 Found 151 hits with Last Name = 'darienzo' and Initial = 'c'
Found 151 hits with Last Name = 'darienzo' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM50205111
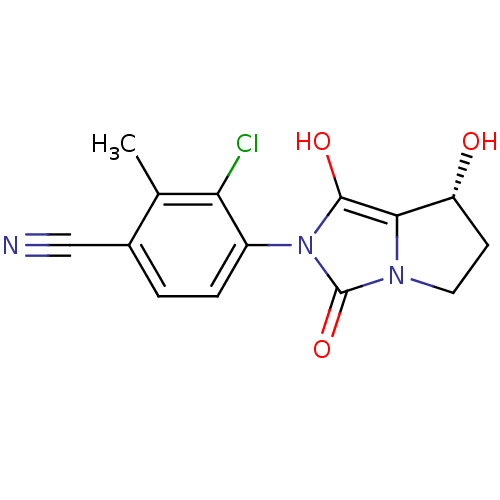
(3-chloro-4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydr...)Show SMILES Cc1c(Cl)c(ccc1C#N)-n1c(O)c2[C@H](O)CCn2c1=O |wU:14.15,(22.97,-37.01,;22.21,-35.67,;20.67,-35.65,;19.89,-36.98,;19.91,-34.31,;20.69,-32.99,;22.22,-32.99,;22.99,-34.34,;24.53,-34.34,;26.08,-34.34,;18.37,-34.31,;17.48,-33.06,;17.96,-31.6,;16.01,-33.53,;14.55,-33.04,;14.08,-31.57,;13.63,-34.28,;14.54,-35.53,;16,-35.06,;17.46,-35.55,;17.93,-37.02,)| Show InChI InChI=1S/C14H12ClN3O3/c1-7-8(6-16)2-3-9(11(7)15)18-13(20)12-10(19)4-5-17(12)14(18)21/h2-3,10,19-20H,4-5H2,1H3/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205116
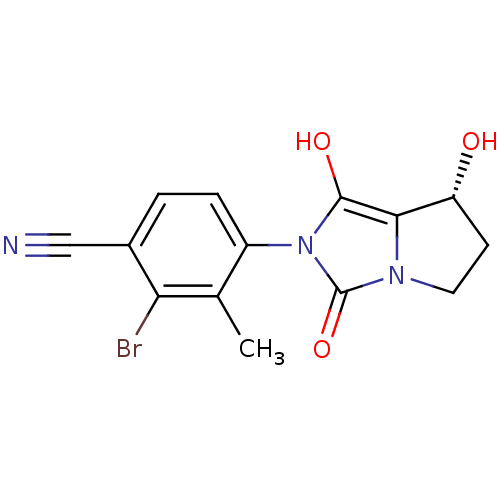
(2-bromo-4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro...)Show SMILES Cc1c(Br)c(ccc1-n1c(O)c2[C@H](O)CCn2c1=O)C#N |wU:12.13,(21.54,-10.86,;22.32,-9.54,;23.86,-9.55,;24.62,-10.89,;24.64,-8.22,;23.87,-6.88,;22.33,-6.87,;21.56,-8.2,;20.02,-8.19,;19.13,-6.94,;19.61,-5.48,;17.66,-7.41,;16.2,-6.92,;15.73,-5.45,;15.28,-8.17,;16.19,-9.42,;17.65,-8.95,;19.11,-9.43,;19.58,-10.9,;26.18,-8.22,;27.72,-8.23,)| Show InChI InChI=1S/C14H12BrN3O3/c1-7-9(3-2-8(6-16)11(7)15)18-13(20)12-10(19)4-5-17(12)14(18)21/h2-3,10,19-20H,4-5H2,1H3/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205114
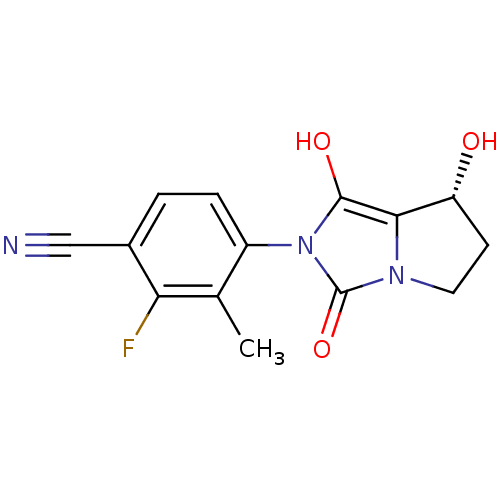
(2-fluoro-4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydr...)Show SMILES Cc1c(F)c(ccc1-n1c(O)c2[C@H](O)CCn2c1=O)C#N |wU:12.13,(21.37,-1.41,;22.15,-.08,;23.69,-.09,;24.45,-1.43,;24.47,1.24,;23.7,2.58,;22.16,2.59,;21.39,1.26,;19.85,1.27,;18.96,2.52,;19.44,3.98,;17.49,2.05,;16.03,2.54,;15.56,4.01,;15.11,1.29,;16.02,.04,;17.48,.51,;18.94,.03,;19.41,-1.44,;26.01,1.24,;27.55,1.23,)| Show InChI InChI=1S/C14H12FN3O3/c1-7-9(3-2-8(6-16)11(7)15)18-13(20)12-10(19)4-5-17(12)14(18)21/h2-3,10,19-20H,4-5H2,1H3/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205096
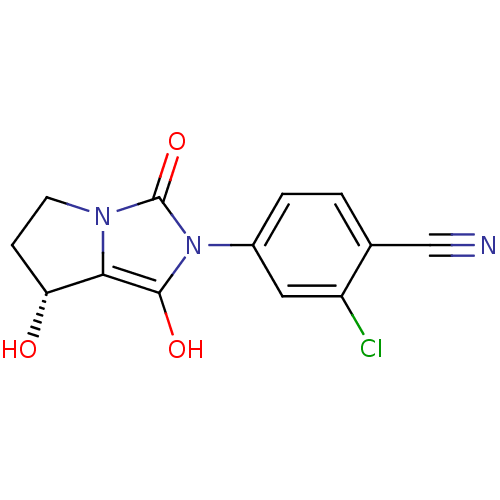
(2-chloro-4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydr...)Show SMILES O[C@@H]1CCn2c1c(O)n(-c1ccc(C#N)c(Cl)c1)c2=O Show InChI InChI=1S/C13H10ClN3O3/c14-9-5-8(2-1-7(9)6-15)17-12(19)11-10(18)3-4-16(11)13(17)20/h1-2,5,10,18-19H,3-4H2/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18173
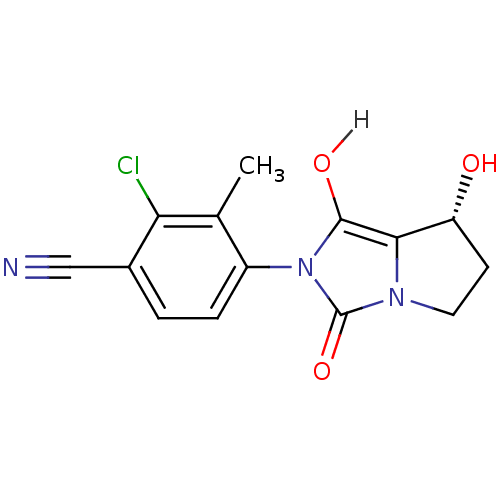
(4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...)Show SMILES Cc1c(Cl)c(ccc1-n1c(O)c2[C@H](O)CCn2c1=O)C#N |r,wU:12.13,(.01,.36,;.81,1.67,;2.35,1.63,;3.09,.28,;3.16,2.94,;2.42,4.3,;.88,4.34,;.08,3.02,;-1.46,3.02,;-1.94,4.49,;-1.03,5.73,;-3.48,4.49,;-4.72,5.39,;-4.72,6.93,;-5.97,4.49,;-5.5,3.02,;-3.95,3.02,;-2.71,2.12,;-2.71,.58,;4.7,2.9,;6.24,2.9,)| Show InChI InChI=1S/C14H12ClN3O3/c1-7-9(3-2-8(6-16)11(7)15)18-13(20)12-10(19)4-5-17(12)14(18)21/h2-3,10,19-20H,4-5H2,1H3/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205100
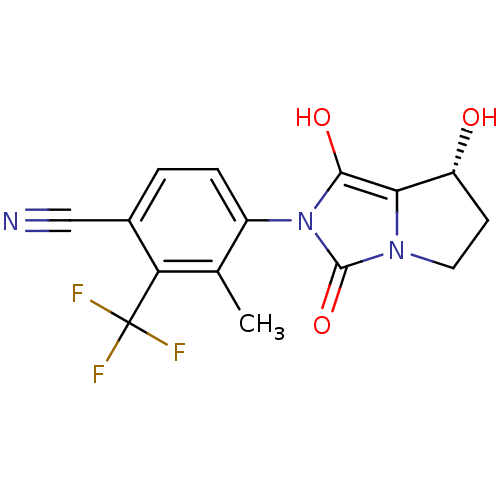
(4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-1H-pyrr...)Show SMILES Cc1c(ccc(C#N)c1C(F)(F)F)-n1c(O)c2[C@H](O)CCn2c1=O |wU:17.18,(-.05,-49.74,;.73,-48.41,;-.03,-47.07,;.75,-45.75,;2.28,-45.75,;3.05,-47.09,;4.59,-47.09,;6.13,-47.1,;2.27,-48.42,;3.03,-49.77,;3.79,-51.1,;1.69,-50.53,;4.38,-49.01,;-1.57,-47.07,;-2.46,-45.82,;-1.98,-44.35,;-3.93,-46.28,;-5.39,-45.8,;-5.86,-44.33,;-6.31,-47.04,;-5.4,-48.29,;-3.94,-47.82,;-2.48,-48.3,;-2.01,-49.77,)| Show InChI InChI=1S/C15H12F3N3O3/c1-7-9(3-2-8(6-19)11(7)15(16,17)18)21-13(23)12-10(22)4-5-20(12)14(21)24/h2-3,10,22-23H,4-5H2,1H3/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205113
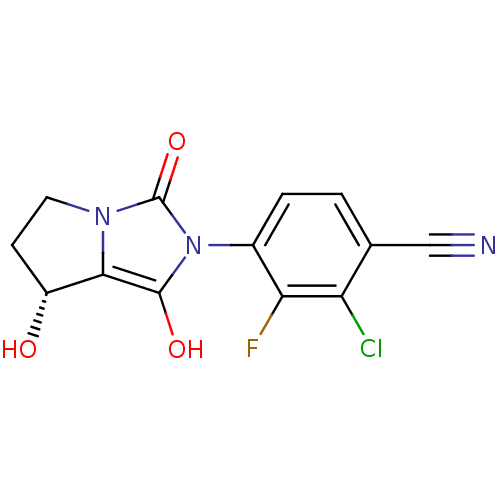
