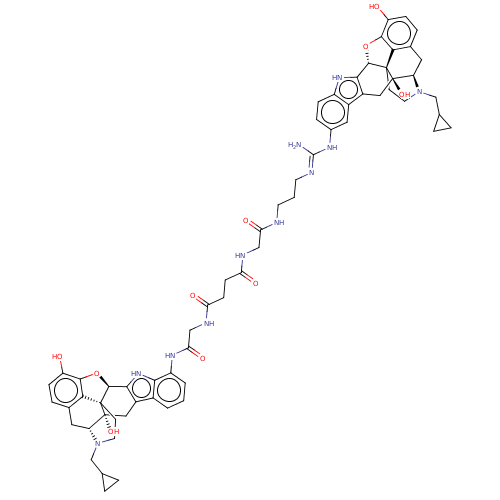 (CHEMBL611140) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand singly expressed with delta or kappa receptors. | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474627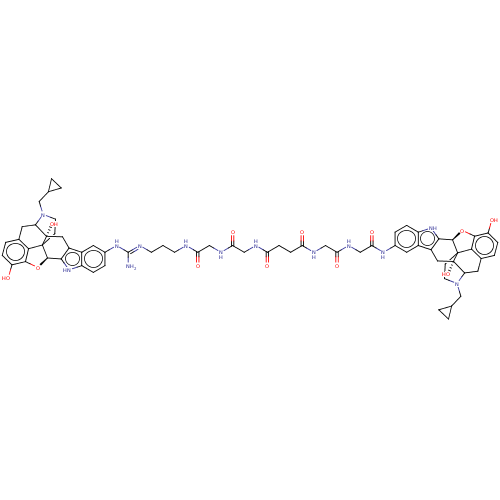 (CHEMBL440626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand singly expressed with delta or kappa receptor | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474628 (CHEMBL386810) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand singly expressed with delta or kappa receptors. | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474629 (CHEMBL415006) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand singly expressed with delta or kappa receptors. | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474623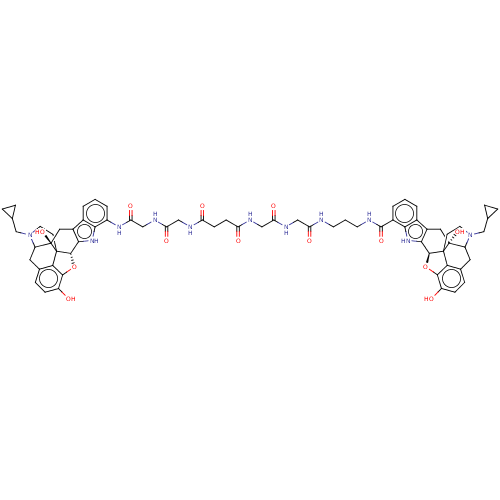 (CHEMBL410574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 199 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand singly expressed with delta or kappa receptor | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474625 (CHEMBL611140) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 315 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474623 (CHEMBL410574) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type/Kappa-type/Mu-type opioid receptor (MOUSE-Mus musculus (Mouse)) | BDBM50474627 (CHEMBL440626) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Compound was tested for binding affinity on intact HEK cells using [3H]diprenorphine as radioligand co-expressed with delta and kappa opioid receptor... | J Med Chem 47: 2969-72 (2004) Article DOI: 10.1021/jm0342358 BindingDB Entry DOI: 10.7270/Q2N58Q39 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM50112349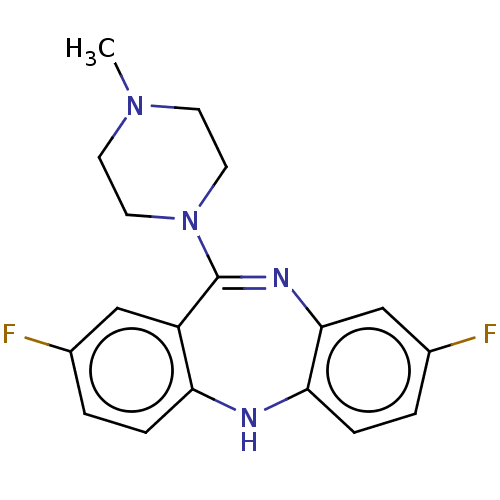 (CHEMBL3609328) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human histamine H1 receptor | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112348 (CHEMBL3609372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50112349 (CHEMBL3609328) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human