 Found 229 hits with Last Name = 'dawson' and Initial = 'mi'
Found 229 hits with Last Name = 'dawson' and Initial = 'mi' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151228
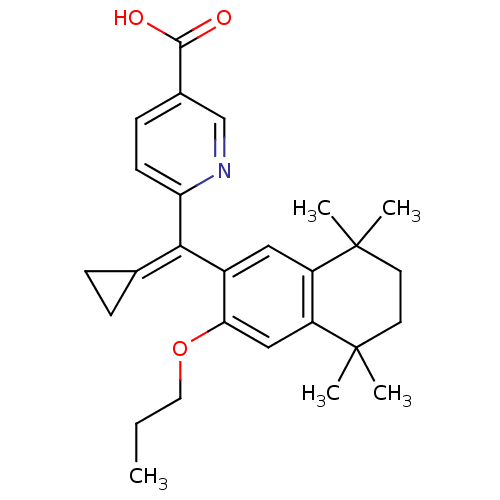
(6-[Cyclopropylidene-(5,5,8,8-tetramethyl-3-propoxy...)Show SMILES [#6]-[#6]-[#6]-[#8]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cn1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C27H33NO3/c1-6-13-31-23-15-21-20(26(2,3)11-12-27(21,4)5)14-19(23)24(17-7-8-17)22-10-9-18(16-28-22)25(29)30/h9-10,14-16H,6-8,11-13H2,1-5H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151230
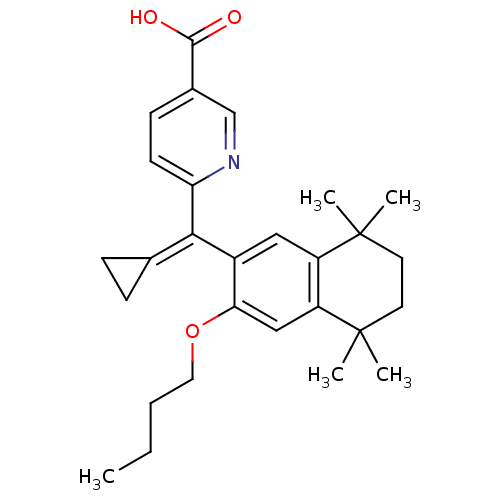
(6-[(3-Butoxy-5,5,8,8-tetramethyl-5,6,7,8-tetrahydr...)Show SMILES [#6]-[#6]-[#6]-[#6]-[#8]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cn1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C28H35NO3/c1-6-7-14-32-24-16-22-21(27(2,3)12-13-28(22,4)5)15-20(24)25(18-8-9-18)23-11-10-19(17-29-23)26(30)31/h10-11,15-17H,6-9,12-14H2,1-5H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151229
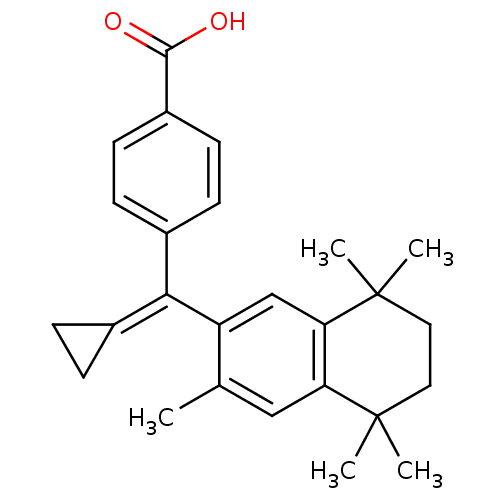
(4-[Cyclopropylidene-(3,5,5,8,8-pentamethyl-5,6,7,8...)Show SMILES [#6]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cc1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C26H30O2/c1-16-14-21-22(26(4,5)13-12-25(21,2)3)15-20(16)23(17-6-7-17)18-8-10-19(11-9-18)24(27)28/h8-11,14-15H,6-7,12-13H2,1-5H3,(H,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151231
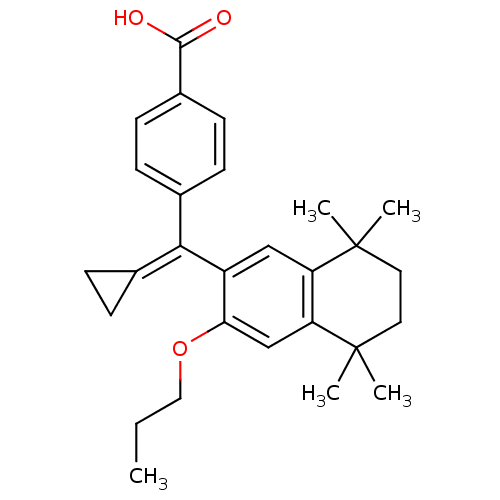
(4-[Cyclopropylidene-(5,5,8,8-tetramethyl-3-propoxy...)Show SMILES [#6]-[#6]-[#6]-[#8]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cc1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C28H34O3/c1-6-15-31-24-17-23-22(27(2,3)13-14-28(23,4)5)16-21(24)25(18-7-8-18)19-9-11-20(12-10-19)26(29)30/h9-12,16-17H,6-8,13-15H2,1-5H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151227
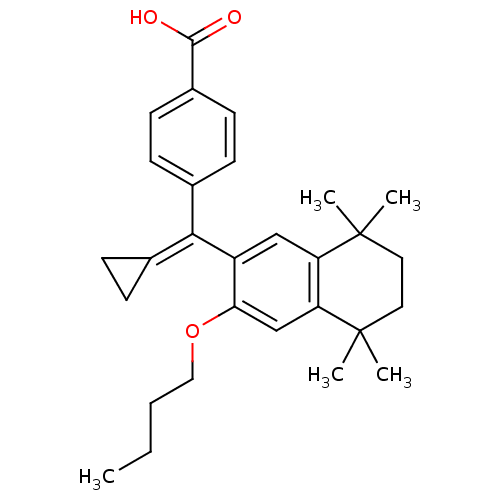
(4-[(3-Butoxy-5,5,8,8-tetramethyl-5,6,7,8-tetrahydr...)Show SMILES [#6]-[#6]-[#6]-[#6]-[#8]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cc1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C29H36O3/c1-6-7-16-32-25-18-24-23(28(2,3)14-15-29(24,4)5)17-22(25)26(19-8-9-19)20-10-12-21(13-11-20)27(30)31/h10-13,17-18H,6-9,14-16H2,1-5H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25127

