 Found 657 hits with Last Name = 'de leon' and Initial = 'p'
Found 657 hits with Last Name = 'de leon' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444982
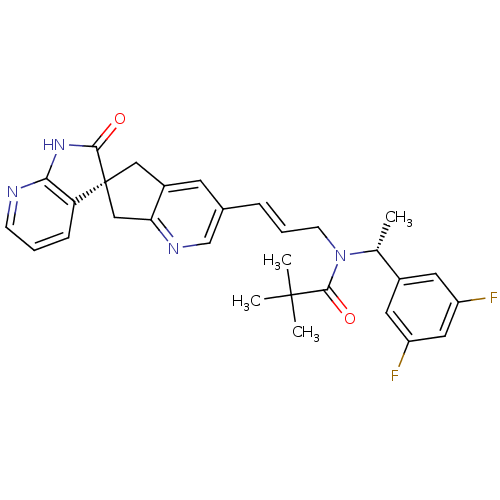
(CHEMBL3099918)Show SMILES C[C@@H](N(C\C=C\c1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C(C)(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H30F2N4O2/c1-18(20-12-22(31)14-23(32)13-20)36(28(38)29(2,3)4)10-6-7-19-11-21-15-30(16-25(21)34-17-19)24-8-5-9-33-26(24)35-27(30)37/h5-9,11-14,17-18H,10,15-16H2,1-4H3,(H,33,35,37)/b7-6+/t18-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444972
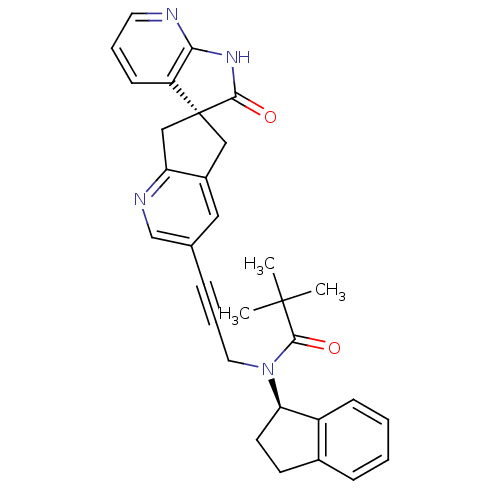
(CHEMBL3099931)Show SMILES CC(C)(C)C(=O)N(CC#Cc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C31H30N4O2/c1-30(2,3)29(37)35(26-13-12-21-9-4-5-10-23(21)26)15-7-8-20-16-22-17-31(18-25(22)33-19-20)24-11-6-14-32-27(24)34-28(31)36/h4-6,9-11,14,16,19,26H,12-13,15,17-18H2,1-3H3,(H,32,34,36)/t26-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM103499

(US8552023, 11)Show SMILES C[C@@H](N(C\C=C\c1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C1(CC1)C(F)(F)F)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H25F5N4O2/c1-17(19-11-21(31)13-22(32)12-19)39(27(41)29(6-7-29)30(33,34)35)9-3-4-18-10-20-14-28(15-24(20)37-16-18)23-5-2-8-36-25(23)38-26(28)40/h2-5,8,10-13,16-17H,6-7,9,14-15H2,1H3,(H,36,38,40)/b4-3+/t17-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444980
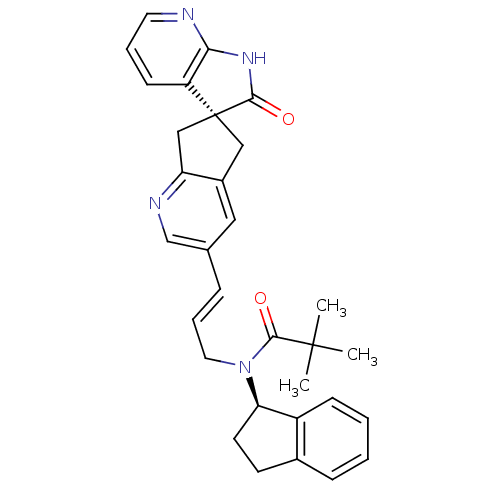
(CHEMBL3099920)Show SMILES CC(C)(C)C(=O)N(C\C=C\c1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C31H32N4O2/c1-30(2,3)29(37)35(26-13-12-21-9-4-5-10-23(21)26)15-7-8-20-16-22-17-31(18-25(22)33-19-20)24-11-6-14-32-27(24)34-28(31)36/h4-11,14,16,19,26H,12-13,15,17-18H2,1-3H3,(H,32,34,36)/b8-7+/t26-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444984
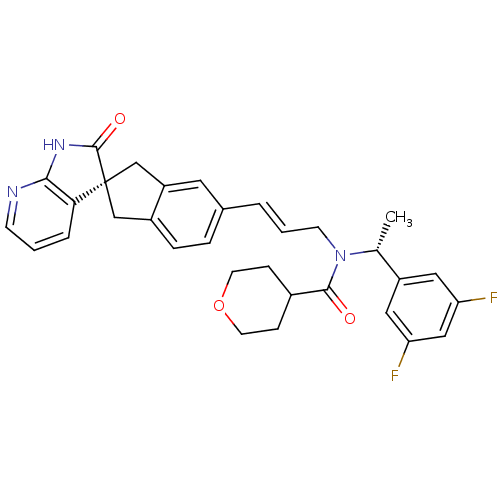
(CHEMBL3099916)Show SMILES C[C@@H](N(C\C=C\c1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C1CCOCC1)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H31F2N3O3/c1-20(24-15-26(33)17-27(34)16-24)37(30(38)22-8-12-40-13-9-22)11-3-4-21-6-7-23-18-32(19-25(23)14-21)28-5-2-10-35-29(28)36-31(32)39/h2-7,10,14-17,20,22H,8-9,11-13,18-19H2,1H3,(H,35,36,39)/b4-3+/t20-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444971
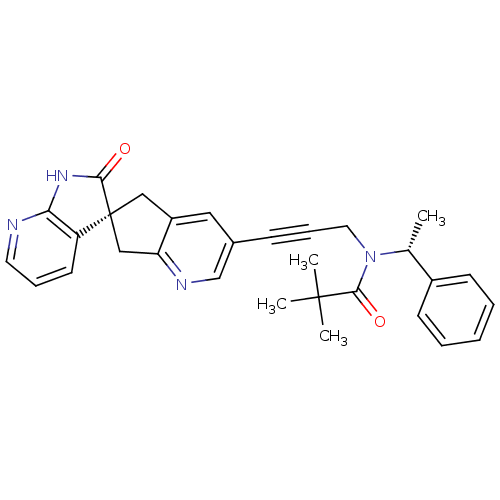
(CHEMBL3099932)Show SMILES C[C@@H](N(CC#Cc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C30H30N4O2/c1-20(22-11-6-5-7-12-22)34(28(36)29(2,3)4)15-9-10-21-16-23-17-30(18-25(23)32-19-21)24-13-8-14-31-26(24)33-27(30)35/h5-8,11-14,16,19-20H,15,17-18H2,1-4H3,(H,31,33,35)/t20-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM103501