(2-chloro-3-fluoro-4-((7R,7aS)-7-hydroxy-1,3-dioxo-...)Show SMILES O[C@@H]1CCn2c1c(O)n(-c1ccc(C#N)c(Cl)c1F)c2=O |wU:1.0,(-7.12,-24.7,;-6.65,-26.17,;-7.57,-27.42,;-6.66,-28.66,;-5.2,-28.19,;-5.19,-26.66,;-3.72,-26.19,;-3.24,-24.73,;-2.83,-27.44,;-1.29,-27.45,;-.52,-26.12,;1.02,-26.12,;1.79,-27.47,;3.33,-27.47,;4.87,-27.48,;1.01,-28.8,;1.77,-30.14,;-.53,-28.78,;-1.31,-30.11,;-3.74,-28.68,;-3.27,-30.15,)| Show InChI InChI=1S/C13H9ClFN3O3/c14-9-6(5-16)1-2-7(10(9)15)18-12(20)11-8(19)3-4-17(11)13(18)21/h1-2,8,19-20H,3-4H2/t8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18171

(4-[(7R,7aS)-7-hydroxy-1,3-dioxo-hexahydro-1H-pyrro...)Show SMILES O[C@@H]1CCn2c1c(O)n(-c1ccc(C#N)c3ccccc13)c2=O |r,wU:1.0,(-7.31,4.33,;-6.4,3.09,;-6.88,1.62,;-5.63,.72,;-4.39,1.62,;-4.86,3.09,;-3.62,3.99,;-3.62,5.53,;-2.37,3.09,;-.83,3.09,;.03,4.36,;1.57,4.25,;2.24,2.87,;3.78,2.76,;5.32,2.76,;1.38,1.59,;2.05,.21,;1.19,-1.07,;-.35,-.96,;-1.02,.43,;-.16,1.7,;-2.85,1.62,;-1.94,.38,)| Show InChI InChI=1S/C17H13N3O3/c18-9-10-5-6-13(12-4-2-1-3-11(10)12)20-16(22)15-14(21)7-8-19(15)17(20)23/h1-6,14,21-22H,7-8H2/t14-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205102
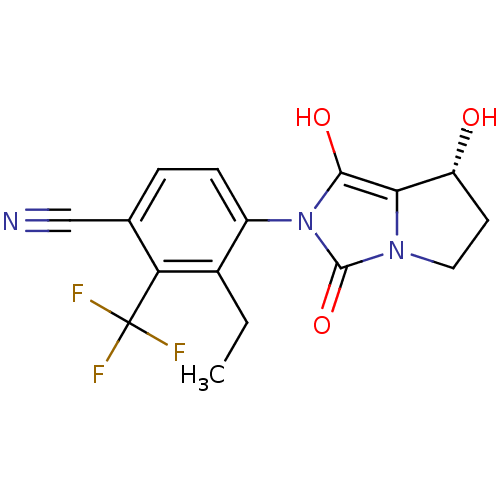
(3-ethyl-4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro...)Show SMILES CCc1c(ccc(C#N)c1C(F)(F)F)-n1c(O)c2[C@H](O)CCn2c1=O |wU:18.19,(4.34,-4.72,;3.58,-3.37,;4.36,-2.05,;3.6,-.71,;4.37,.62,;5.91,.61,;6.68,-.73,;8.22,-.73,;9.76,-.74,;5.9,-2.06,;6.66,-3.4,;7.42,-4.73,;5.32,-4.17,;8,-2.65,;2.06,-.7,;1.16,.55,;1.65,2.01,;-.3,.08,;-1.76,.57,;-2.23,2.04,;-2.68,-.68,;-1.77,-1.93,;-.31,-1.46,;1.15,-1.94,;1.62,-3.41,)| Show InChI InChI=1S/C16H14F3N3O3/c1-2-9-10(4-3-8(7-20)12(9)16(17,18)19)22-14(24)13-11(23)5-6-21(13)15(22)25/h3-4,11,23-24H,2,5-6H2,1H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205103
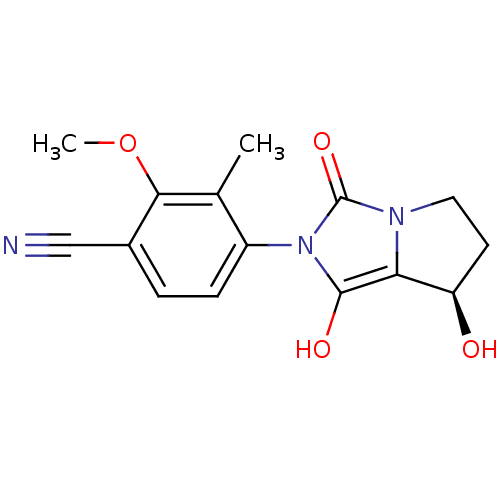
(4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-1H-pyrr...)Show SMILES COc1c(C)c(ccc1C#N)-n1c(O)c2[C@H](O)CCn2c1=O |wU:15.16,(.83,-2.32,;1.61,-.99,;.85,.35,;-.69,.37,;-1.47,-.96,;-1.45,1.7,;-.68,3.03,;.86,3.03,;1.63,1.68,;3.17,1.68,;4.71,1.67,;-2.99,1.71,;-3.89,2.96,;-3.4,4.42,;-5.35,2.49,;-6.81,2.98,;-7.28,4.45,;-7.73,1.73,;-6.83,.49,;-5.36,.96,;-3.9,.47,;-3.44,-1,)| Show InChI InChI=1S/C15H15N3O4/c1-8-10(4-3-9(7-16)13(8)22-2)18-14(20)12-11(19)5-6-17(12)15(18)21/h3-4,11,19-20H,5-6H2,1-2H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205104
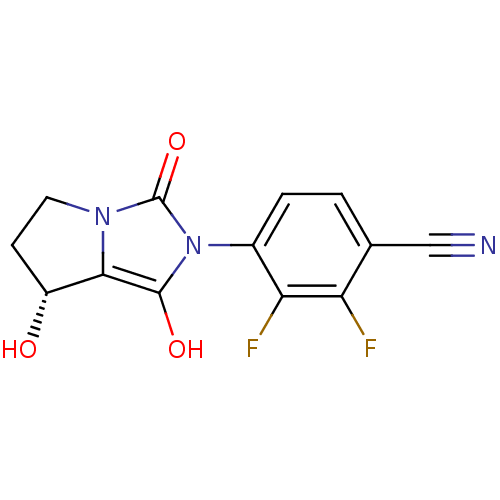
(2,3-difluoro-4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetra...)Show SMILES O[C@@H]1CCn2c1c(O)n(-c1ccc(C#N)c(F)c1F)c2=O |wU:1.0,(16.12,-15.47,;16.59,-16.94,;15.67,-18.19,;16.58,-19.44,;18.04,-18.97,;18.05,-17.43,;19.52,-16.97,;20,-15.5,;20.41,-18.21,;21.95,-18.22,;22.73,-16.89,;24.26,-16.9,;25.03,-18.24,;26.57,-18.24,;28.11,-18.25,;24.25,-19.57,;25.01,-20.91,;22.71,-19.56,;21.93,-20.89,;19.5,-19.45,;19.97,-20.92,)| Show InChI InChI=1S/C13H9F2N3O3/c14-9-6(5-16)1-2-7(10(9)15)18-12(20)11-8(19)3-4-17(11)13(18)21/h1-2,8,19-20H,3-4H2/t8-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205098

(4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-1H-pyrr...)Show SMILES Cc1c(C)c(ccc1C#N)-n1c(O)c2[C@H](O)CCn2c1=O |wU:14.15,(1.66,-40.91,;.9,-39.57,;-.64,-39.56,;-1.42,-40.88,;-1.4,-38.22,;-.62,-36.89,;.91,-36.9,;1.68,-38.24,;3.22,-38.24,;4.76,-38.25,;-2.94,-38.21,;-3.83,-36.96,;-3.35,-35.5,;-5.3,-37.43,;-6.76,-36.94,;-7.23,-35.47,;-7.68,-38.19,;-6.77,-39.44,;-5.31,-38.97,;-3.85,-39.45,;-3.38,-40.92,)| Show InChI InChI=1S/C15H15N3O3/c1-8-9(2)11(4-3-10(8)7-16)18-14(20)13-12(19)5-6-17(13)15(18)21/h3-4,12,19-20H,5-6H2,1-2H3/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205107
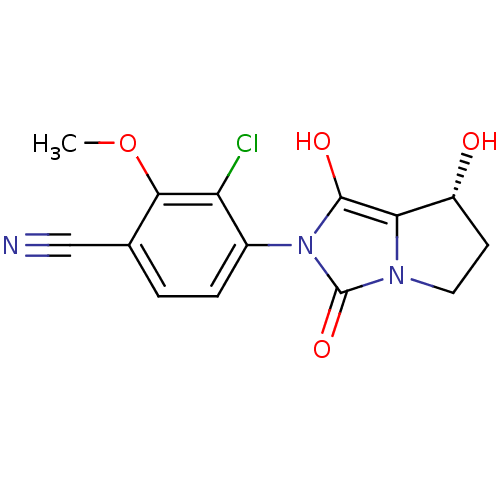