muscarinic M1 receptor | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112347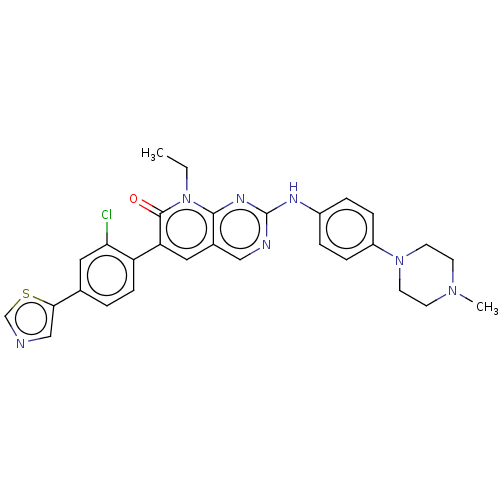 (CHEMBL3609327 | FRAX597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PAK1 by Z'-LYTE assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112355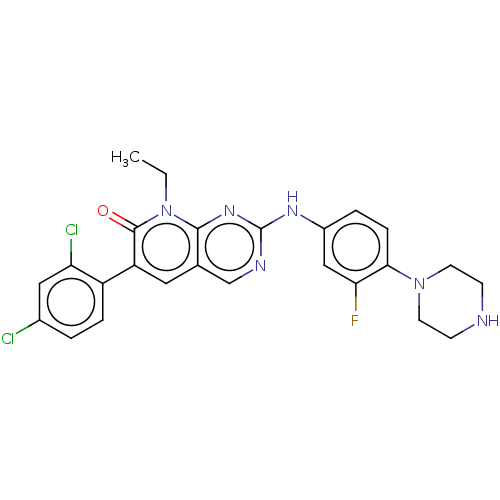 (CHEMBL3609326) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of full length PAK1 (unknown origin) by Z'-LYTE assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50112347 (CHEMBL3609327 | FRAX597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PAK2 by Z'-LYTE assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM101618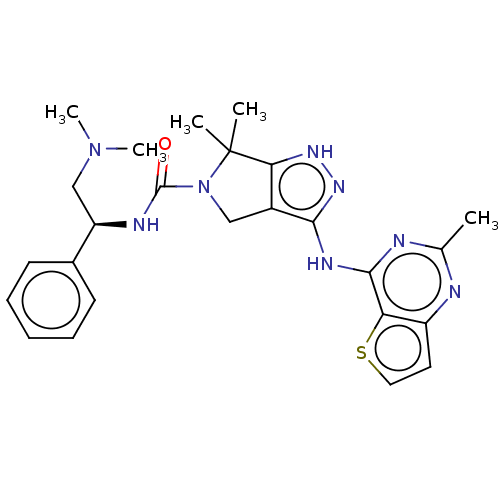 (US8530652, 114) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PAK1 (unknown origin) using Syntide2 peptide as substrate by pyruvate kinase/lactate dehydrogenase coupled assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 6 (Homo sapiens (Human)) | BDBM101618 (US8530652, 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PAK6 (unknown origin) using peptide 7 as substrate by pyruvate kinase/lactate dehydrogenase coupled assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 5 (Homo sapiens (Human)) | BDBM101618 (US8530652, 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PAK5 (unknown origin) using peptide 7 as substrate by pyruvate kinase/lactate dehydrogenase coupled assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112352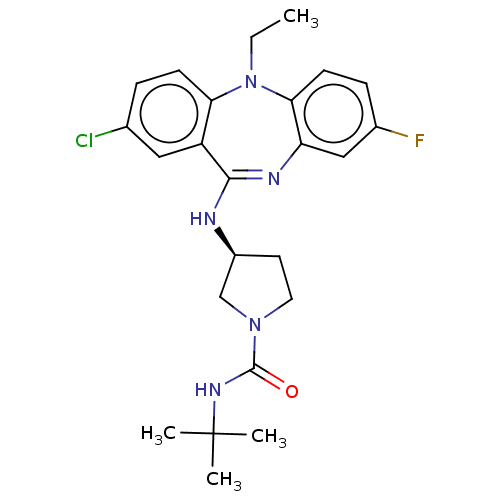 (CHEMBL3609371) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM101618 (US8530652, 114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged recombinant human PAK4 kinase domain (300 to 591) using peptide 7 as substrate by pyruvate kinase/lactate dehydr... | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 3 (Homo sapiens (Human)) | BDBM50112347 (CHEMBL3609327 | FRAX597) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PAK3 by Z'-LYTE assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM50112355 (CHEMBL3609326) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of full length