(US8552023, 13)Show SMILES C[C@@H](N(C\C=C\c1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C(C)(C)C)c1ccc(C)c(F)c1 |r| Show InChI InChI=1S/C31H33FN4O2/c1-19-10-11-22(15-25(19)32)20(2)36(29(38)30(3,4)5)13-7-8-21-14-23-16-31(17-26(23)34-18-21)24-9-6-12-33-27(24)35-28(31)37/h6-12,14-15,18,20H,13,16-17H2,1-5H3,(H,33,35,37)/b8-7+/t20-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444976
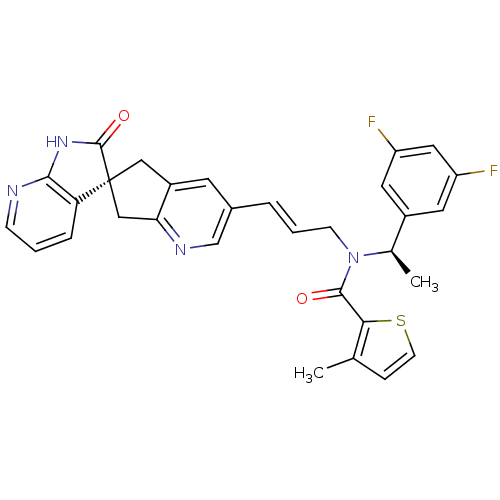
(CHEMBL3099926)Show SMILES C[C@@H](N(C\C=C\c1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)c1sccc1C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H26F2N4O2S/c1-18-7-10-40-27(18)29(38)37(19(2)21-12-23(32)14-24(33)13-21)9-4-5-20-11-22-15-31(16-26(22)35-17-20)25-6-3-8-34-28(25)36-30(31)39/h3-8,10-14,17,19H,9,15-16H2,1-2H3,(H,34,36,39)/b5-4+/t19-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444975
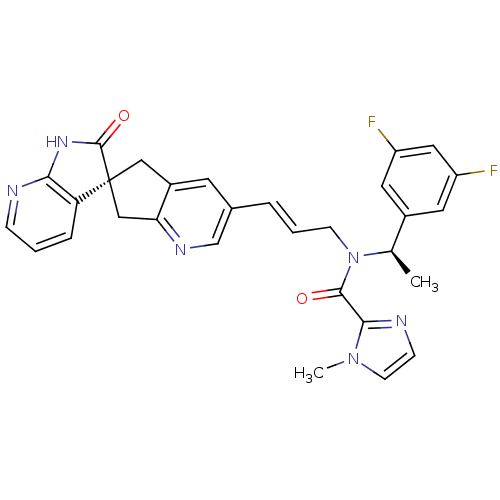
(CHEMBL3099927)Show SMILES C[C@@H](N(C\C=C\c1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)c1nccn1C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H26F2N6O2/c1-18(20-12-22(31)14-23(32)13-20)38(28(39)27-34-8-10-37(27)2)9-4-5-19-11-21-15-30(16-25(21)35-17-19)24-6-3-7-33-26(24)36-29(30)40/h3-8,10-14,17-18H,9,15-16H2,1-2H3,(H,33,36,40)/b5-4+/t18-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444987
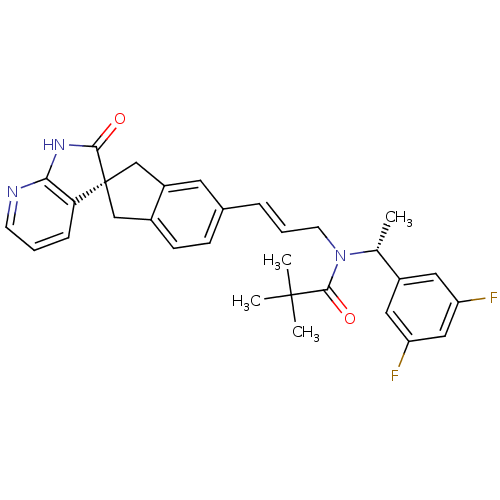
(CHEMBL3099939)Show SMILES C[C@@H](N(C\C=C\c1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C(C)(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H31F2N3O2/c1-19(22-14-24(32)16-25(33)15-22)36(29(38)30(2,3)4)12-6-7-20-9-10-21-17-31(18-23(21)13-20)26-8-5-11-34-27(26)35-28(31)37/h5-11,13-16,19H,12,17-18H2,1-4H3,(H,34,35,37)/b7-6+/t19-,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444986
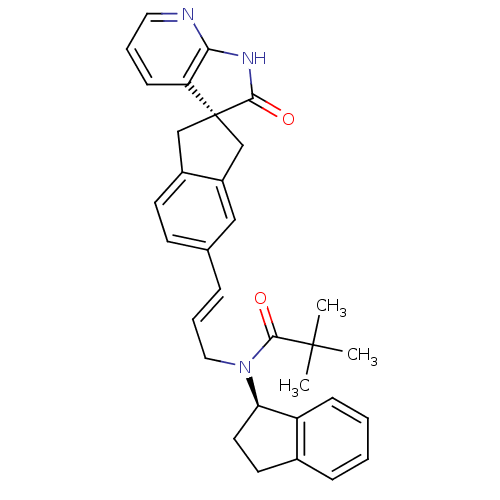
(CHEMBL3099914)Show SMILES CC(C)(C)C(=O)N(C\C=C\c1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C32H33N3O2/c1-31(2,3)30(37)35(27-15-14-22-9-4-5-10-25(22)27)17-7-8-21-12-13-23-19-32(20-24(23)18-21)26-11-6-16-33-28(26)34-29(32)36/h4-13,16,18,27H,14-15,17,19-20H2,1-3H3,(H,33,34,36)/b8-7+/t27-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444974
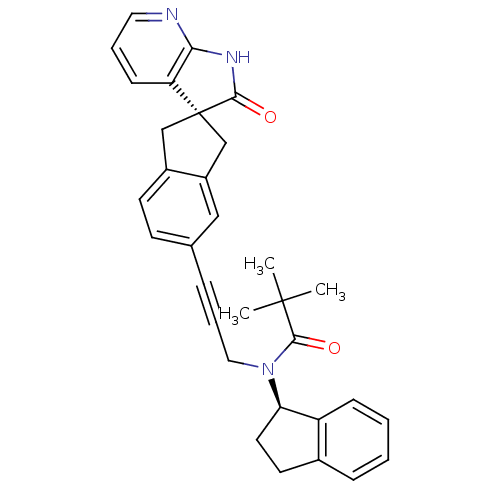
(CHEMBL3099929)Show SMILES CC(C)(C)C(=O)N(CC#Cc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C32H31N3O2/c1-31(2,3)30(37)35(27-15-14-22-9-4-5-10-25(22)27)17-7-8-21-12-13-23-19-32(20-24(23)18-21)26-11-6-16-33-28(26)34-29(32)36/h4-6,9-13,16,18,27H,14-15,17,19-20H2,1-3H3,(H,33,34,36)/t27-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444968
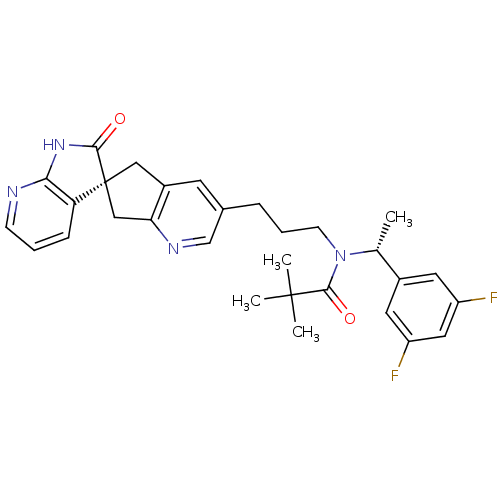
(CHEMBL3099935)Show SMILES C[C@@H](N(CCCc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C(C)(C)C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H32F2N4O2/c1-18(20-12-22(31)14-23(32)13-20)36(28(38)29(2,3)4)10-6-7-19-11-21-15-30(16-25(21)34-17-19)24-8-5-9-33-26(24)35-27(30)37/h5,8-9,11-14,17-18H,6-7,10,15-16H2,1-4H3,(H,33,35,37)/t18-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444981
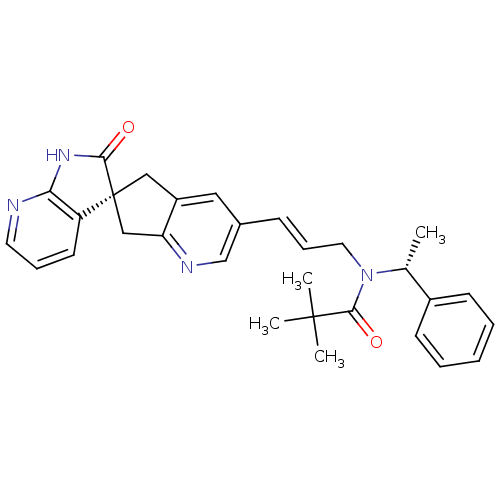
(CHEMBL3099919)Show SMILES C[C@@H](N(C\C=C\c1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C30H32N4O2/c1-20(22-11-6-5-7-12-22)34(28(36)29(2,3)4)15-9-10-21-16-23-17-30(18-25(23)32-19-21)24-13-8-14-31-26(24)33-27(30)35/h5-14,16,19-20H,15,17-18H2,1-4H3,(H,31,33,35)/b10-9+/t20-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444977