(3-chloro-4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydr...)Show SMILES COc1c(Cl)c(ccc1C#N)-n1c(O)c2[C@H](O)CCn2c1=O |wU:15.16,(1.24,-38.75,;2.02,-37.42,;1.26,-36.08,;-.28,-36.06,;-1.06,-37.39,;-1.04,-34.73,;-.27,-33.4,;1.27,-33.4,;2.04,-34.75,;3.58,-34.75,;5.12,-34.76,;-2.58,-34.72,;-3.48,-33.47,;-2.99,-32.01,;-4.94,-33.94,;-6.4,-33.45,;-6.87,-31.98,;-7.32,-34.7,;-6.42,-35.94,;-4.95,-35.47,;-3.49,-35.96,;-3.02,-37.43,)| Show InChI InChI=1S/C14H12ClN3O4/c1-22-12-7(6-16)2-3-8(10(12)15)18-13(20)11-9(19)4-5-17(11)14(18)21/h2-3,9,19-20H,4-5H2,1H3/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205101
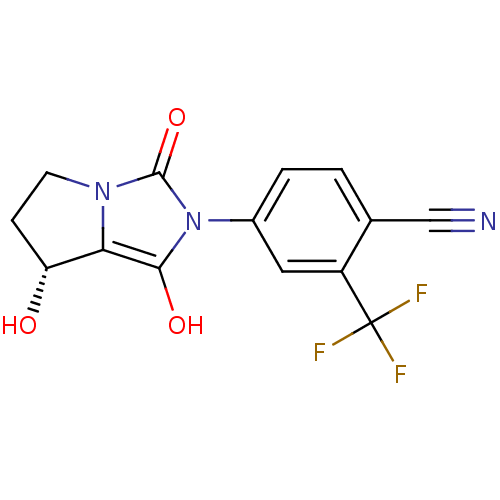
(4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-1H-pyrr...)Show SMILES O[C@@H]1CCn2c1c(O)n(-c1ccc(C#N)c(c1)C(F)(F)F)c2=O Show InChI InChI=1S/C14H10F3N3O3/c15-14(16,17)9-5-8(2-1-7(9)6-18)20-12(22)11-10(21)3-4-19(11)13(20)23/h1-2,5,10,21-22H,3-4H2/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205110
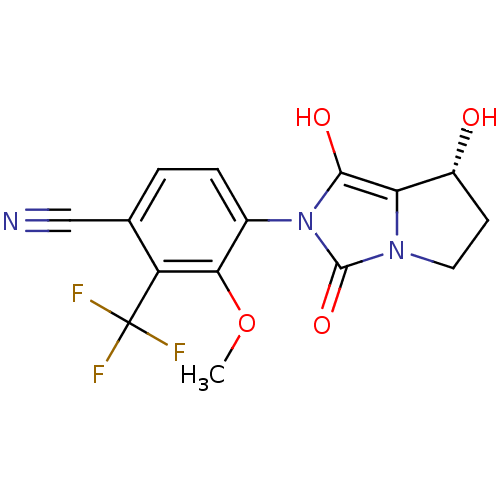
(4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-1H-pyrr...)Show SMILES COc1c(ccc(C#N)c1C(F)(F)F)-n1c(O)c2[C@H](O)CCn2c1=O |wU:18.19,(13.93,-47.01,;13.17,-45.67,;13.95,-44.34,;13.19,-43,;13.96,-41.68,;15.5,-41.68,;16.27,-43.03,;17.81,-43.03,;19.35,-43.03,;15.49,-44.36,;16.25,-45.7,;17.01,-47.03,;14.91,-46.46,;17.6,-44.95,;11.65,-43,;10.76,-41.75,;11.24,-40.29,;9.29,-42.22,;7.83,-41.73,;7.36,-40.26,;6.91,-42.97,;7.82,-44.22,;9.28,-43.75,;10.74,-44.24,;11.21,-45.71,)| Show InChI InChI=1S/C15H12F3N3O4/c1-25-12-8(3-2-7(6-19)10(12)15(16,17)18)21-13(23)11-9(22)4-5-20(11)14(21)24/h2-3,9,22-23H,4-5H2,1H3/t9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205112
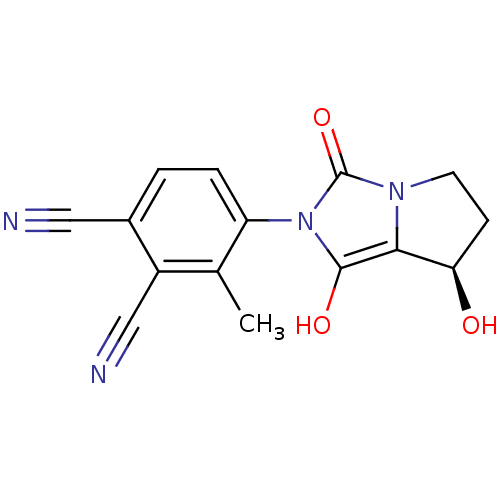
(4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-1H-pyrr...)Show SMILES Cc1c(ccc(C#N)c1C#N)-n1c(O)c2[C@H](O)CCn2c1=O |wU:15.16,(-.55,-20.76,;.23,-19.43,;-.53,-18.1,;.25,-16.77,;1.78,-16.77,;2.56,-18.12,;4.1,-18.12,;5.64,-18.12,;1.77,-19.45,;2.53,-20.79,;3.29,-22.13,;-2.07,-18.09,;-2.96,-16.84,;-2.48,-15.38,;-4.43,-17.31,;-5.89,-16.82,;-6.36,-15.35,;-6.8,-18.07,;-5.9,-19.31,;-4.44,-18.84,;-2.98,-19.33,;-2.51,-20.8,)| Show InChI InChI=1S/C15H12N4O3/c1-8-10(7-17)9(6-16)2-3-11(8)19-14(21)13-12(20)4-5-18(13)15(19)22/h2-3,12,20-21H,4-5H2,1H3/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205099

(4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-1H-pyrr...)Show SMILES COc1c(C)c(ccc1-n1c(O)c2[C@H](O)CCn2c1=O)C#N |wU:13.14,(-2.46,-47.7,;-3.22,-46.35,;-2.44,-45.03,;-.9,-45.04,;-.14,-46.38,;-.12,-43.71,;-.89,-42.37,;-2.42,-42.36,;-3.2,-43.69,;-4.74,-43.68,;-5.63,-42.44,;-5.15,-40.97,;-7.1,-42.9,;-8.56,-42.41,;-9.03,-40.94,;-9.47,-43.66,;-8.57,-44.91,;-7.11,-44.44,;-5.65,-44.92,;-5.18,-46.39,;1.42,-43.71,;2.97,-43.72,)| Show InChI InChI=1S/C15H15N3O4/c1-8-9(7-16)3-4-10(13(8)22-2)18-14(20)12-11(19)5-6-17(12)15(18)21/h3-4,11,19-20H,5-6H2,1-2H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205115
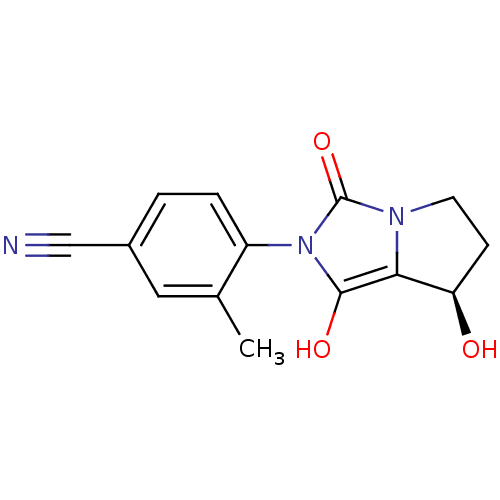
(4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-1H-pyrr...)Show SMILES Cc1cc(ccc1-n1c(O)c2[C@H](O)CCn2c1=O)C#N |wU:11.12,(19.99,-30.33,;20.77,-29,;22.31,-29.02,;23.09,-27.69,;22.32,-26.34,;20.79,-26.34,;20.01,-27.66,;18.47,-27.66,;17.58,-26.41,;18.06,-24.95,;16.11,-26.88,;14.65,-26.39,;14.18,-24.92,;13.73,-27.63,;14.64,-28.88,;16.1,-28.41,;17.56,-28.9,;18.03,-30.37,;24.63,-27.69,;26.18,-27.69,)| Show InChI InChI=1S/C14H13N3O3/c1-8-6-9(7-15)2-3-10(8)17-13(19)12-11(18)4-5-16(12)14(17)20/h2-3,6,11,18-19H,4-5H2,1H3/t11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205097
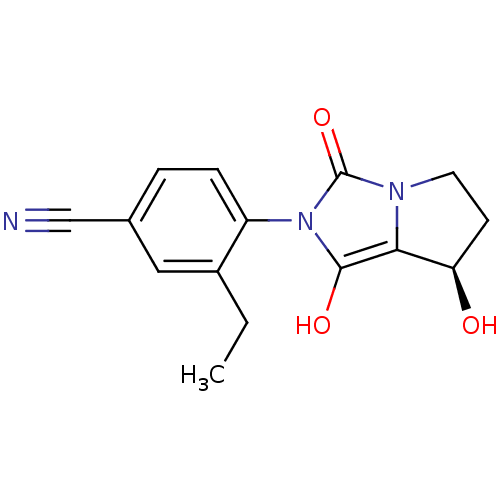
(3-ethyl-4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro...)Show SMILES CCc1cc(ccc1-n1c(O)c2[C@H](O)CCn2c1=O)C#N |wU:12.13,(4.34,-4.72,;3.58,-3.37,;4.36,-2.05,;5.9,-2.06,;6.68,-.73,;5.91,.61,;4.37,.62,;3.6,-.71,;2.06,-.7,;1.16,.55,;1.65,2.01,;-.3,.08,;-1.76,.57,;-2.23,2.04,;-2.68,-.68,;-1.77,-1.93,;-.31,-1.46,;1.15,-1.94,;1.62,-3.41,;8.22,-.73,;9.76,-.74,)| Show InChI InChI=1S/C15H15N3O3/c1-2-10-7-9(8-16)3-4-11(10)18-14(20)13-12(19)5-6-17(13)15(18)21/h3-4,7,12,19-20H,2,5-6H2,1H3/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205108