((2E)-3-{3-chloro-4-[3-(3,3-dimethylbut-1-yn-1-yl)-...)Show SMILES CC(C)(C)C#Cc1cc(ccc1O)-c1ccc(\C=C\C(O)=O)cc1Cl Show InChI InChI=1S/C21H19ClO3/c1-21(2,3)11-10-16-13-15(6-8-19(16)23)17-7-4-14(12-18(17)22)5-9-20(24)25/h4-9,12-13,23H,1-3H3,(H,24,25)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50151226
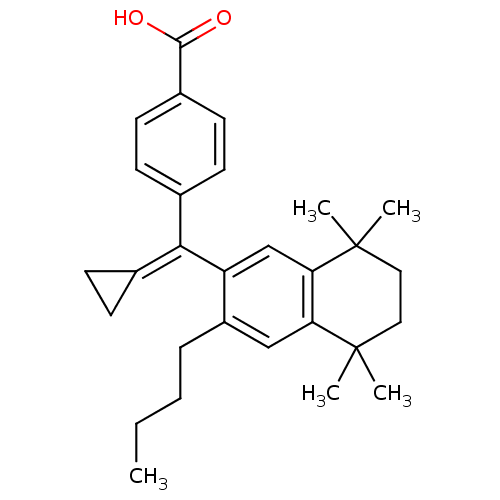
(4-[(3-Butyl-5,5,8,8-tetramethyl-5,6,7,8-tetrahydro...)Show SMILES [#6]-[#6]-[#6]-[#6]-c1cc2c(cc1\[#6](=[#6]-1\[#6]-[#6]-1)-c1ccc(cc1)-[#6](-[#8])=O)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C29H36O2/c1-6-7-8-22-17-24-25(29(4,5)16-15-28(24,2)3)18-23(22)26(19-9-10-19)20-11-13-21(14-12-20)27(30)31/h11-14,17-18H,6-10,15-16H2,1-5H3,(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-9-cis-retinoic acid binding to human retinoid X receptor alpha ligand-binding domain expressed in E. coli |
J Med Chem 47: 4360-72 (2004)
Article DOI: 10.1021/jm030651g
BindingDB Entry DOI: 10.7270/Q24749B0 |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50212333

(5-[3'-(1-adamantyl)-2-chloro-4'-hydroxy-4-biphenyl...)Show SMILES Oc1ccc(cc1C12CC3CC(CC(C3)C1)C2)-c1ccc(cc1Cl)-c1nnn[nH]1 |TLB:6:7:10:14.13.12,THB:8:9:12:16.7.15,8:7:10.9.14:12,15:7:10:14.13.12,15:13:10:16.8.7| Show InChI InChI=1S/C23H23ClN4O/c24-20-9-17(22-25-27-28-26-22)1-3-18(20)16-2-4-21(29)19(8-16)23-10-13-5-14(11-23)7-15(6-13)12-23/h1-4,8-9,13-15,29H,5-7,10-12H2,(H,25,26,27,28) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of CD45 PTP by fluorescence spectrometry |
J Med Chem 50: 2622-39 (2007)
Article DOI: 10.1021/jm0613323
BindingDB Entry DOI: 10.7270/Q2XS5V3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50212333