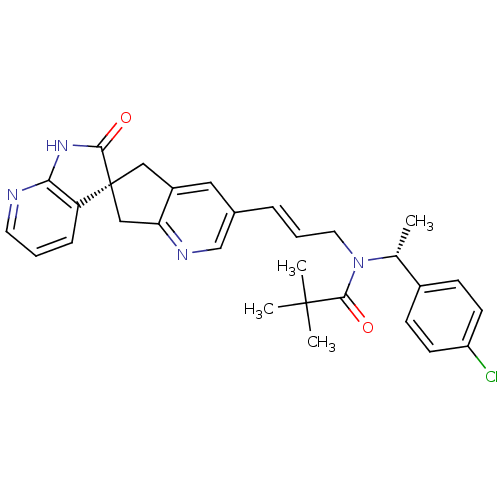
(CHEMBL3099924)Show SMILES C[C@@H](N(C\C=C\c1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C(C)(C)C)c1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H31ClN4O2/c1-19(21-9-11-23(31)12-10-21)35(28(37)29(2,3)4)14-6-7-20-15-22-16-30(17-25(22)33-18-20)24-8-5-13-32-26(24)34-27(30)36/h5-13,15,18-19H,14,16-17H2,1-4H3,(H,32,34,36)/b7-6+/t19-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444988
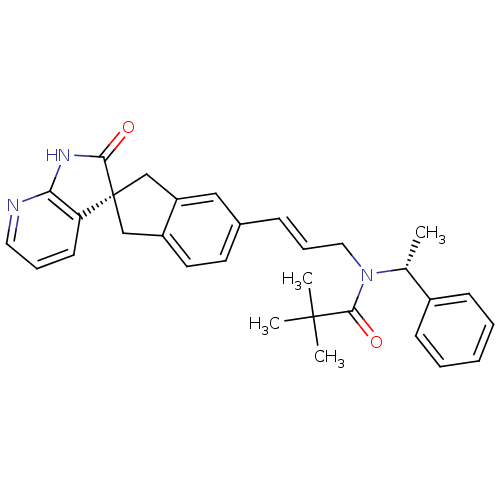
(CHEMBL3099913)Show SMILES C[C@@H](N(C\C=C\c1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C31H33N3O2/c1-21(23-11-6-5-7-12-23)34(29(36)30(2,3)4)17-9-10-22-14-15-24-19-31(20-25(24)18-22)26-13-8-16-32-27(26)33-28(31)35/h5-16,18,21H,17,19-20H2,1-4H3,(H,32,33,35)/b10-9+/t21-,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM103497
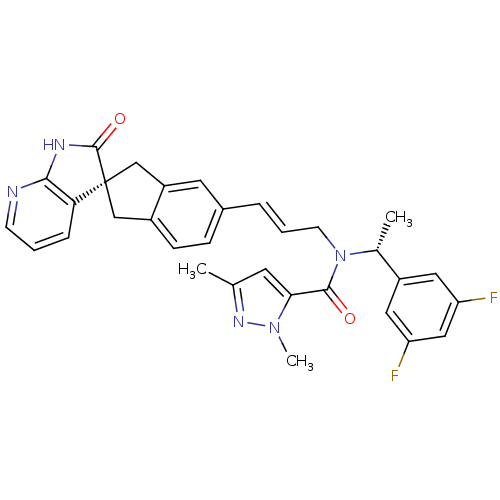
(US8552023, 29)Show SMILES C[C@@H](N(C\C=C\c1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)c1cc(C)nn1C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H29F2N5O2/c1-19-12-28(38(3)37-19)30(40)39(20(2)23-14-25(33)16-26(34)15-23)11-5-6-21-8-9-22-17-32(18-24(22)13-21)27-7-4-10-35-29(27)36-31(32)41/h4-10,12-16,20H,11,17-18H2,1-3H3,(H,35,36,41)/b6-5+/t20-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444967
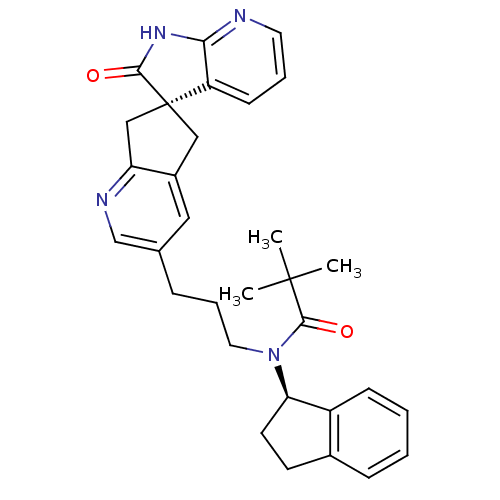
(CHEMBL3099936)Show SMILES CC(C)(C)C(=O)N(CCCc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C31H34N4O2/c1-30(2,3)29(37)35(26-13-12-21-9-4-5-10-23(21)26)15-7-8-20-16-22-17-31(18-25(22)33-19-20)24-11-6-14-32-27(24)34-28(31)36/h4-6,9-11,14,16,19,26H,7-8,12-13,15,17-18H2,1-3H3,(H,32,34,36)/t26-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444973
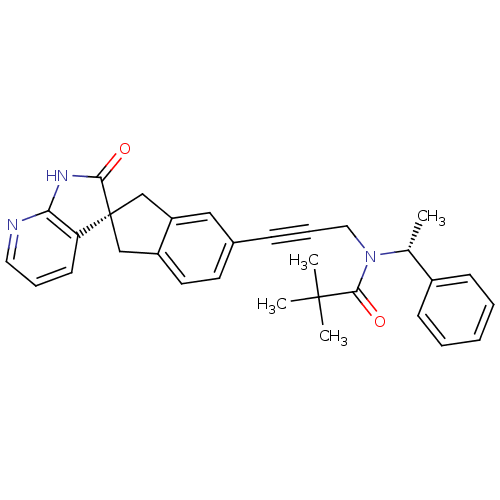
(CHEMBL3099930)Show SMILES C[C@@H](N(CC#Cc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C31H31N3O2/c1-21(23-11-6-5-7-12-23)34(29(36)30(2,3)4)17-9-10-22-14-15-24-19-31(20-25(24)18-22)26-13-8-16-32-27(26)33-28(31)35/h5-8,11-16,18,21H,17,19-20H2,1-4H3,(H,32,33,35)/t21-,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444978
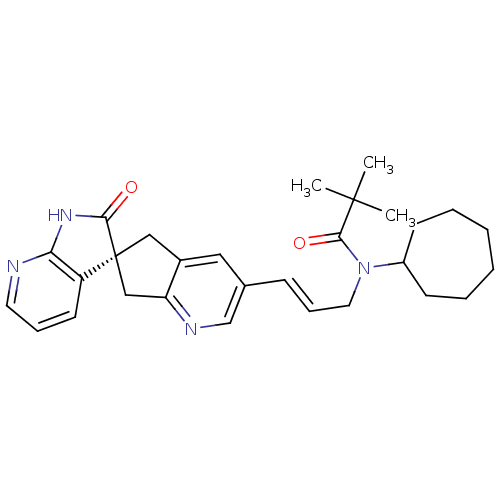
(CHEMBL3099922)Show SMILES CC(C)(C)C(=O)N(C\C=C\c1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C1CCCCCC1 |r| Show InChI InChI=1S/C29H36N4O2/c1-28(2,3)27(35)33(22-11-6-4-5-7-12-22)15-9-10-20-16-21-17-29(18-24(21)31-19-20)23-13-8-14-30-25(23)32-26(29)34/h8-10,13-14,16,19,22H,4-7,11-12,15,17-18H2,1-3H3,(H,30,32,34)/b10-9+/t29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444979
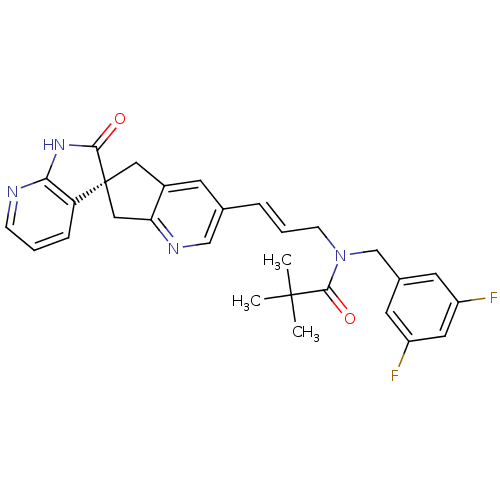
(CHEMBL3099921)Show SMILES CC(C)(C)C(=O)N(C\C=C\c1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)Cc1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C29H28F2N4O2/c1-28(2,3)27(37)35(17-19-11-21(30)13-22(31)12-19)9-5-6-18-10-20-14-29(15-24(20)33-16-18)23-7-4-8-32-25(23)34-26(29)36/h4-8,10-13,16H,9,14-15,17H2,1-3H3,(H,32,34,36)/b6-5+/t29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444985
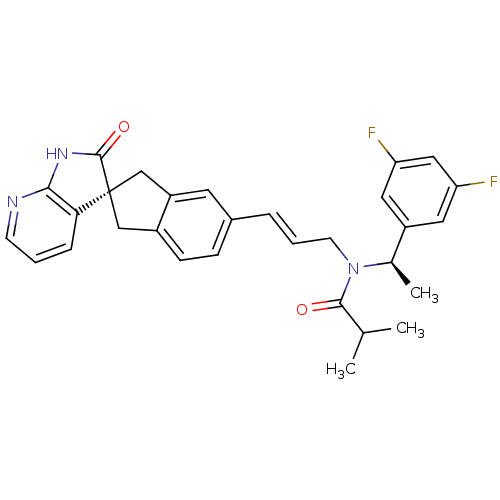