(8-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-1H-pyrr...)Show SMILES O[C@@H]1CCn2c1c(O)n(-c1ccc(C#N)c3cccnc13)c2=O |wU:1.0,(15.06,5.91,;15.52,4.44,;14.61,3.19,;15.51,1.94,;16.98,2.41,;16.99,3.95,;18.45,4.42,;18.94,5.88,;19.35,3.17,;20.89,3.16,;21.66,4.49,;23.2,4.48,;23.97,3.14,;25.51,3.14,;27.05,3.13,;23.19,1.81,;23.96,.47,;23.18,-.86,;21.63,-.85,;20.87,.49,;21.65,1.82,;18.44,1.93,;18.9,.46,)| Show InChI InChI=1S/C16H12N4O3/c17-8-9-3-4-11(13-10(9)2-1-6-18-13)20-15(22)14-12(21)5-7-19(14)16(20)23/h1-4,6,12,21-22H,5,7H2/t12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205106
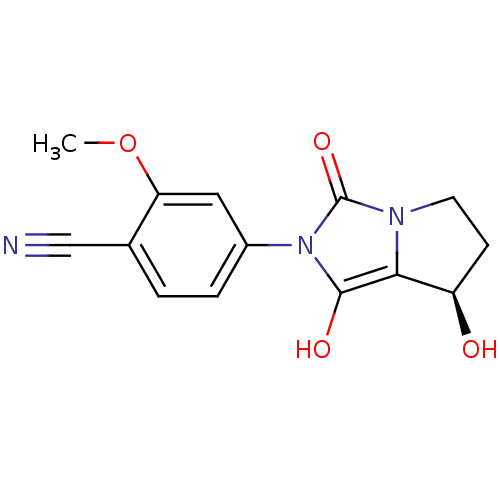
(4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-1H-pyrr...)Show InChI InChI=1S/C14H13N3O4/c1-21-11-6-9(3-2-8(11)7-15)17-13(19)12-10(18)4-5-16(12)14(17)20/h2-3,6,10,18-19H,4-5H2,1H3/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205105
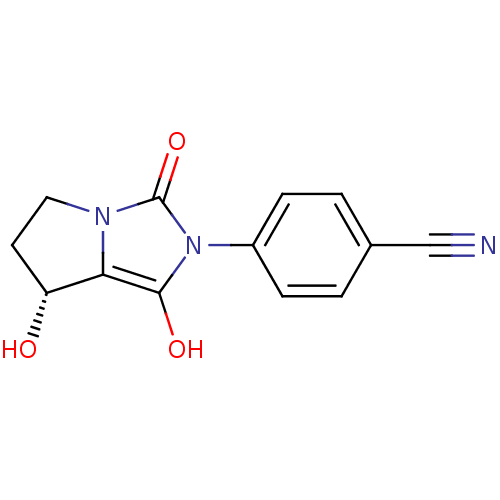
(4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-1H-pyrr...)Show InChI InChI=1S/C13H11N3O3/c14-7-8-1-3-9(4-2-8)16-12(18)11-10(17)5-6-15(11)13(16)19/h1-4,10,17-18H,5-6H2/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205109
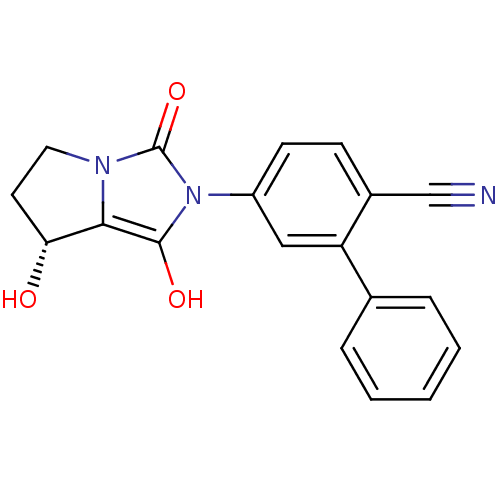
(5-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydro-pyrrolo...)Show SMILES O[C@@H]1CCn2c1c(O)n(-c1ccc(C#N)c(c1)-c1ccccc1)c2=O Show InChI InChI=1S/C19H15N3O3/c20-11-13-6-7-14(10-15(13)12-4-2-1-3-5-12)22-18(24)17-16(23)8-9-21(17)19(22)25/h1-7,10,16,23-24H,8-9H2/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM22158

((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[methyl(1-methy...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)c1nnnn1C |r| Show InChI InChI=1S/C23H28FN7O4/c1-13(2)20-18(10-9-16(32)11-17(33)12-19(34)35)21(14-5-7-15(24)8-6-14)26-22(25-20)30(3)23-27-28-29-31(23)4/h5-10,13,16-17,32-33H,11-12H2,1-4H3,(H,34,35)/b10-9+/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | 1.40 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM22157
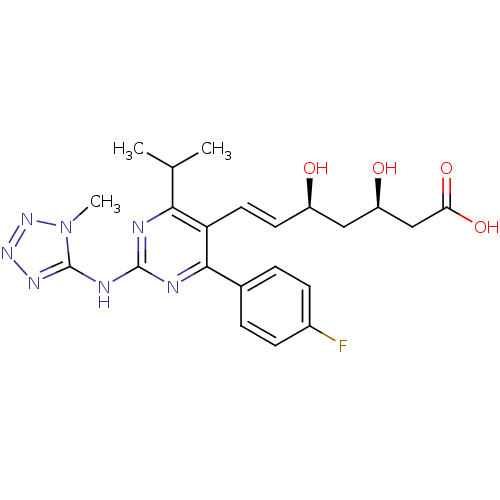
((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[(1-methyl-1H-1...)Show SMILES CC(C)c1nc(Nc2nnnn2C)nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C22H26FN7O4/c1-12(2)19-17(9-8-15(31)10-16(32)11-18(33)34)20(13-4-6-14(23)7-5-13)25-21(24-19)26-22-27-28-29-30(22)3/h4-9,12,15-16,31-32H,10-11H2,1-3H3,(H,33,34)(H,24,25,26,27,29)/b9-8+/t15-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | 1.5 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM22160
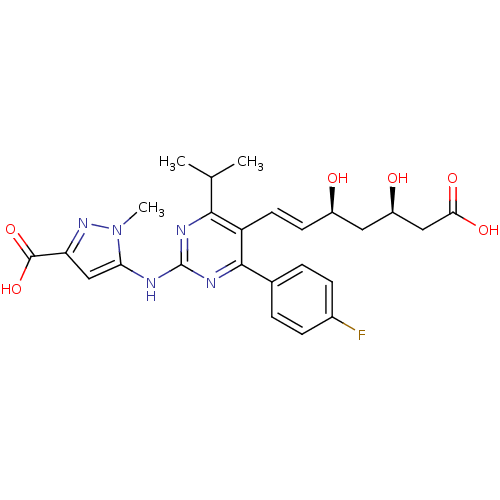
(pyrazole compound, 14a | sodium 5-({5-[(1E,3S,5R)-...)Show SMILES CC(C)c1nc(Nc2cc(nn2C)C(O)=O)nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C25H28FN5O6/c1-13(2)22-18(9-8-16(32)10-17(33)11-21(34)35)23(14-4-6-15(26)7-5-14)29-25(28-22)27-20-12-19(24(36)37)30-31(20)3/h4-9,12-13,16-17,32-33H,10-11H2,1-3H3,(H,34,35)(H,36,37)(H,27,28,29)/b9-8+/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | 13.7 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24457
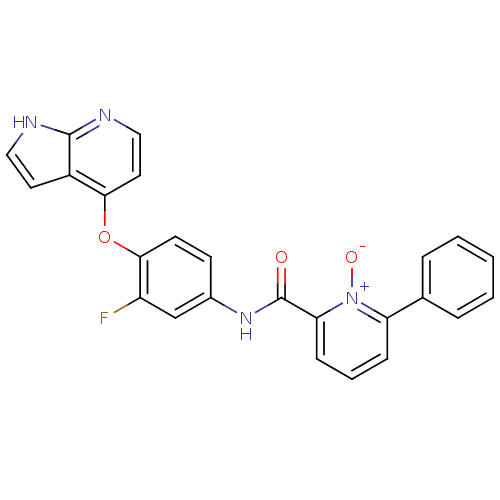
(2-[(3-fluoro-4-{1H-pyrrolo[2,3-b]pyridin-4-yloxy}p...)Show SMILES [O-][n+]1c(cccc1-c1ccccc1)C(=O)Nc1ccc(Oc2ccnc3[nH]ccc23)c(F)c1 Show InChI InChI=1S/C25H17FN4O3/c26-19-15-17(9-10-23(19)33-22-12-14-28-24-18(22)11-13-27-24)29-25(31)21-8-4-7-20(30(21)32)16-5-2-1-3-6-16/h1-15H,(H,27,28)(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company
| Assay Description
Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate. Dose response curves were generated to determine... |
J Med Chem 51: 5330-41 (2008)
Article DOI: 10.1021/jm800476q
BindingDB Entry DOI: 10.7270/Q2K35RZG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM22163