(5-[3'-(1-adamantyl)-2-chloro-4'-hydroxy-4-biphenyl...)Show SMILES Oc1ccc(cc1C12CC3CC(CC(C3)C1)C2)-c1ccc(cc1Cl)-c1nnn[nH]1 |TLB:6:7:10:14.13.12,THB:8:9:12:16.7.15,8:7:10.9.14:12,15:7:10:14.13.12,15:13:10:16.8.7| Show InChI InChI=1S/C23H23ClN4O/c24-20-9-17(22-25-27-28-26-22)1-3-18(20)16-2-4-21(29)19(8-16)23-10-13-5-14(11-23)7-15(6-13)12-23/h1-4,8-9,13-15,29H,5-7,10-12H2,(H,25,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of SHP2 PTP by fluorescence spectroscopy |
J Med Chem 50: 2622-39 (2007)
Article DOI: 10.1021/jm0613323
BindingDB Entry DOI: 10.7270/Q2XS5V3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25124

((2E)-3-{4-[3-(adamantan-1-yl)-4-aminophenyl]-3-chl...)Show SMILES Nc1ccc(cc1C12CC3CC(CC(C3)C1)C2)-c1ccc(\C=C\C(O)=O)cc1Cl |TLB:14:9:16:13.12.15,14:13:9.10.8:16,THB:12:11:8:13.14.15,12:13:8:11.10.16| Show InChI InChI=1S/C25H26ClNO2/c26-22-10-15(2-6-24(28)29)1-4-20(22)19-3-5-23(27)21(11-19)25-12-16-7-17(13-25)9-18(8-16)14-25/h1-6,10-11,16-18H,7-9,12-14,27H2,(H,28,29)/b6-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25122

((2E)-3-{4-[3-(adamantan-1-yl)-4-hydroxyphenyl]-3-c...)Show SMILES OC(=O)\C=C\c1ccc(c(Cl)c1)-c1ccc(O)c(c1)C12CC3CC(CC(C3)C1)C2 |TLB:26:21:28:25.24.27,26:25:21.22.20:28,THB:24:23:20:25.26.27,24:25:20:23.22.28| Show InChI InChI=1S/C25H25ClO3/c26-22-10-15(2-6-24(28)29)1-4-20(22)19-3-5-23(27)21(11-19)25-12-16-7-17(13-25)9-18(8-16)14-25/h1-6,10-11,16-18,27H,7-9,12-14H2,(H,28,29)/b6-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25125
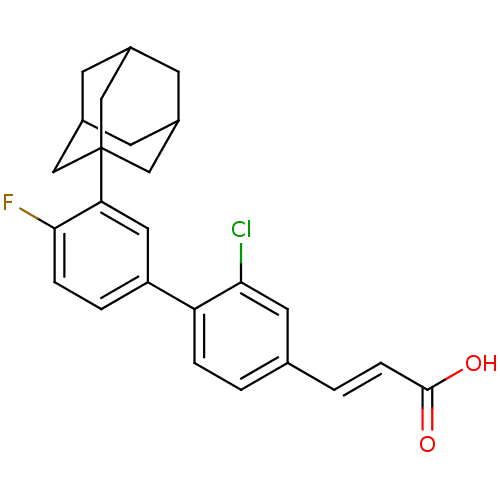
((2E)-3-{4-[3-(adamantan-1-yl)-4-fluorophenyl]-3-ch...)Show SMILES OC(=O)\C=C\c1ccc(c(Cl)c1)-c1ccc(F)c(c1)C12CC3CC(CC(C3)C1)C2 |TLB:26:21:28:25.24.27,26:25:21.22.20:28,THB:24:23:20:25.26.27,24:25:20:23.22.28| Show InChI InChI=1S/C25H24ClFO2/c26-22-10-15(2-6-24(28)29)1-4-20(22)19-3-5-23(27)21(11-19)25-12-16-7-17(13-25)9-18(8-16)14-25/h1-6,10-11,16-18H,7-9,12-14H2,(H,28,29)/b6-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.11E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11
(Homo sapiens (Human)) | BDBM50212331
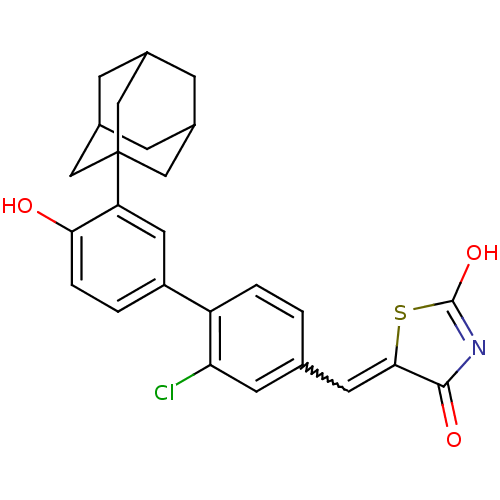
(5-[1-(3'-adamantan-1-yl-2-chloro-4'-hydroxy-biphen...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(c(Cl)c1)-c1ccc(O)c(c1)C12CC3CC(CC(C3)C1)C2 |w:7.8,t:1,TLB:20:22:25:29.28.27,THB:23:24:27:31.22.30,23:22:25.24.29:27,30:22:25:29.28.27,30:28:25:31.23.22| Show InChI InChI=1S/C26H24ClNO3S/c27-21-8-14(9-23-24(30)28-25(31)32-23)1-3-19(21)18-2-4-22(29)20(10-18)26-11-15-5-16(12-26)7-17(6-15)13-26/h1-4,8-10,15-17,29H,5-7,11-13H2,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of SHP2 PTP by fluorescence spectroscopy |
J Med Chem 50: 2622-39 (2007)
Article DOI: 10.1021/jm0613323
BindingDB Entry DOI: 10.7270/Q2XS5V3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25131