(CHEMBL3099915)Show SMILES CC(C)C(=O)N(C\C=C\c1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)[C@H](C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C30H29F2N3O2/c1-18(2)28(36)35(19(3)22-13-24(31)15-25(32)14-22)11-5-6-20-8-9-21-16-30(17-23(21)12-20)26-7-4-10-33-27(26)34-29(30)37/h4-10,12-15,18-19H,11,16-17H2,1-3H3,(H,33,34,37)/b6-5+/t19-,30-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444966
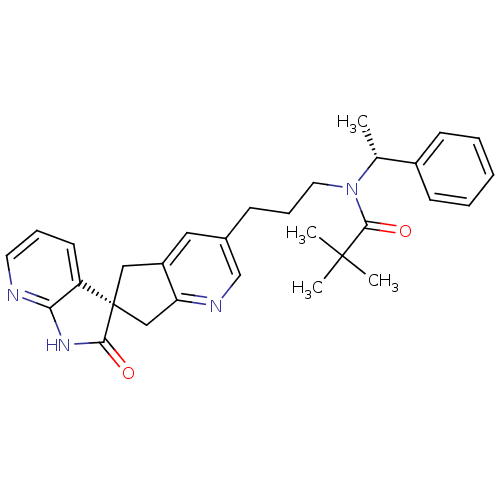
(CHEMBL3099937)Show SMILES C[C@@H](N(CCCc1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C30H34N4O2/c1-20(22-11-6-5-7-12-22)34(28(36)29(2,3)4)15-9-10-21-16-23-17-30(18-25(23)32-19-21)24-13-8-14-31-26(24)33-27(30)35/h5-8,11-14,16,19-20H,9-10,15,17-18H2,1-4H3,(H,31,33,35)/t20-,30+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444970
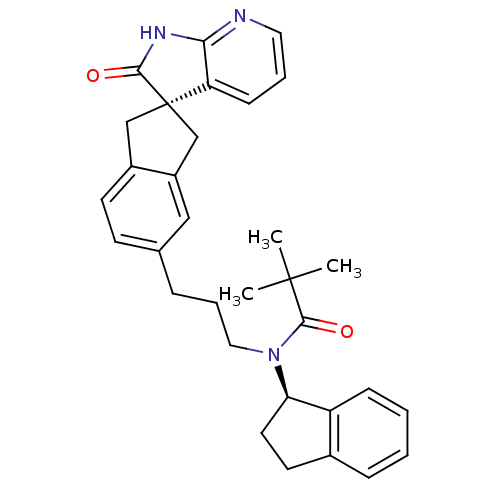
(CHEMBL3099933)Show SMILES CC(C)(C)C(=O)N(CCCc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C32H35N3O2/c1-31(2,3)30(37)35(27-15-14-22-9-4-5-10-25(22)27)17-7-8-21-12-13-23-19-32(20-24(23)18-21)26-11-6-16-33-28(26)34-29(32)36/h4-6,9-13,16,18,27H,7-8,14-15,17,19-20H2,1-3H3,(H,33,34,36)/t27-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM103498
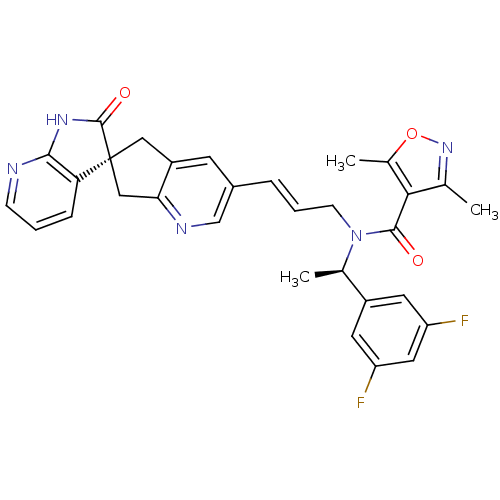
(US8552023, 30)Show SMILES C[C@@H](N(C\C=C\c1cnc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)c1c(C)noc1C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C31H27F2N5O3/c1-17-27(19(3)41-37-17)29(39)38(18(2)21-11-23(32)13-24(33)12-21)9-5-6-20-10-22-14-31(15-26(22)35-16-20)25-7-4-8-34-28(25)36-30(31)40/h4-8,10-13,16,18H,9,14-15H2,1-3H3,(H,34,36,40)/b6-5+/t18-,31+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444983
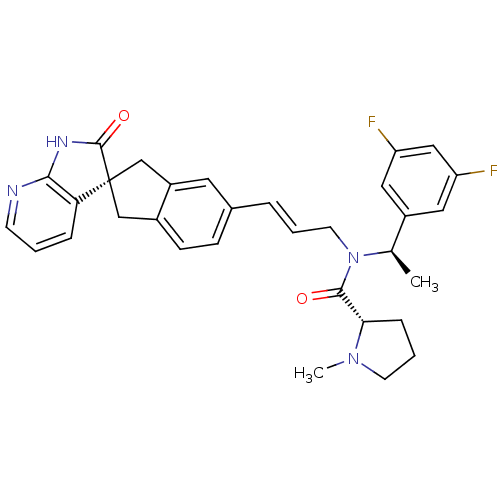
(CHEMBL3098148)Show SMILES C[C@@H](N(C\C=C\c1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)[C@@H]1CCCN1C)c1cc(F)cc(F)c1 |r| Show InChI InChI=1S/C32H32F2N4O2/c1-20(23-15-25(33)17-26(34)16-23)38(30(39)28-8-5-12-37(28)2)13-4-6-21-9-10-22-18-32(19-24(22)14-21)27-7-3-11-35-29(27)36-31(32)40/h3-4,6-7,9-11,14-17,20,28H,5,8,12-13,18-19H2,1-2H3,(H,35,36,40)/b6-4+/t20-,28+,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Calcitonin gene-related peptide type 1 receptor/Receptor activity-modifying protein 1
(Homo sapiens (Human)) | BDBM50444969
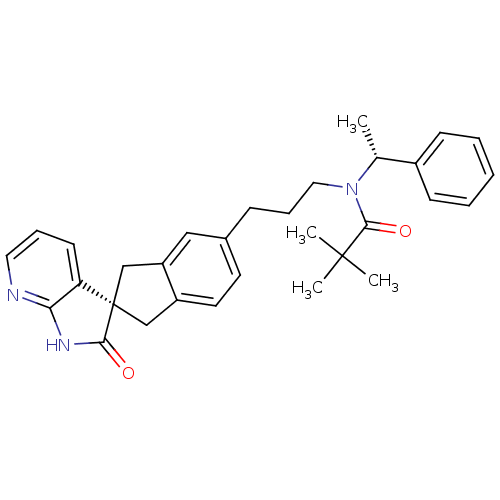
(CHEMBL3099934)Show SMILES C[C@@H](N(CCCc1ccc2C[C@]3(Cc2c1)C(=O)Nc1ncccc31)C(=O)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C31H35N3O2/c1-21(23-11-6-5-7-12-23)34(29(36)30(2,3)4)17-9-10-22-14-15-24-19-31(20-25(24)18-22)26-13-8-16-32-27(26)33-28(31)35/h5-8,11-16,18,21H,9-10,17,19-20H2,1-4H3,(H,32,33,35)/t21-,31-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck& Co.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-hCGRP from human CALCRL/RAMP1 expressed in HEK293 cell membranes |
Bioorg Med Chem Lett 24: 258-61 (2013)
Article DOI: 10.1016/j.bmcl.2013.11.027
BindingDB Entry DOI: 10.7270/Q2DB8393 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213143
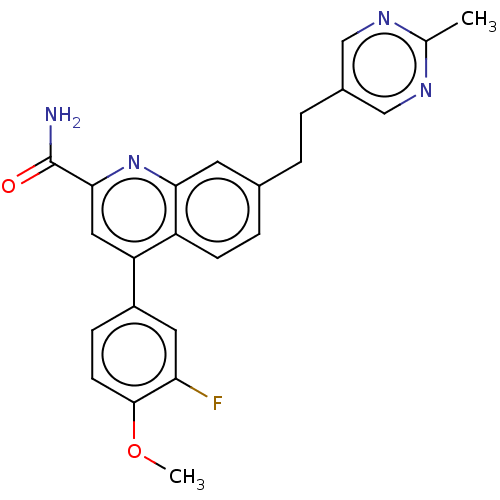
(US9278960, 8-50 | US9636337, 8-50)Show SMILES COc1ccc(cc1F)-c1cc(nc2cc(CCc3cnc(C)nc3)ccc12)C(N)=O Show InChI InChI=1S/C24H21FN4O2/c1-14-27-12-16(13-28-14)4-3-15-5-7-18-19(11-22(24(26)30)29-21(18)9-15)17-6-8-23(31-2)20(25)10-17/h5-13H,3-4H2,1-2H3,(H2,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213143