(BMS-644950 | sodium (3R,5S,6E)-7-[4-(4-fluoropheny...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)c1ncnn1C |r| Show InChI InChI=1S/C24H29FN6O4/c1-14(2)21-19(10-9-17(32)11-18(33)12-20(34)35)22(15-5-7-16(25)8-6-15)29-23(28-21)30(3)24-26-13-27-31(24)4/h5-10,13-14,17-18,32-33H,11-12H2,1-4H3,(H,34,35)/b10-9+/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | 3.95 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM22154

((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[(1-methyl-1H-p...)Show SMILES CC(C)c1nc(Nc2ccn(C)n2)nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C24H28FN5O4/c1-14(2)22-19(9-8-17(31)12-18(32)13-21(33)34)23(15-4-6-16(25)7-5-15)28-24(27-22)26-20-10-11-30(3)29-20/h4-11,14,17-18,31-32H,12-13H2,1-3H3,(H,33,34)(H,26,27,28,29)/b9-8+/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | 3.70 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24458
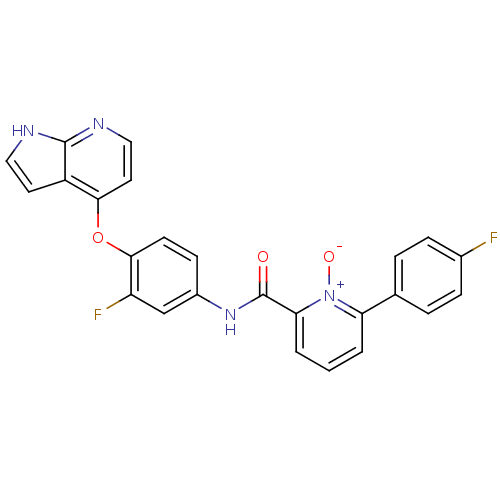
(2-[(3-fluoro-4-{1H-pyrrolo[2,3-b]pyridin-4-yloxy}p...)Show SMILES [O-][n+]1c(cccc1-c1ccc(F)cc1)C(=O)Nc1ccc(Oc2ccnc3[nH]ccc23)c(F)c1 Show InChI InChI=1S/C25H16F2N4O3/c26-16-6-4-15(5-7-16)20-2-1-3-21(31(20)33)25(32)30-17-8-9-23(19(27)14-17)34-22-11-13-29-24-18(22)10-12-28-24/h1-14H,(H,28,29)(H,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company
| Assay Description
Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate. Dose response curves were generated to determine... |
J Med Chem 51: 5330-41 (2008)
Article DOI: 10.1021/jm800476q
BindingDB Entry DOI: 10.7270/Q2K35RZG |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24440
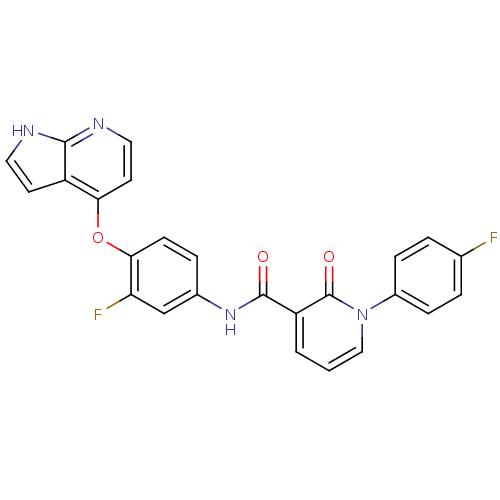
(2-pyridone analogue, 2 | JMC521251 Compound 1 | N-...)Show SMILES Fc1ccc(cc1)-n1cccc(C(=O)Nc2ccc(Oc3ccnc4[nH]ccc34)c(F)c2)c1=O Show InChI InChI=1S/C25H16F2N4O3/c26-15-3-6-17(7-4-15)31-13-1-2-19(25(31)33)24(32)30-16-5-8-22(20(27)14-16)34-21-10-12-29-23-18(21)9-11-28-23/h1-14H,(H,28,29)(H,30,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company
| Assay Description
Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate. Dose response curves were generated to determine... |
J Med Chem 51: 5330-41 (2008)
Article DOI: 10.1021/jm800476q
BindingDB Entry DOI: 10.7270/Q2K35RZG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24441
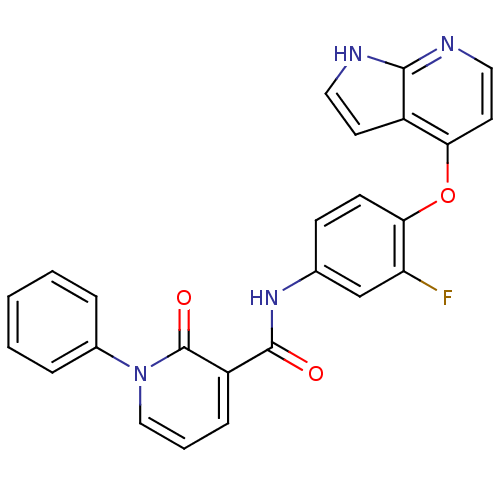
(2-pyridone analogue, 13 | N-(3-fluoro-4-{1H-pyrrol...)Show SMILES Fc1cc(NC(=O)c2cccn(-c3ccccc3)c2=O)ccc1Oc1ccnc2[nH]ccc12 Show InChI InChI=1S/C25H17FN4O3/c26-20-15-16(8-9-22(20)33-21-11-13-28-23-18(21)10-12-27-23)29-24(31)19-7-4-14-30(25(19)32)17-5-2-1-3-6-17/h1-15H,(H,27,28)(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company
| Assay Description
Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate. Dose response curves were generated to determine... |
J Med Chem 51: 5330-41 (2008)
Article DOI: 10.1021/jm800476q
BindingDB Entry DOI: 10.7270/Q2K35RZG |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50550026
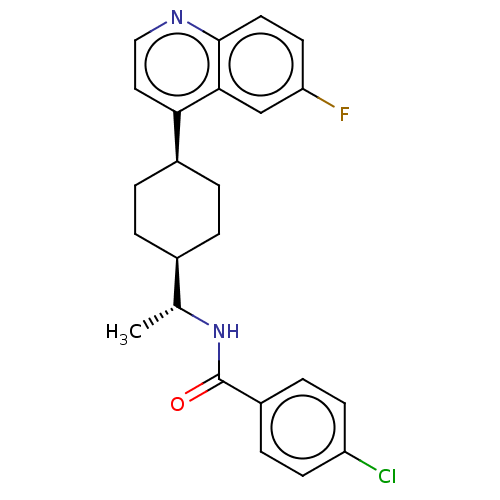
(CHEMBL4786690)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)NC(=O)c1ccc(Cl)cc1 |r,wU:4.7,18.21,wD:1.0,(55.56,-25.24,;55.58,-26.78,;54.24,-26.01,;52.9,-26.78,;52.91,-28.32,;54.25,-29.09,;55.57,-28.32,;51.57,-29.08,;50.24,-28.32,;48.91,-29.1,;48.92,-30.63,;50.25,-31.38,;50.25,-32.93,;51.58,-33.7,;52.92,-32.93,;54.25,-33.69,;52.91,-31.38,;51.58,-30.61,;56.91,-26.01,;56.91,-24.47,;58.24,-26.78,;58.24,-28.32,;56.9,-29.08,;59.58,-29.09,;59.57,-30.63,;60.9,-31.4,;62.24,-30.63,;63.57,-31.4,;62.23,-29.09,;60.9,-28.32,)| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated mouse M109 cells preincubated for 2 hrs followed by IFNgamma stimulation and measured after 18 hrs by |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00668
BindingDB Entry DOI: 10.7270/Q2TM7FR5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50550030