((2E)-3-{3-chloro-4-[3-(3,5-dimethyladamantan-1-yl)...)Show SMILES CC12CC3CC(C)(C1)CC(C3)(C2)c1cc(ccc1O)-c1ccc(\C=C\C(O)=O)cc1Cl |TLB:4:3:11:7.8.5,4:5:11:3.2.10,THB:2:1:8:3.4.10,2:3:1.7.11:8| Show InChI InChI=1S/C27H29ClO3/c1-25-11-18-12-26(2,14-25)16-27(13-18,15-25)21-10-19(5-7-23(21)29)20-6-3-17(9-22(20)28)4-8-24(30)31/h3-10,18,29H,11-16H2,1-2H3,(H,30,31)/b8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.27E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Protein tyrosine phosphatase receptor type C-associated protein
(Homo sapiens (Human)) | BDBM50212331

(5-[1-(3'-adamantan-1-yl-2-chloro-4'-hydroxy-biphen...)Show SMILES OC1=NC(=O)C(S1)=Cc1ccc(c(Cl)c1)-c1ccc(O)c(c1)C12CC3CC(CC(C3)C1)C2 |w:7.8,t:1,TLB:20:22:25:29.28.27,THB:23:24:27:31.22.30,23:22:25.24.29:27,30:22:25:29.28.27,30:28:25:31.23.22| Show InChI InChI=1S/C26H24ClNO3S/c27-21-8-14(9-23-24(30)28-25(31)32-23)1-3-19(21)18-2-4-22(29)20(10-18)26-11-15-5-16(12-26)7-17(6-15)13-26/h1-4,8-10,15-17,29H,5-7,11-13H2,(H,28,30,31) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Institute for Medical Research
Curated by ChEMBL
| Assay Description
Inhibition of CD45 PTP by fluorescence spectrometry |
J Med Chem 50: 2622-39 (2007)
Article DOI: 10.1021/jm0613323
BindingDB Entry DOI: 10.7270/Q2XS5V3H |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25129
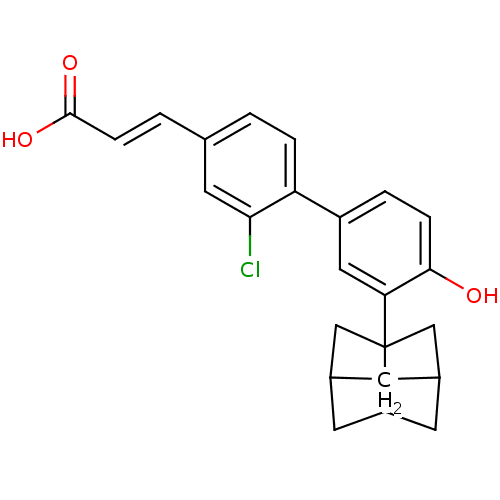
((2E)-3-[3-chloro-4-(4-hydroxy-3-{tricyclo[3.3.1.0^...)Show SMILES OC(=O)\C=C\c1ccc(c(Cl)c1)-c1ccc(O)c(c1)C12CC3CC1CC(C2)C3 |THB:24:23:20:25.27.26,24:25:23.22:20| Show InChI InChI=1S/C24H23ClO3/c25-21-10-14(2-6-23(27)28)1-4-19(21)17-3-5-22(26)20(11-17)24-12-15-7-16(13-24)9-18(24)8-15/h1-6,10-11,15-16,18,26H,7-9,12-13H2,(H,27,28)/b6-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.91E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25123