(US9278960, 8-50 | US9636337, 8-50)Show SMILES COc1ccc(cc1F)-c1cc(nc2cc(CCc3cnc(C)nc3)ccc12)C(N)=O Show InChI InChI=1S/C24H21FN4O2/c1-14-27-12-16(13-28-14)4-3-15-5-7-18-19(11-22(24(26)30)29-21(18)9-15)17-6-8-23(31-2)20(25)10-17/h5-13H,3-4H2,1-2H3,(H2,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9663506 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34H4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213110
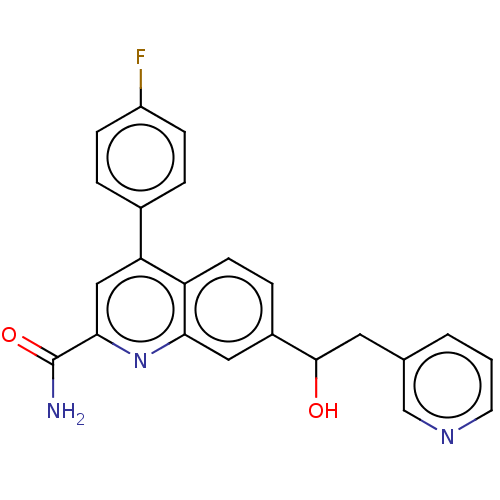
(US9278960, 8-17 | US9278960, 8-18 | US9636337, 8-1...)Show SMILES NC(=O)c1cc(-c2ccc(F)cc2)c2ccc(cc2n1)C(O)Cc1cccnc1 Show InChI InChI=1S/C23H18FN3O2/c24-17-6-3-15(4-7-17)19-12-21(23(25)29)27-20-11-16(5-8-18(19)20)22(28)10-14-2-1-9-26-13-14/h1-9,11-13,22,28H,10H2,(H2,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213110