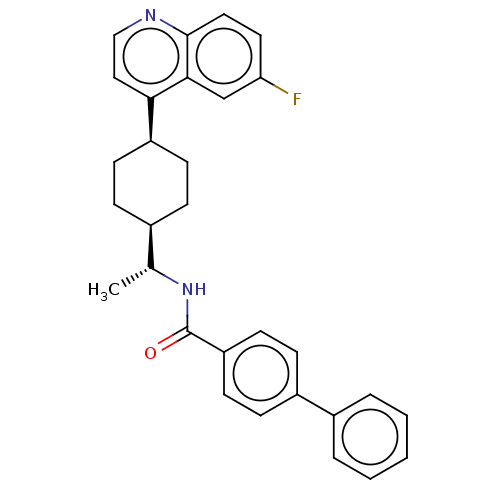
(CHEMBL4743409)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccccc1 |r,wU:4.7,18.21,wD:1.0,(9.78,-48.44,;9.8,-49.98,;8.46,-49.21,;7.12,-49.98,;7.13,-51.52,;8.47,-52.29,;9.79,-51.52,;5.79,-52.29,;4.46,-51.52,;3.14,-52.3,;3.15,-53.83,;4.47,-54.58,;4.47,-56.13,;5.8,-56.9,;7.14,-56.13,;8.47,-56.9,;7.13,-54.58,;5.8,-53.82,;11.13,-49.21,;11.13,-47.67,;12.47,-49.98,;12.46,-51.52,;11.12,-52.29,;13.8,-52.3,;13.79,-53.83,;15.12,-54.6,;16.46,-53.83,;16.46,-52.29,;15.12,-51.52,;17.78,-54.6,;17.78,-56.14,;19.11,-56.91,;20.45,-56.14,;20.45,-54.59,;19.11,-53.83,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by IFNgamma stimulation and measured after 18 hrs by |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00668
BindingDB Entry DOI: 10.7270/Q2TM7FR5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50550026

(CHEMBL4786690)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)NC(=O)c1ccc(Cl)cc1 |r,wU:4.7,18.21,wD:1.0,(55.56,-25.24,;55.58,-26.78,;54.24,-26.01,;52.9,-26.78,;52.91,-28.32,;54.25,-29.09,;55.57,-28.32,;51.57,-29.08,;50.24,-28.32,;48.91,-29.1,;48.92,-30.63,;50.25,-31.38,;50.25,-32.93,;51.58,-33.7,;52.92,-32.93,;54.25,-33.69,;52.91,-31.38,;51.58,-30.61,;56.91,-26.01,;56.91,-24.47,;58.24,-26.78,;58.24,-28.32,;56.9,-29.08,;59.58,-29.09,;59.57,-30.63,;60.9,-31.4,;62.24,-30.63,;63.57,-31.4,;62.23,-29.09,;60.9,-28.32,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by IFNgamma stimulation and measured after 18 hrs by |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00668
BindingDB Entry DOI: 10.7270/Q2TM7FR5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50285416
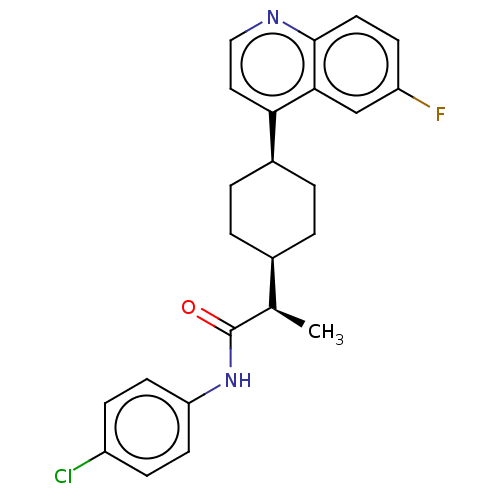
(CHEMBL4161733)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)C(=O)Nc1ccc(Cl)cc1 |r,wU:18.21,1.0,wD:4.7,(66.16,-9.22,;66.17,-7.68,;66.16,-6.15,;67.5,-5.38,;68.84,-6.15,;68.83,-7.69,;67.5,-8.46,;70.18,-5.39,;71.5,-6.16,;72.84,-5.4,;72.84,-3.85,;71.5,-3.08,;71.5,-1.55,;70.17,-.79,;68.84,-1.56,;67.5,-.8,;68.85,-3.09,;70.18,-3.85,;64.83,-8.46,;64.84,-10,;63.5,-7.69,;63.49,-6.14,;62.16,-8.46,;60.83,-7.69,;59.49,-8.47,;58.15,-7.69,;58.16,-6.14,;56.82,-5.38,;59.49,-5.38,;60.83,-6.14,)| Show InChI InChI=1S/C24H24ClFN2O/c1-15(24(29)28-20-9-6-18(25)7-10-20)16-2-4-17(5-3-16)21-12-13-27-23-11-8-19(26)14-22(21)23/h6-17H,2-5H2,1H3,(H,28,29)/t15-,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated human HeLa cells preincubated for 2 hrs followed by IFNgamma stimulation and measured after 18 hrs by |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00668
BindingDB Entry DOI: 10.7270/Q2TM7FR5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM22152

((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[N-(1-methyl-1H...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(c1ccnn1C)S(C)(=O)=O |r| Show InChI InChI=1S/C25H30FN5O6S/c1-15(2)23-20(10-9-18(32)13-19(33)14-22(34)35)24(16-5-7-17(26)8-6-16)29-25(28-23)31(38(4,36)37)21-11-12-27-30(21)3/h5-12,15,18-19,32-33H,13-14H2,1-4H3,(H,34,35)/b10-9+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | 5.40 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM22147

((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(1,2-oxazol-3-y...)Show SMILES CC(C)c1nc(Nc2ccon2)nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C23H25FN4O5/c1-13(2)21-18(8-7-16(29)11-17(30)12-20(31)32)22(14-3-5-15(24)6-4-14)27-23(26-21)25-19-9-10-33-28-19/h3-10,13,16-17,29-30H,11-12H2,1-2H3,(H,31,32)(H,25,26,27,28)/b8-7+/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | 1.40 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24454
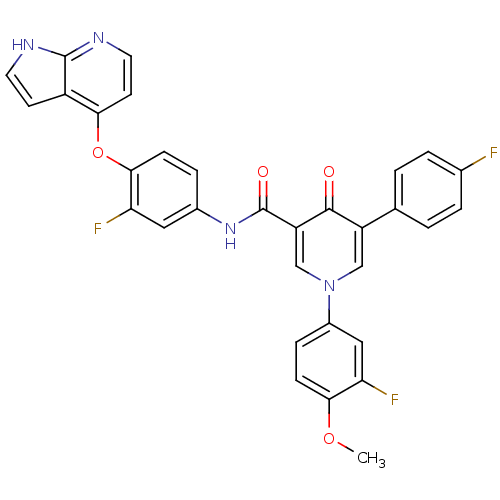