PAK2 (unknown origin) by Z'-LYTE assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 3 (Homo sapiens (Human)) | BDBM50112355 (CHEMBL3609326) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of full length PAK3 (unknown origin) by Z'-LYTE assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 3 (Homo sapiens (Human)) | BDBM101618 (US8530652, 114) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 99 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PAK3 (unknown origin) | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50157570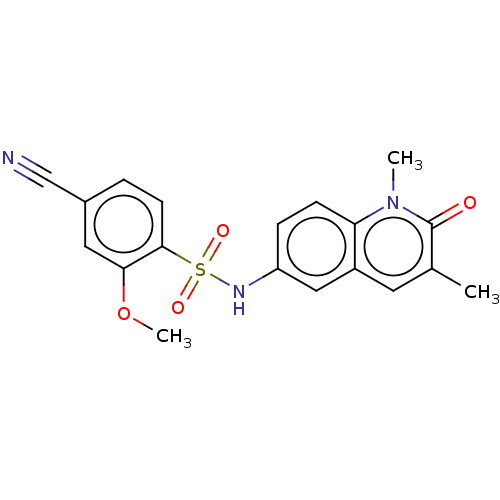 (4-cyano-N-(1,3-dimethyl-2-oxoquinolin-6-yl)-2-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 114 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50032927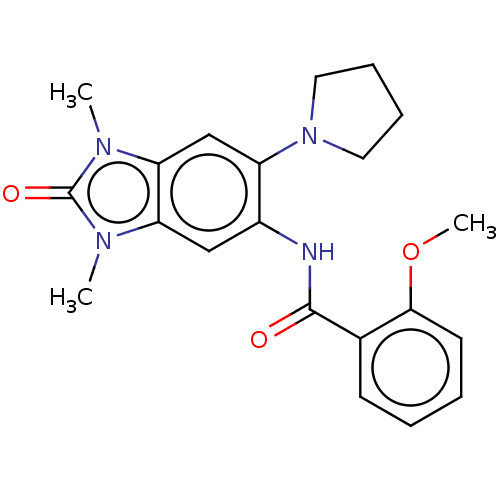 (CHEMBL3356143 | N-[2,3-Dihydro-1,3-dimethyl-2-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50078631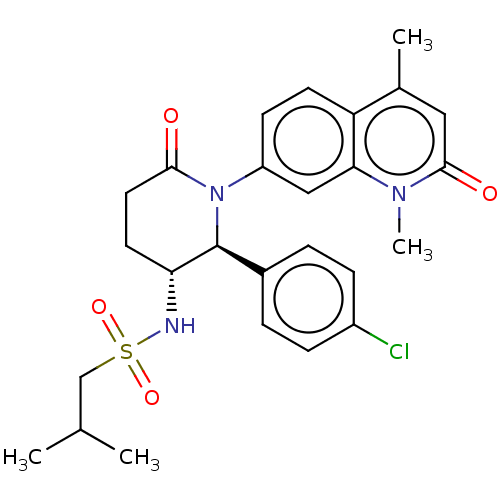 (CHEMBL4650212) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 189 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Homogeneous Time Resolved Fluorescence (HTRF) assay. Domain start/stop: L14-Q143 | Angew Chem Int Ed Engl 54: 6217-21 (2015) Article DOI: 10.1002/anie.201501394 BindingDB Entry DOI: 10.7270/Q20R9T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112350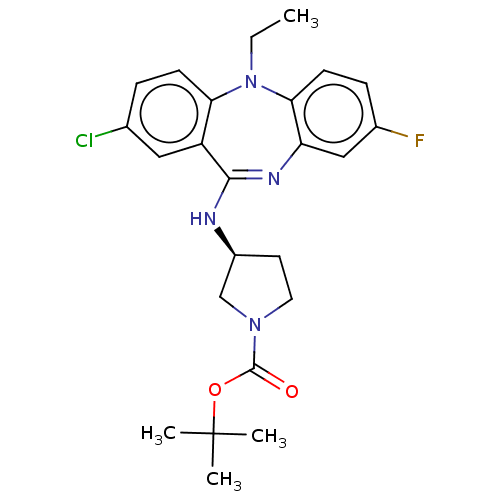 (CHEMBL3609370) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 2 (Homo sapiens (Human)) | BDBM101618 (US8530652, 114) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PAK2 (unknown origin) | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peregrin (Homo sapiens (Human)) | BDBM50148549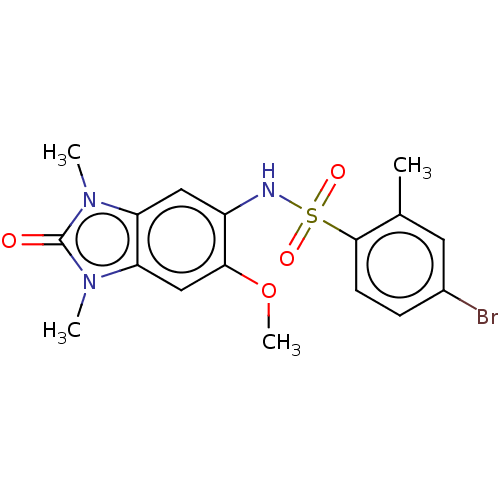 (4-Bromo-N-(2,3-dihydro-6-methoxy-1,3-dimethyl-2-ox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112351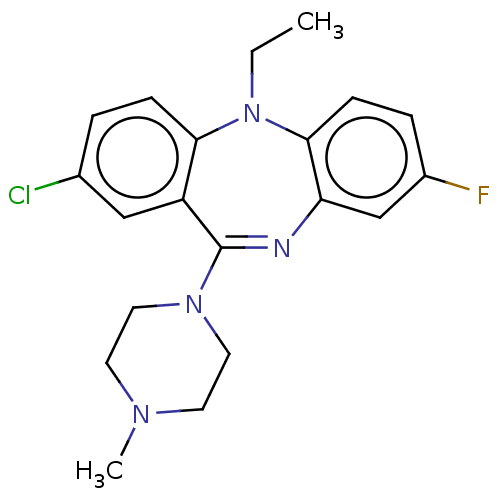 (CHEMBL3609330) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 323 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| CREB-binding protein (Homo sapiens (Human)) | BDBM50266292 (CHEMBL3906203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Alphascreen