(US9278960, 8-17 | US9278960, 8-18 | US9636337, 8-1...)Show SMILES NC(=O)c1cc(-c2ccc(F)cc2)c2ccc(cc2n1)C(O)Cc1cccnc1 Show InChI InChI=1S/C23H18FN3O2/c24-17-6-3-15(4-7-17)19-12-21(23(25)29)27-20-11-16(5-8-18(19)20)22(28)10-14-2-1-9-26-13-14/h1-9,11-13,22,28H,10H2,(H2,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213133

(US9278960, 8-40 | US9278960, 8-41 | US9636337, 8-4...)Show SMILES COc1ccc(cc1)-c1cc(nc2cc(ccc12)C(C)Cc1cccnc1)C(N)=O Show InChI InChI=1S/C25H23N3O2/c1-16(12-17-4-3-11-27-15-17)19-7-10-21-22(18-5-8-20(30-2)9-6-18)14-24(25(26)29)28-23(21)13-19/h3-11,13-16H,12H2,1-2H3,(H2,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213144
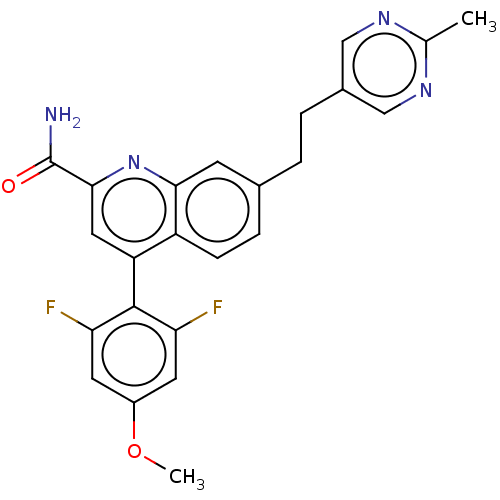
(US9278960, 8-51 | US9636337, 8-51)Show SMILES COc1cc(F)c(c(F)c1)-c1cc(nc2cc(CCc3cnc(C)nc3)ccc12)C(N)=O |(5.33,-6.16,;4,-5.39,;4,-3.85,;5.33,-3.08,;5.33,-1.54,;6.67,-.77,;4,-.77,;2.67,-1.54,;1.33,-.77,;2.67,-3.08,;4,.77,;5.33,1.54,;5.33,3.08,;4,3.85,;2.67,3.08,;1.33,3.85,;,3.08,;-1.33,3.85,;-2.67,3.08,;-4,3.85,;-4,5.39,;-5.33,6.16,;-6.67,5.39,;-8,6.16,;-6.67,3.85,;-5.33,3.08,;,1.54,;1.33,.77,;2.67,1.54,;6.67,3.85,;8,3.08,;6.67,5.39,)| Show InChI InChI=1S/C24H20F2N4O2/c1-13-28-11-15(12-29-13)4-3-14-5-6-17-18(10-22(24(27)31)30-21(17)7-14)23-19(25)8-16(32-2)9-20(23)26/h5-12H,3-4H2,1-2H3,(H2,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213110