(1-(3-fluoro-4-methoxyphenyl)-N-(3-fluoro-4-{1H-pyr...)Show SMILES COc1ccc(cc1F)-n1cc(C(=O)Nc2ccc(Oc3ccnc4[nH]ccc34)c(F)c2)c(=O)c(c1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H21F3N4O4/c1-42-28-9-7-21(15-26(28)35)39-16-23(18-2-4-19(33)5-3-18)30(40)24(17-39)32(41)38-20-6-8-29(25(34)14-20)43-27-11-13-37-31-22(27)10-12-36-31/h2-17H,1H3,(H,36,37)(H,38,41) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company
| Assay Description
Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate. Dose response curves were generated to determine... |
J Med Chem 51: 5330-41 (2008)
Article DOI: 10.1021/jm800476q
BindingDB Entry DOI: 10.7270/Q2K35RZG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
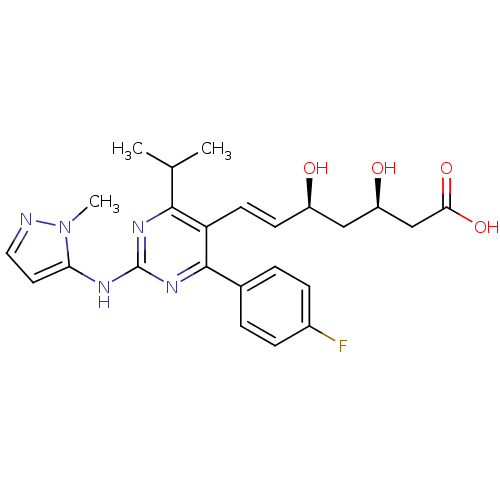
(Rattus norvegicus (rat)) | BDBM22150
((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[(1-methyl-1H-p...)Show SMILES CC(C)c1nc(Nc2ccnn2C)nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C24H28FN5O4/c1-14(2)22-19(9-8-17(31)12-18(32)13-21(33)34)23(15-4-6-16(25)7-5-15)29-24(28-22)27-20-10-11-26-30(20)3/h4-11,14,17-18,31-32H,12-13H2,1-3H3,(H,33,34)(H,27,28,29)/b9-8+/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | 1.30 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM22155

((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[methyl(1-methy...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)c1ccn(C)n1 |r| Show InChI InChI=1S/C25H30FN5O4/c1-15(2)23-20(10-9-18(32)13-19(33)14-22(34)35)24(16-5-7-17(26)8-6-16)28-25(27-23)31(4)21-11-12-30(3)29-21/h5-12,15,18-19,32-33H,13-14H2,1-4H3,(H,34,35)/b10-9+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | 1.20 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
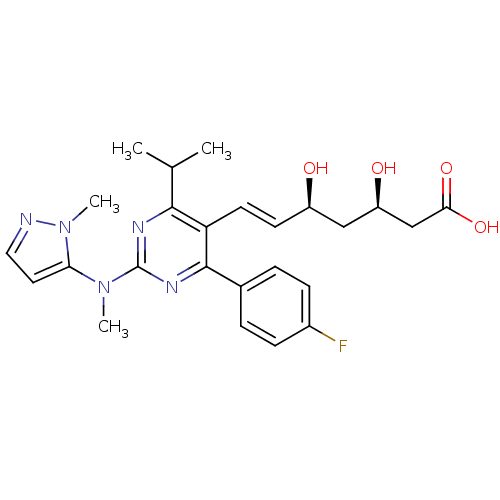
(Rattus norvegicus (rat)) | BDBM22151
((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[methyl(1-methy...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)c1ccnn1C |r| Show InChI InChI=1S/C25H30FN5O4/c1-15(2)23-20(10-9-18(32)13-19(33)14-22(34)35)24(16-5-7-17(26)8-6-16)29-25(28-23)30(3)21-11-12-27-31(21)4/h5-12,15,18-19,32-33H,13-14H2,1-4H3,(H,34,35)/b10-9+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | 2.5 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
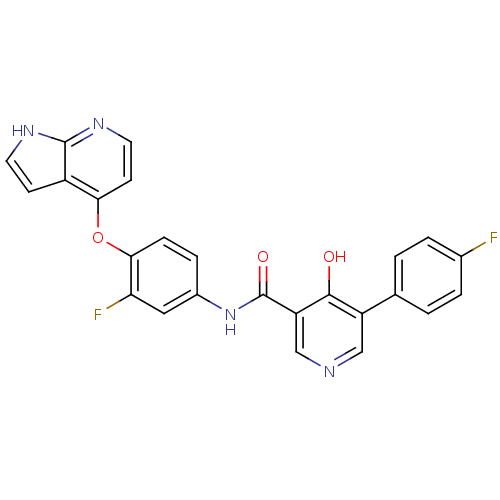
(Homo sapiens (Human)) | BDBM24449
(4-pyridone analogue, 3 | N-(3-fluoro-4-{1H-pyrrolo...)Show SMILES Oc1c(cncc1-c1ccc(F)cc1)C(=O)Nc1ccc(Oc2ccnc3[nH]ccc23)c(F)c1 Show InChI InChI=1S/C25H16F2N4O3/c26-15-3-1-14(2-4-15)18-12-28-13-19(23(18)32)25(33)31-16-5-6-22(20(27)11-16)34-21-8-10-30-24-17(21)7-9-29-24/h1-13H,(H,28,32)(H,29,30)(H,31,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company
| Assay Description
Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate. Dose response curves were generated to determine... |
J Med Chem 51: 5330-41 (2008)
Article DOI: 10.1021/jm800476q
BindingDB Entry DOI: 10.7270/Q2K35RZG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM22162
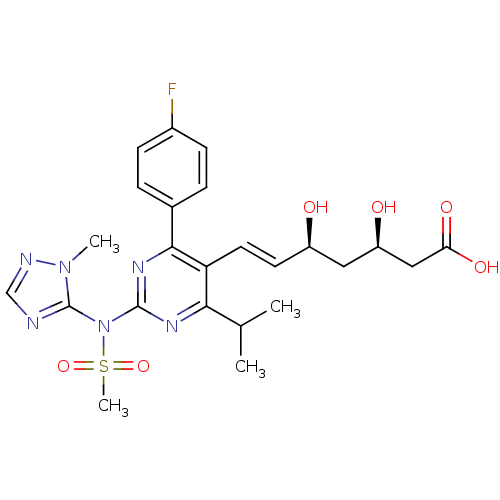
(sodium (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[N-(1-me...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(c1ncnn1C)S(C)(=O)=O |r| Show InChI InChI=1S/C24H29FN6O6S/c1-14(2)21-19(10-9-17(32)11-18(33)12-20(34)35)22(15-5-7-16(25)8-6-15)29-23(28-21)31(38(4,36)37)24-26-13-27-30(24)3/h5-10,13-14,17-18,32-33H,11-12H2,1-4H3,(H,34,35)/b10-9+/t17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | 7.5 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM22161

(sodium (3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[(1-meth...)Show SMILES CC(C)c1nc(Nc2ncnn2C)nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O |r| Show InChI InChI=1S/C23H27FN6O4/c1-13(2)20-18(9-8-16(31)10-17(32)11-19(33)34)21(14-4-6-15(24)7-5-14)28-22(27-20)29-23-25-12-26-30(23)3/h4-9,12-13,16-17,31-32H,10-11H2,1-3H3,(H,33,34)(H,25,26,27,28,29)/b9-8+/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | 3.70 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Mus musculus) | BDBM50550030