((2E)-3-{4-[3-(adamantan-1-yl)-4-acetamidophenyl]-3...)Show SMILES CC(=O)Nc1ccc(cc1C12CC3CC(CC(C3)C1)C2)-c1ccc(\C=C\C(O)=O)cc1Cl |TLB:17:12:19:16.15.18,17:16:12.13.11:19,THB:15:14:11:16.17.18,15:16:11:14.13.19| Show InChI InChI=1S/C27H28ClNO3/c1-16(30)29-25-6-4-21(22-5-2-17(11-24(22)28)3-7-26(31)32)12-23(25)27-13-18-8-19(14-27)10-20(9-18)15-27/h2-7,11-12,18-20H,8-10,13-15H2,1H3,(H,29,30)(H,31,32)/b7-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.55E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25133

((2E)-3-{3-chloro-4-[3-(3-ethylpentan-3-yl)-4-hydro...)Show SMILES CCC(CC)(CC)c1cc(ccc1O)-c1ccc(\C=C\C(O)=O)cc1Cl Show InChI InChI=1S/C22H25ClO3/c1-4-22(5-2,6-3)18-14-16(9-11-20(18)24)17-10-7-15(13-19(17)23)8-12-21(25)26/h7-14,24H,4-6H2,1-3H3,(H,25,26)/b12-8+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.37E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25130
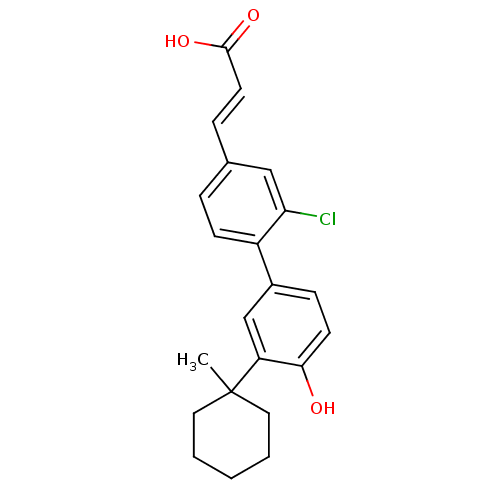
((2E)-3-{3-chloro-4-[4-hydroxy-3-(1-methylcyclohexy...)Show SMILES CC1(CCCCC1)c1cc(ccc1O)-c1ccc(\C=C\C(O)=O)cc1Cl Show InChI InChI=1S/C22H23ClO3/c1-22(11-3-2-4-12-22)18-14-16(7-9-20(18)24)17-8-5-15(13-19(17)23)6-10-21(25)26/h5-10,13-14,24H,2-4,11-12H2,1H3,(H,25,26)/b10-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25132

((2E)-3-{3-chloro-4-[4-hydroxy-3-(2-methyl-1,3-dith...)Show SMILES CC1(SCCCS1)c1cc(ccc1O)-c1ccc(\C=C\C(O)=O)cc1Cl Show InChI InChI=1S/C20H19ClO3S2/c1-20(25-9-2-10-26-20)16-12-14(5-7-18(16)22)15-6-3-13(11-17(15)21)4-8-19(23)24/h3-8,11-12,22H,2,9-10H2,1H3,(H,23,24)/b8-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25128

((2E)-3-{3-chloro-4-[3-(2,6-dimethylphenyl)-4-hydro...)Show SMILES Cc1cccc(C)c1-c1cc(ccc1O)-c1ccc(\C=C\C(O)=O)cc1Cl |(-4.83,6.88,;-6.17,6.11,;-7.5,6.88,;-8.83,6.11,;-8.83,4.57,;-7.5,3.8,;-7.5,2.26,;-6.17,4.57,;-4.83,3.8,;-3.5,4.57,;-2.17,3.8,;-2.17,2.26,;-3.5,1.49,;-4.83,2.26,;-5.6,.93,;-.83,4.57,;-.83,6.11,;.5,6.88,;1.83,6.11,;3.17,6.88,;4.5,6.11,;5.84,6.88,;7.17,6.11,;5.84,8.42,;1.83,4.57,;.5,3.8,;.5,2.26,)| Show InChI InChI=1S/C23H19ClO3/c1-14-4-3-5-15(2)23(14)19-13-17(8-10-21(19)25)18-9-6-16(12-20(18)24)7-11-22(26)27/h3-13,25H,1-2H3,(H,26,27)/b11-7+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.99E+4 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 11 [205-593]
(Homo sapiens (Human)) | BDBM25126