(US9278960, 8-17 | US9278960, 8-18 | US9636337, 8-1...)Show SMILES NC(=O)c1cc(-c2ccc(F)cc2)c2ccc(cc2n1)C(O)Cc1cccnc1 Show InChI InChI=1S/C23H18FN3O2/c24-17-6-3-15(4-7-17)19-12-21(23(25)29)27-20-11-16(5-8-18(19)20)22(28)10-14-2-1-9-26-13-14/h1-9,11-13,22,28H,10H2,(H2,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9663506 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34H4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213110

(US9278960, 8-17 | US9278960, 8-18 | US9636337, 8-1...)Show SMILES NC(=O)c1cc(-c2ccc(F)cc2)c2ccc(cc2n1)C(O)Cc1cccnc1 Show InChI InChI=1S/C23H18FN3O2/c24-17-6-3-15(4-7-17)19-12-21(23(25)29)27-20-11-16(5-8-18(19)20)22(28)10-14-2-1-9-26-13-14/h1-9,11-13,22,28H,10H2,(H2,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9663506 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34H4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213133

(US9278960, 8-40 | US9278960, 8-41 | US9636337, 8-4...)Show SMILES COc1ccc(cc1)-c1cc(nc2cc(ccc12)C(C)Cc1cccnc1)C(N)=O Show InChI InChI=1S/C25H23N3O2/c1-16(12-17-4-3-11-27-15-17)19-7-10-21-22(18-5-8-20(30-2)9-6-18)14-24(25(26)29)28-23(21)13-19/h3-11,13-16H,12H2,1-2H3,(H2,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9663506 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34H4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213144

(US9278960, 8-51 | US9636337, 8-51)Show SMILES COc1cc(F)c(c(F)c1)-c1cc(nc2cc(CCc3cnc(C)nc3)ccc12)C(N)=O |(5.33,-6.16,;4,-5.39,;4,-3.85,;5.33,-3.08,;5.33,-1.54,;6.67,-.77,;4,-.77,;2.67,-1.54,;1.33,-.77,;2.67,-3.08,;4,.77,;5.33,1.54,;5.33,3.08,;4,3.85,;2.67,3.08,;1.33,3.85,;,3.08,;-1.33,3.85,;-2.67,3.08,;-4,3.85,;-4,5.39,;-5.33,6.16,;-6.67,5.39,;-8,6.16,;-6.67,3.85,;-5.33,3.08,;,1.54,;1.33,.77,;2.67,1.54,;6.67,3.85,;8,3.08,;6.67,5.39,)| Show InChI InChI=1S/C24H20F2N4O2/c1-13-28-11-15(12-29-13)4-3-14-5-6-17-18(10-22(24(27)31)30-21(17)7-14)23-19(25)8-16(32-2)9-20(23)26/h5-12H,3-4H2,1-2H3,(H2,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9663506 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34H4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM212882

(US9278960, 2-29 | US9663506, Example 2-29)Show SMILES Cc1ccc(cc1)-c1cc(nc2cc(CN3C(=O)CCC3=O)ccc12)C(N)=O Show InChI InChI=1S/C22H19N3O3/c1-13-2-5-15(6-3-13)17-11-19(22(23)28)24-18-10-14(4-7-16(17)18)12-25-20(26)8-9-21(25)27/h2-7,10-11H,8-9,12H2,1H3,(H2,23,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM212882

