

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50292438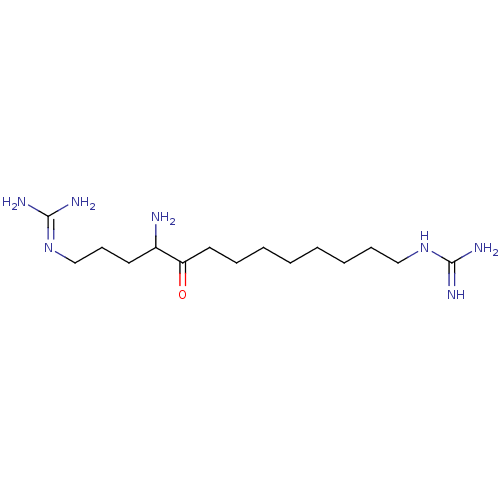 (1,-(4-amino-5-oxotridecane-1,13-diyl)diguanidine |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of QNB from muscarinic M2 receptor | J Nat Prod 58: 843-847 (1995) Article DOI: 10.1021/np50120a004 BindingDB Entry DOI: 10.7270/Q2FJ2GS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50292438 (1,-(4-amino-5-oxotridecane-1,13-diyl)diguanidine |...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of QNB from muscarinic M1 receptor | J Nat Prod 58: 843-847 (1995) Article DOI: 10.1021/np50120a004 BindingDB Entry DOI: 10.7270/Q2FJ2GS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50292438 (1,-(4-amino-5-oxotridecane-1,13-diyl)diguanidine |...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of QNB from muscarinic M4 receptor | J Nat Prod 58: 843-847 (1995) Article DOI: 10.1021/np50120a004 BindingDB Entry DOI: 10.7270/Q2FJ2GS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50197834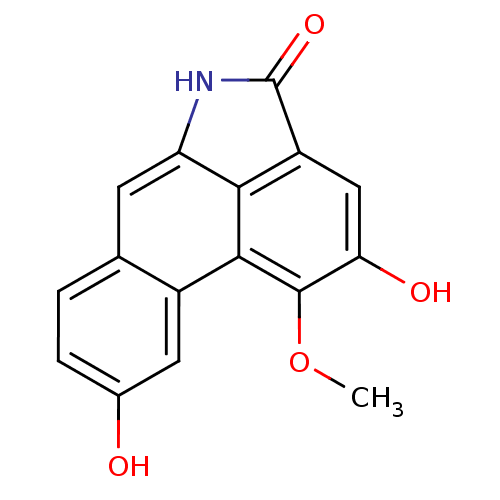 (2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of CDK2 | Bioorg Med Chem Lett 20: 1344-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.018 BindingDB Entry DOI: 10.7270/Q2BZ664X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 1 (Homo sapiens (Human)) | BDBM50197834 (2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 214 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of CDC2 | Bioorg Med Chem Lett 20: 1344-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.018 BindingDB Entry DOI: 10.7270/Q2BZ664X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50135740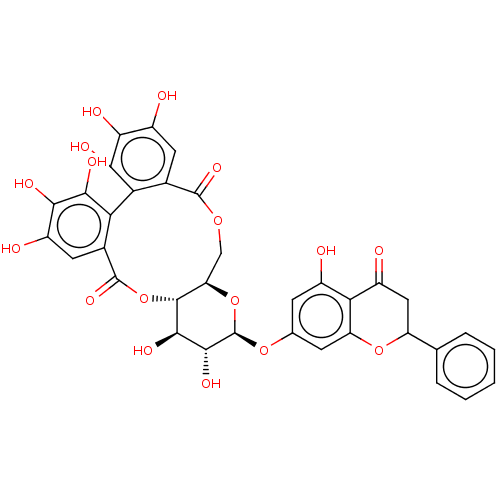 (2,3,4,5,6,7,14,15-octahydroxy-13-(5-hydroxy-4-oxo-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound evaluated in the HCV NS3 protease activity assay | Bioorg Med Chem Lett 13: 2925-8 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50070315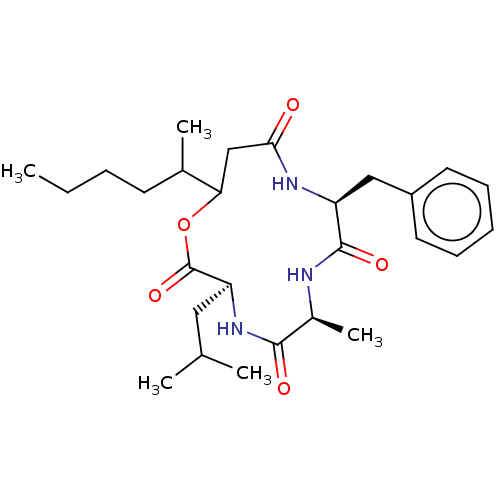 ((3S,9S)-9-Benzyl-3-isobutyl-6-methyl-13-(1-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory activity against cholesteryl ester transfer protein | Bioorg Med Chem Lett 8: 1277-80 (1999) BindingDB Entry DOI: 10.7270/Q25X282F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50135739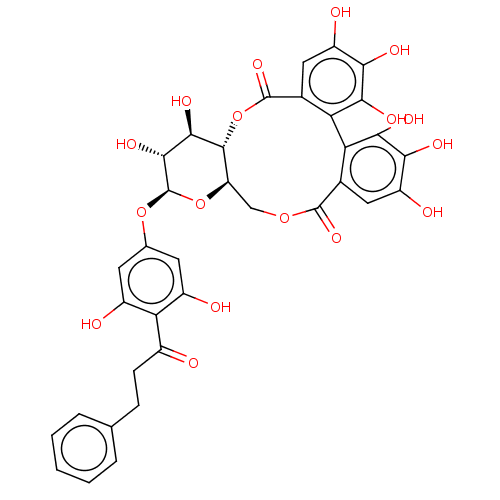 (13-[3,5-dihydroxy-4-(3-phenylpropanoyl)phenoxy]-2,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound evaluated in the HCV NS3 protease assay | Bioorg Med Chem Lett 13: 2925-8 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50292439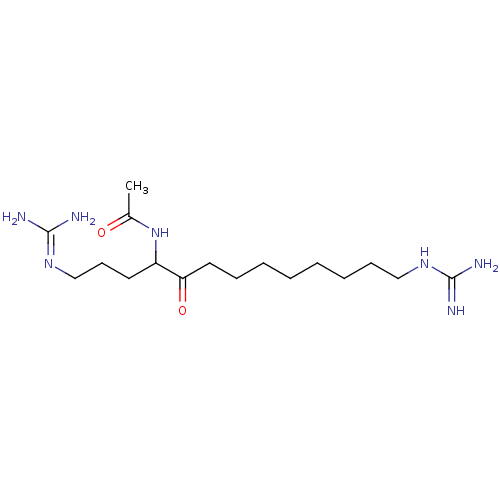 (CHEMBL479224 | N-(1,13-diguanidino-5-oxotridecan-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of QNB from muscarinic M4 receptor | J Nat Prod 58: 843-847 (1995) Article DOI: 10.1021/np50120a004 BindingDB Entry DOI: 10.7270/Q2FJ2GS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50292439 (CHEMBL479224 | N-(1,13-diguanidino-5-oxotridecan-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of QNB from muscarinic M2 receptor | J Nat Prod 58: 843-847 (1995) Article DOI: 10.1021/np50120a004 BindingDB Entry DOI: 10.7270/Q2FJ2GS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50292439 (CHEMBL479224 | N-(1,13-diguanidino-5-oxotridecan-4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Displacement of QNB from muscarinic M1 receptor | J Nat Prod 58: 843-847 (1995) Article DOI: 10.1021/np50120a004 BindingDB Entry DOI: 10.7270/Q2FJ2GS1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4 (Homo sapiens (Human)) | BDBM50197834 (2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of CDK4 | Bioorg Med Chem Lett 20: 1344-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.018 BindingDB Entry DOI: 10.7270/Q2BZ664X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM50197834 (2,9-dihydroxy-1-methoxydibenzo[cd,f]indol-4(5H)-on...