assay. Binding to CREBBPA (domain start/stop: R1081-G1197) by alphascreen assay | Cancer Res 75: 5106-5119 (2015) Article DOI: 10.1158/0008-5472.CAN-15-0236 BindingDB Entry DOI: 10.7270/Q2HQ43GJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 1 (Homo sapiens (Human)) | BDBM50157570 (4-cyano-N-(1,3-dimethyl-2-oxoquinolin-6-yl)-2-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 619 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 4 (Homo sapiens (Human)) | BDBM50112355 (CHEMBL3609326) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 779 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of full length PAK4 (unknown origin) by Z'-LYTE assay | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112353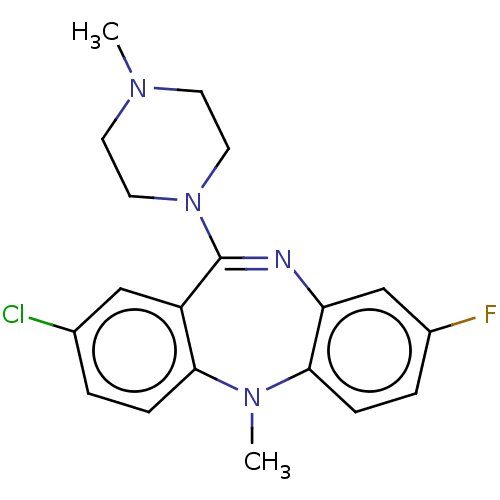 (CHEMBL3609329) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of wild type dephosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH a... | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112353 (CHEMBL3609329) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PAK1 (unknown origin) in presence of 1.5 uM of ATP | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain and PHD finger-containing protein 3 (Homo sapiens (Human)) | BDBM50157570 (4-cyano-N-(1,3-dimethyl-2-oxoquinolin-6-yl)-2-meth...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.01E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 1 (Homo sapiens (Human)) | BDBM50148549 (4-Bromo-N-(2,3-dihydro-6-methoxy-1,3-dimethyl-2-ox...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bromodomain-containing protein 9 (Homo sapiens (Human)) | BDBM50078631 (CHEMBL4650212) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Alphascreen assay. Binding to BRD9A (domain start/stop: L14-Q143) by alphascreen assay | Angew Chem Int Ed Engl 54: 6217-21 (2015) Article DOI: 10.1002/anie.201501394 BindingDB Entry DOI: 10.7270/Q20R9T0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50266292 (CHEMBL3906203) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 2.