((2E)-3-[3-chloro-4-(4-fluorophenyl)phenyl]prop-2-e...)Show InChI InChI=1S/C15H10ClFO2/c16-14-9-10(2-8-15(18)19)1-7-13(14)11-3-5-12(17)6-4-11/h1-9H,(H,18,19)/b8-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | 6.0 | 30 |
Burnham Institute for Medical Research
| Assay Description
The phosphatase-catalyzed hydrolysis of 6,8-difluoro-4-methylumbelliferyl phosphate was assayed in the presence of each test compound. The fluorescen... |
J Med Chem 51: 5650-62 (2008)
Article DOI: 10.1021/jm800456k
BindingDB Entry DOI: 10.7270/Q24T6GNQ |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50033075
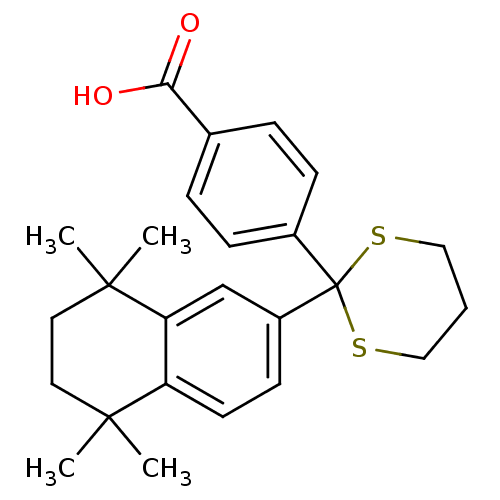
(4-[2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1(SCCCS1)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H30O2S2/c1-23(2)12-13-24(3,4)21-16-19(10-11-20(21)23)25(28-14-5-15-29-25)18-8-6-17(7-9-18)22(26)27/h6-11,16H,5,12-15H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 630 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50033070

(4-[2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1(OCCCO1)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C25H30O4/c1-23(2)12-13-24(3,4)21-16-19(10-11-20(21)23)25(28-14-5-15-29-25)18-8-6-17(7-9-18)22(26)27/h6-11,16H,5,12-15H2,1-4H3,(H,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 750 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50033063
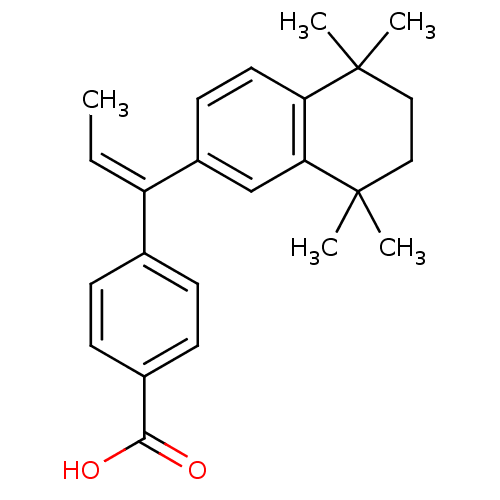
(4-[(Z)-1-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES C\C=C(\c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-6-19(16-7-9-17(10-8-16)22(25)26)18-11-12-20-21(15-18)24(4,5)14-13-23(20,2)3/h6-12,15H,13-14H2,1-5H3,(H,25,26)/b19-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 55 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50033066

(4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H24O3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)19(23)14-5-7-15(8-6-14)20(24)25/h5-10,13H,11-12H2,1-4H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50033062
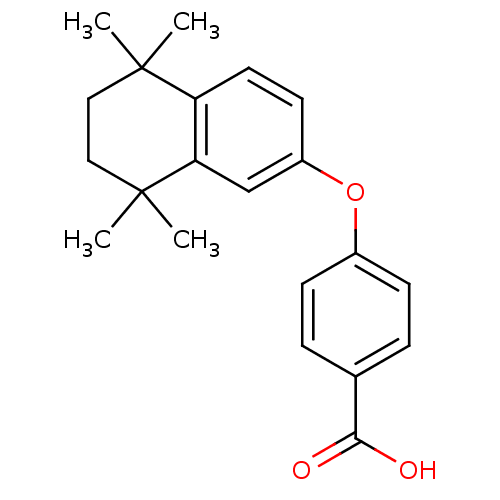
(4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(Oc3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C21H24O3/c1-20(2)11-12-21(3,4)18-13-16(9-10-17(18)20)24-15-7-5-14(6-8-15)19(22)23/h5-10,13H,11-12H2,1-4H3,(H,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50033063