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of Aurora 2 kinase | Bioorg Med Chem Lett 20: 1344-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.018 BindingDB Entry DOI: 10.7270/Q2BZ664X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50135740 (2,3,4,5,6,7,14,15-octahydroxy-13-(5-hydroxy-4-oxo-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibitory activity of the compound evaluated in the HCV protease binding assay | Bioorg Med Chem Lett 13: 2925-8 (2003) BindingDB Entry DOI: 10.7270/Q2HX1C2D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1beta ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1beta ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153764 ((Z)-1-((1S,2S)-2-((S)-hydroxy((R)-6-oxo-3,6-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1beta ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of 50% of RANTES co-receptor of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1alpha ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES co-receptor of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153764 ((Z)-1-((1S,2S)-2-((S)-hydroxy((R)-6-oxo-3,6-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1alpha ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153762 (3-(4-Methoxy-phenyl)-acrylic acid 2-{(1S,2S)-2-[(S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of MIP-1alpha ligand of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50153764 ((Z)-1-((1S,2S)-2-((S)-hydroxy((R)-6-oxo-3,6-dihydr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of RANTES co-receptor of chemokine receptor 5 induced calcium signal in U-87-CCR5 cells by calcium mobilization assay | Bioorg Med Chem Lett 14: 5339-42 (2004) Article DOI: 10.1016/j.bmcl.2004.08.021 BindingDB Entry DOI: 10.7270/Q2SJ1K31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50306911 (8-((2S,3R,4S,5S,6R)-3,4,5-Trihydroxy-6-hydroxymeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of CDK2 | Bioorg Med Chem Lett 20: 1344-6 (2010) Article DOI: 10.1016/j.bmcl.2010.01.018 BindingDB Entry DOI: 10.7270/Q2BZ664X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50070315 ((3S,9S)-9-Benzyl-3-isobutyl-6-methyl-13-(1-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description Tested for inhibition of substrate hydrolysis in the presence of porcine kidney esterase at a concentration of 45 nM | Bioorg Med Chem Lett 8: 1277-80 (1999) BindingDB Entry DOI: 10.7270/Q25X282F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesteryl ester transfer protein (Homo sapiens (Human)) | BDBM50070315 ((3S,9S)-9-Benzyl-3-isobutyl-6-methyl-13-(1-methyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering Plough Research Institute Curated by ChEMBL | Assay Description pA2 value towards endothelin receptor A was determined as functional ETA antagonism | Bioorg Med Chem Lett 8: 1277-80 (1999) BindingDB Entry DOI: 10.7270/Q25X282F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||