(CHEMBL4743409)Show SMILES [H][C@]1(CC[C@@H](CC1)c1ccnc2ccc(F)cc12)[C@@H](C)NC(=O)c1ccc(cc1)-c1ccccc1 |r,wU:4.7,18.21,wD:1.0,(9.78,-48.44,;9.8,-49.98,;8.46,-49.21,;7.12,-49.98,;7.13,-51.52,;8.47,-52.29,;9.79,-51.52,;5.79,-52.29,;4.46,-51.52,;3.14,-52.3,;3.15,-53.83,;4.47,-54.58,;4.47,-56.13,;5.8,-56.9,;7.14,-56.13,;8.47,-56.9,;7.13,-54.58,;5.8,-53.82,;11.13,-49.21,;11.13,-47.67,;12.47,-49.98,;12.46,-51.52,;11.12,-52.29,;13.8,-52.3,;13.79,-53.83,;15.12,-54.6,;16.46,-53.83,;16.46,-52.29,;15.12,-51.52,;17.78,-54.6,;17.78,-56.14,;19.11,-56.91,;20.45,-56.14,;20.45,-54.59,;19.11,-53.83,)| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 in IFNgamma-stimulated mouse M109 cells preincubated for 2 hrs followed by IFNgamma stimulation and measured after 18 hrs by |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.0c00668
BindingDB Entry DOI: 10.7270/Q2TM7FR5 |
More data for this
Ligand-Target Pair | |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24450
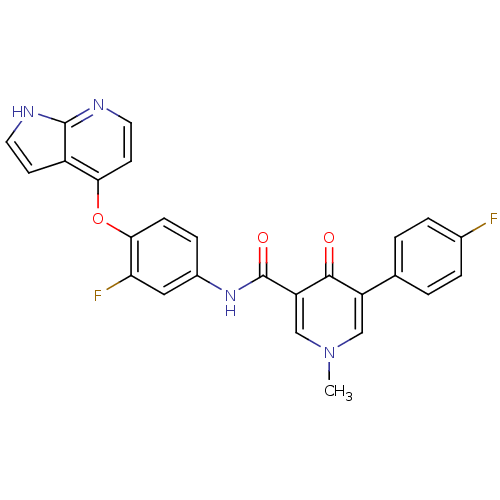
(4-pyridone analogue, 34 | N-(3-fluoro-4-{1H-pyrrol...)Show SMILES Cn1cc(C(=O)Nc2ccc(Oc3ccnc4[nH]ccc34)c(F)c2)c(=O)c(c1)-c1ccc(F)cc1 Show InChI InChI=1S/C26H18F2N4O3/c1-32-13-19(15-2-4-16(27)5-3-15)24(33)20(14-32)26(34)31-17-6-7-23(21(28)12-17)35-22-9-11-30-25-18(22)8-10-29-25/h2-14H,1H3,(H,29,30)(H,31,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company
| Assay Description
Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate. Dose response curves were generated to determine... |
J Med Chem 51: 5330-41 (2008)
Article DOI: 10.1021/jm800476q
BindingDB Entry DOI: 10.7270/Q2K35RZG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM18372
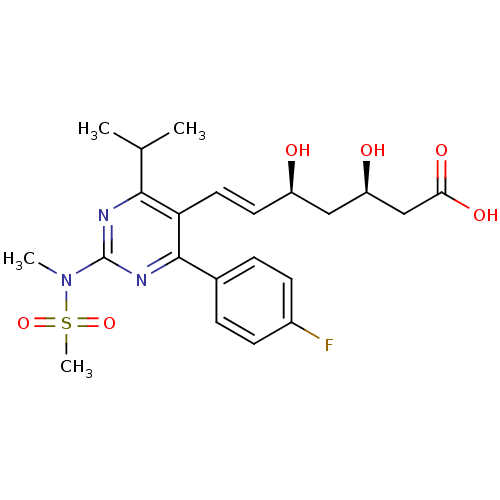
((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-(N-methylmethan...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(C)S(C)(=O)=O |r| Show InChI InChI=1S/C22H28FN3O6S/c1-13(2)20-18(10-9-16(27)11-17(28)12-19(29)30)21(14-5-7-15(23)8-6-14)25-22(24-20)26(3)33(4,31)32/h5-10,13,16-17,27-28H,11-12H2,1-4H3,(H,29,30)/b10-9+/t16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3.10 | n/a | 0.600 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hepatocyte growth factor receptor
(Homo sapiens (Human)) | BDBM24452
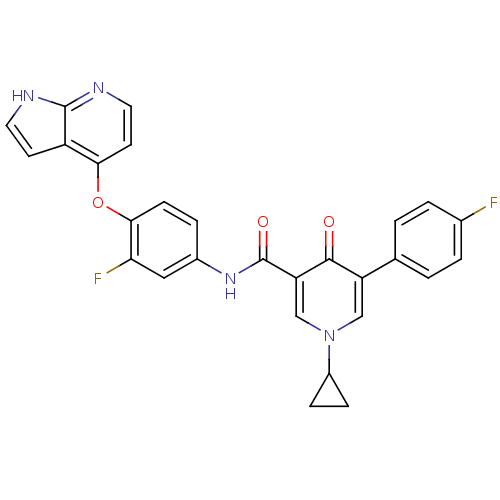
(1-cyclopropyl-N-(3-fluoro-4-{1H-pyrrolo[2,3-b]pyri...)Show SMILES Fc1ccc(cc1)-c1cn(cc(C(=O)Nc2ccc(Oc3ccnc4[nH]ccc34)c(F)c2)c1=O)C1CC1 Show InChI InChI=1S/C28H20F2N4O3/c29-17-3-1-16(2-4-17)21-14-34(19-6-7-19)15-22(26(21)35)28(36)33-18-5-8-25(23(30)13-18)37-24-10-12-32-27-20(24)9-11-31-27/h1-5,8-15,19H,6-7H2,(H,31,32)(H,33,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Bristol-Myers Squibb Company
| Assay Description
Kinase activity was assayed using baculovirus expressed GST-Met, and poly(Glu/Tyr) as the substrate. Dose response curves were generated to determine... |
J Med Chem 51: 5330-41 (2008)
Article DOI: 10.1021/jm800476q
BindingDB Entry DOI: 10.7270/Q2K35RZG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM22156
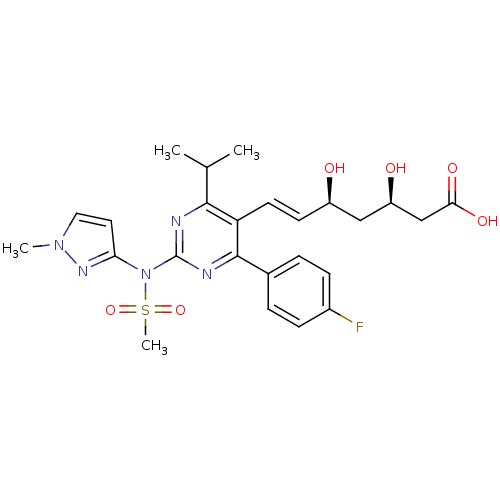
((3R,5S,6E)-7-[4-(4-fluorophenyl)-2-[N-(1-methyl-1H...)Show SMILES CC(C)c1nc(nc(-c2ccc(F)cc2)c1\C=C\[C@@H](O)C[C@@H](O)CC(O)=O)N(c1ccn(C)n1)S(C)(=O)=O |r| Show InChI InChI=1S/C25H30FN5O6S/c1-15(2)23-20(10-9-18(32)13-19(33)14-22(34)35)24(16-5-7-17(26)8-6-16)28-25(27-23)31(38(4,36)37)21-11-12-30(3)29-21/h5-12,15,18-19,32-33H,13-14H2,1-4H3,(H,34,35)/b10-9+/t18-,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | 7.70 | n/a | n/a | 7.0 | 37 |
Bristol-Myers Squibb Company
| Assay Description
Enzyme Assay for HMG-CoA reductase was based on the conversion of isotopically labeled HMG-CoA to mevalonic acid using rat liver microsomes as enzyme... |
J Med Chem 51: 2722-33 (2008)
Article DOI: 10.1021/jm800001n
BindingDB Entry DOI: 10.7270/Q2FT8JB2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data