(4-[(Z)-1-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES C\C=C(\c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-6-19(16-7-9-17(10-8-16)22(25)26)18-11-12-20-21(15-18)24(4,5)14-13-23(20,2)3/h6-12,15H,13-14H2,1-5H3,(H,25,26)/b19-6- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 160 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50032667
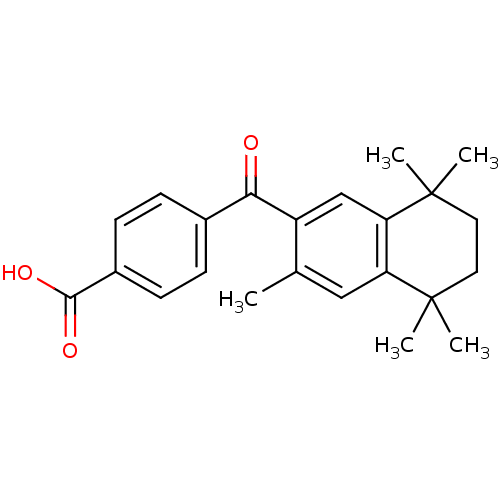
(4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1C(=O)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H26O3/c1-14-12-18-19(23(4,5)11-10-22(18,2)3)13-17(14)20(24)15-6-8-16(9-7-15)21(25)26/h6-9,12-13H,10-11H2,1-5H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 360 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50032667

(4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1C(=O)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H26O3/c1-14-12-18-19(23(4,5)11-10-22(18,2)3)13-17(14)20(24)15-6-8-16(9-7-15)21(25)26/h6-9,12-13H,10-11H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-gamma |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50033064
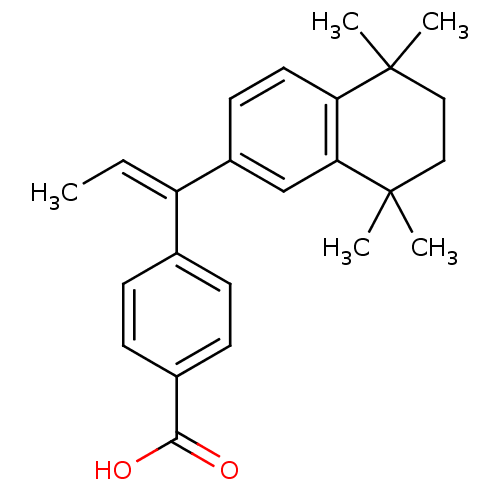
(4-[(E)-1-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES C\C=C(/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-6-19(16-7-9-17(10-8-16)22(25)26)18-11-12-20-21(15-18)24(4,5)14-13-23(20,2)3/h6-12,15H,13-14H2,1-5H3,(H,25,26)/b19-6+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50033065
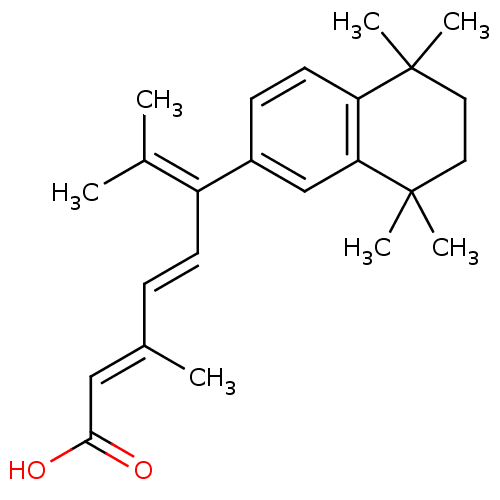
((2E,4E)-3,7-Dimethyl-6-(5,5,8,8-tetramethyl-5,6,7,...)Show SMILES [#6]\[#6](-[#6])=[#6](\[#6]=[#6]\[#6](\[#6])=[#6]\[#6](-[#8])=O)/c1ccc2c(c1)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C24H32O2/c1-16(2)19(10-8-17(3)14-22(25)26)18-9-11-20-21(15-18)24(6,7)13-12-23(20,4)5/h8-11,14-15H,12-13H2,1-7H3,(H,25,26)/b10-8+,17-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 33 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50033065

((2E,4E)-3,7-Dimethyl-6-(5,5,8,8-tetramethyl-5,6,7,...)Show SMILES [#6]\[#6](-[#6])=[#6](\[#6]=[#6]\[#6](\[#6])=[#6]\[#6](-[#8])=O)/c1ccc2c(c1)C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C24H32O2/c1-16(2)19(10-8-17(3)14-22(25)26)18-9-11-20-21(15-18)24(6,7)13-12-23(20,4)5/h8-11,14-15H,12-13H2,1-7H3,(H,25,26)/b10-8+,17-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 380 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50033066

(4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H24O3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)19(23)14-5-7-15(8-6-14)20(24)25/h5-10,13H,11-12H2,1-4H3,(H,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50033063

(4-[(Z)-1-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES C\C=C(\c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-6-19(16-7-9-17(10-8-16)22(25)26)18-11-12-20-21(15-18)24(4,5)14-13-23(20,2)3/h6-12,15H,13-14H2,1-5H3,(H,25,26)/b19-6- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 370 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-gamma |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50032667