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Alphascreen assay. Binding to BRD4A (domain start/stop: N44-E168) by alphascreen assay | Cancer Res 75: 5106-5119 (2015) Article DOI: 10.1158/0008-5472.CAN-15-0236 BindingDB Entry DOI: 10.7270/Q2HQ43GJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112354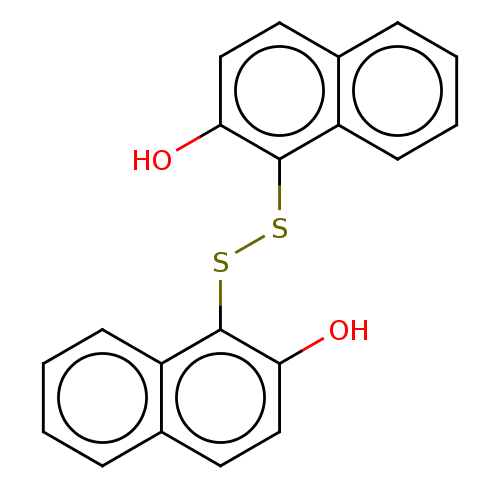 (CHEMBL472940) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Non-ATP competitive inhibition of full-length human PAK1 assessed as phosphate incorporation onto MBP preincubated for 5 mins followed by Cdc42, MBP,... | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 1 (Homo sapiens (Human)) | BDBM50032927 (CHEMBL3356143 | N-[2,3-Dihydro-1,3-dimethyl-2-oxo-...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.52E+3 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Oxford University | Assay Description Assays were performed as described previously with minor modifications(Philpott et al., 2011). All reagents were diluted in 25 mM HEPES, 100 mM NaCl,... | ACS Chem Biol 12: 2619-2630 (2017) Article DOI: 10.1021/acschembio.7b00481 BindingDB Entry DOI: 10.7270/Q2HM56MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112353 (CHEMBL3609329) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PAK1 (unknown origin) in presence of 15 uM of ATP | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 1 (Homo sapiens (Human)) | BDBM50112353 (CHEMBL3609329) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of wild type phosphorylated form of PAK1 (249 to 545) (unknown origin) expressed in Escherichia coli using 5-Fluo-Ahx-AKRRRLSSLRA-COOH as ... | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase PAK 6 (Homo sapiens (Human)) | BDBM50112354 (CHEMBL472940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of full length human PAK6 assessed as phosphate incorporation onto MBP preincubated for 5 mins followed by Cdc42, MBP, and mixture of ATP ... | ACS Med Chem Lett 6: 776-81 (2015) Article DOI: 10.1021/acsmedchemlett.5b00102 BindingDB Entry DOI: 10.7270/Q25X2BQS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 384 total ) | Next | Last >> |