(US9278960, 2-29 | US9663506, Example 2-29)Show SMILES Cc1ccc(cc1)-c1cc(nc2cc(CN3C(=O)CCC3=O)ccc12)C(N)=O Show InChI InChI=1S/C22H19N3O3/c1-13-2-5-15(6-3-13)17-11-19(22(23)28)24-18-10-14(4-7-16(17)18)12-25-20(26)8-9-21(25)27/h2-7,10-11H,8-9,12H2,1H3,(H2,23,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9663506 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34H4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM325996
(4-(2-fluoro-4-methoxyphenyl)-7-[1-(2-methylpyrimid...)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2cc(ccc12)[C@H](C)Cc1cnc(C)nc1)C(N)=O Show InChI InChI=1S/C25H23FN4O2/c1-14(8-16-12-28-15(2)29-13-16)17-4-6-20-21(11-24(25(27)31)30-23(20)9-17)19-7-5-18(32-3)10-22(19)26/h4-7,9-14H,8H2,1-3H3,(H2,27,31)/t14-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213113

(US9278960, 8-20 | US9278960, 8-27 | US9278960, 8-2...)Show SMILES COc1ccc(cc1)-c1cc(nc2cc(ccc12)C(O)Cc1ccc(Cl)nc1)C(N)=O Show InChI InChI=1S/C24H20ClN3O3/c1-31-17-6-3-15(4-7-17)19-12-21(24(26)30)28-20-11-16(5-8-18(19)20)22(29)10-14-2-9-23(25)27-13-14/h2-9,11-13,22,29H,10H2,1H3,(H2,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213122

(US9278960, 8-29 | US9636337, 8-29)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2cc(CCc3cnc(C)nc3)ccc12)C(N)=O Show InChI InChI=1S/C24H21FN4O2/c1-14-27-12-16(13-28-14)4-3-15-5-7-19-20(11-23(24(26)30)29-22(19)9-15)18-8-6-17(31-2)10-21(18)25/h5-13H,3-4H2,1-2H3,(H2,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213126

(US9278960, 8-33 | US9636337, 8-33)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2cc(CCc3cnc(nc3)C#N)ccc12)C(N)=O Show InChI InChI=1S/C24H18FN5O2/c1-32-16-5-7-17(20(25)9-16)19-10-22(24(27)31)30-21-8-14(4-6-18(19)21)2-3-15-12-28-23(11-26)29-13-15/h4-10,12-13H,2-3H2,1H3,(H2,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM329281

((S)-4-(2-fluoro-4-methoxyphenyl)-7-[1-(2-methylpyr...)Show SMILES COC(=O)c1cc(-c2ccc(OC)cc2F)c2ccc(cc2n1)[C@@H](C)Cc1cnc(C)nc1 |r| Show InChI InChI=1S/C26H24FN3O3/c1-15(9-17-13-28-16(2)29-14-17)18-5-7-21-22(20-8-6-19(32-3)11-23(20)27)12-25(26(31)33-4)30-24(21)10-18/h5-8,10-15H,9H2,1-4H3/t15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9663506 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34H4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213113

(US9278960, 8-20 | US9278960, 8-27 | US9278960, 8-2...)Show SMILES COc1ccc(cc1)-c1cc(nc2cc(ccc12)C(O)Cc1ccc(Cl)nc1)C(N)=O Show InChI InChI=1S/C24H20ClN3O3/c1-31-17-6-3-15(4-7-17)19-12-21(24(26)30)28-20-11-16(5-8-18(19)20)22(29)10-14-2-9-23(25)27-13-14/h2-9,11-13,22,29H,10H2,1H3,(H2,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9663506 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34H4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213122

(US9278960, 8-29 | US9636337, 8-29)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2cc(CCc3cnc(C)nc3)ccc12)C(N)=O Show InChI InChI=1S/C24H21FN4O2/c1-14-27-12-16(13-28-14)4-3-15-5-7-19-20(11-23(24(26)30)29-22(19)9-15)18-8-6-17(31-2)10-21(18)25/h5-13H,3-4H2,1-2H3,(H2,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9663506 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34H4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213126

(US9278960, 8-33 | US9636337, 8-33)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2cc(CCc3cnc(nc3)C#N)ccc12)C(N)=O Show InChI InChI=1S/C24H18FN5O2/c1-32-16-5-7-17(20(25)9-16)19-10-22(24(27)31)30-21-8-14(4-6-18(19)21)2-3-15-12-28-23(11-26)29-13-15/h4-10,12-13H,2-3H2,1H3,(H2,27,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9663506 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34H4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213074
(US9278960, 6-21 | US9663506, Example 6-21)Show SMILES Cn1cc(cn1)-c1cc(nc2cc(Cc3ccnc(c3)C(F)(F)F)ccc12)C(N)=O Show InChI InChI=1S/C21H16F3N5O/c1-29-11-14(10-27-29)16-9-18(20(25)30)28-17-7-12(2-3-15(16)17)6-13-4-5-26-19(8-13)21(22,23)24/h2-5,7-11H,6H2,1H3,(H2,25,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM213074

(US9278960, 6-21 | US9663506, Example 6-21)Show SMILES Cn1cc(cn1)-c1cc(nc2cc(Cc3ccnc(c3)C(F)(F)F)ccc12)C(N)=O Show InChI InChI=1S/C21H16F3N5O/c1-29-11-14(10-27-29)16-9-18(20(25)30)28-17-7-12(2-3-15(16)17)6-13-4-5-26-19(8-13)21(22,23)24/h2-5,7-11H,6H2,1H3,(H2,25,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9663506 (2017)
BindingDB Entry DOI: 10.7270/Q2PC34H4 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 2
(Homo sapiens (Human)) | BDBM325995

(4-(2-fluoro-4-methoxyphenyl)-7-[1-(2-methylpyrimid...)Show SMILES COc1ccc(c(F)c1)-c1cc(nc2cc(ccc12)[C@@H](C)Cc1cnc(C)nc1)C(N)=O Show InChI InChI=1S/C25H23FN4O2/c1-14(8-16-12-28-15(2)29-13-16)17-4-6-20-21(11-24(25(27)31)30-23(20)9-17)19-7-5-18(32-3)10-22(19)26/h4-7,9-14H,8H2,1-3H3,(H2,27,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
The utility of the compounds in accordance with the present invention as antagonists of metabotropic glutamate receptor activity, in particular mGluR... |
US Patent US9636337 (2017)
BindingDB Entry DOI: 10.7270/Q2F191TK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data