(4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro-naphth...)Show SMILES Cc1cc2c(cc1C(=O)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C23H26O3/c1-14-12-18-19(23(4,5)11-10-22(18,2)3)13-17(14)20(24)15-6-8-16(9-7-15)21(25)26/h6-9,12-13H,10-11H2,1-5H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 210 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM31892

(9-cis retinoic acid | 9C-RA | CHEMBL705 | Panretin...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C\C=C\C(\C)=C\C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8-,16-14+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50033067
(4-[2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1(SCCS1)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H28O2S2/c1-22(2)11-12-23(3,4)20-15-18(9-10-19(20)22)24(27-13-14-28-24)17-7-5-16(6-8-17)21(25)26/h5-10,15H,11-14H2,1-4H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-gamma |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50032675

(4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydronaph...)Show SMILES Cc1cc2c(cc1C(=C)c1ccc(cc1)C(O)=O)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-15-13-20-21(24(5,6)12-11-23(20,3)4)14-19(15)16(2)17-7-9-18(10-8-17)22(25)26/h7-10,13-14H,2,11-12H2,1,3-6H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | n/a | n/a | 112 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50033068
((2E,4E)-3-Methyl-5-[2-(3,5,5,8,8-pentamethyl-5,6,7...)Show SMILES C\C(\C=C\C1(OCCO1)c1cc2c(cc1C)C(C)(C)CCC2(C)C)=C/C(O)=O Show InChI InChI=1S/C24H32O4/c1-16(13-21(25)26)7-8-24(27-11-12-28-24)18-15-20-19(14-17(18)2)22(3,4)9-10-23(20,5)6/h7-8,13-15H,9-12H2,1-6H3,(H,25,26)/b8-7+,16-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-gamma |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50033069
((2E,4E)-3,7-Dimethyl-6-(3,5,5,8,8-pentamethyl-5,6,...)Show SMILES [#6]\[#6](-[#6])=[#6](\[#6]=[#6]\[#6](\[#6])=[#6]\[#6](-[#8])=O)/c1cc2c(cc1-[#6])C([#6])([#6])[#6]-[#6]C2([#6])[#6] Show InChI InChI=1S/C25H34O2/c1-16(2)19(10-9-17(3)13-23(26)27)20-15-22-21(14-18(20)4)24(5,6)11-12-25(22,7)8/h9-10,13-15H,11-12H2,1-8H3,(H,26,27)/b10-9+,17-13+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 140 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50033064

(4-[(E)-1-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...)Show SMILES C\C=C(/c1ccc(cc1)C(O)=O)c1ccc2c(c1)C(C)(C)CCC2(C)C Show InChI InChI=1S/C24H28O2/c1-6-19(16-7-9-17(10-8-16)22(25)26)18-11-12-20-21(15-18)24(4,5)14-13-23(20,2)3/h6-12,15H,13-14H2,1-5H3,(H,25,26)/b19-6+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-beta
(Homo sapiens (Human)) | BDBM50033071
(4-[2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C1(OCCS1)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H28O3S/c1-22(2)11-12-23(3,4)20-15-18(9-10-19(20)22)24(27-13-14-28-24)17-7-5-16(6-8-17)21(25)26/h5-10,15H,11-14H2,1-4H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| n/a | n/a | n/a | n/a | 180 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-beta |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM31883

(9-cis-retinoic acid (9cRA) | ALL-TRANS-RETINOIC AC...)Show SMILES C\C(\C=C\C1=C(C)CCCC1(C)C)=C/C=C/C(/C)=C/C(O)=O |c:4| Show InChI InChI=1S/C20H28O2/c1-15(8-6-9-16(2)14-19(21)22)11-12-18-17(3)10-7-13-20(18,4)5/h6,8-9,11-12,14H,7,10,13H2,1-5H3,(H,21,22)/b9-6+,12-11+,15-8+,16-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 530 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-gamma
(Homo sapiens (Human)) | BDBM50033066

(4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(=O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H24O3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)19(23)14-5-7-15(8-6-14)20(24)25/h5-10,13H,11-12H2,1-4H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentrations against Retinoic acid receptor RXR-gamma |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor RXR-alpha
(Homo sapiens (Human)) | BDBM50033072
(4-[Hydroxy-(5,5,8,8-tetramethyl-5,6,7,8-tetrahydro...)Show SMILES CC1(C)CCC(C)(C)c2cc(ccc12)C(O)c1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H26O3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)19(23)14-5-7-15(8-6-14)20(24)25/h5-10,13,19,23H,11-12H2,1-4H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a |
SRI International
Curated by ChEMBL
| Assay Description
Effective concentration against Retinoic acid receptor RXR-alpha |
J Med Chem 38: 3368-83 (1995)
BindingDB Entry DOI: 10.7270/Q24748WR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data