

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007522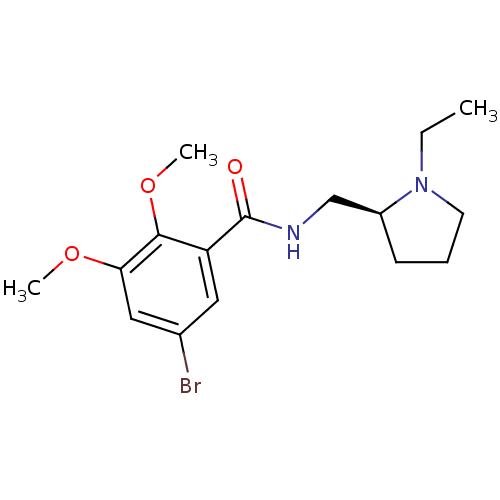 (5-Bromo-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,3-dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368060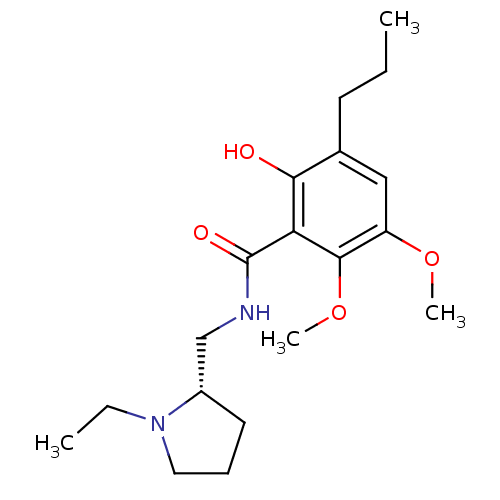 (CHEMBL1907695) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007508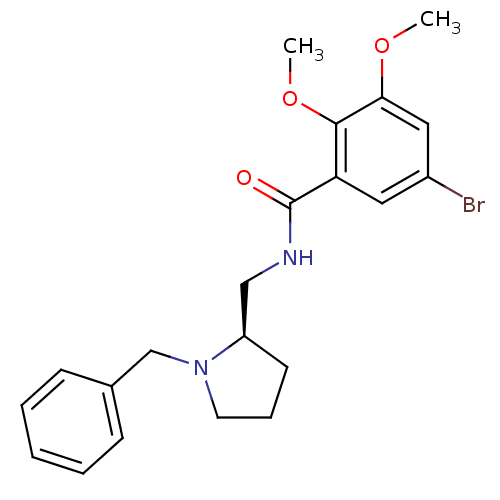 ((R) N-(1-Benzyl-pyrrolidin-2-ylmethyl)-5-bromo-2,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368067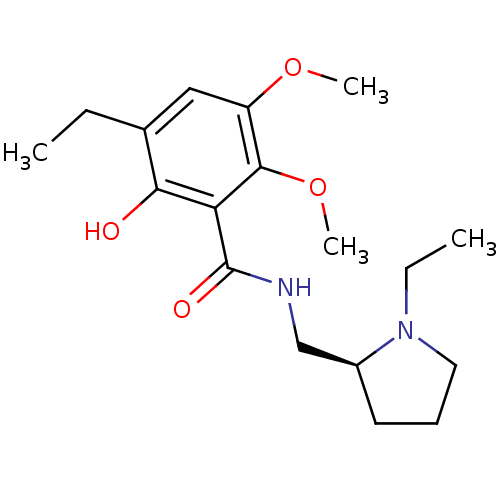 (CHEMBL1907702) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by PDSP Ki Database | Synapse 15: 169-76 (1993) Article DOI: 10.1002/syn.890150302 BindingDB Entry DOI: 10.7270/Q2MS3R8Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by PDSP Ki Database | Synapse 15: 169-76 (1993) Article DOI: 10.1002/syn.890150302 BindingDB Entry DOI: 10.7270/Q2MS3R8Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by PDSP Ki Database | Synapse 15: 169-76 (1993) Article DOI: 10.1002/syn.890150302 BindingDB Entry DOI: 10.7270/Q2MS3R8Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007517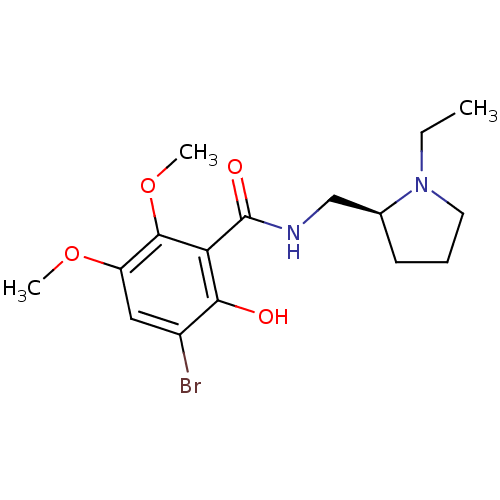 ((S)-3-bromo-N-((1-ethylpyrrolidin-2-yl)methyl)-2-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007517 ((S)-3-bromo-N-((1-ethylpyrrolidin-2-yl)methyl)-2-h...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50368065 (CHEMBL1907692) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center Curated by PDSP Ki Database | J Pharmacol Exp Ther 252: 1108-16 (1990) BindingDB Entry DOI: 10.7270/Q2TD9VV5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50008782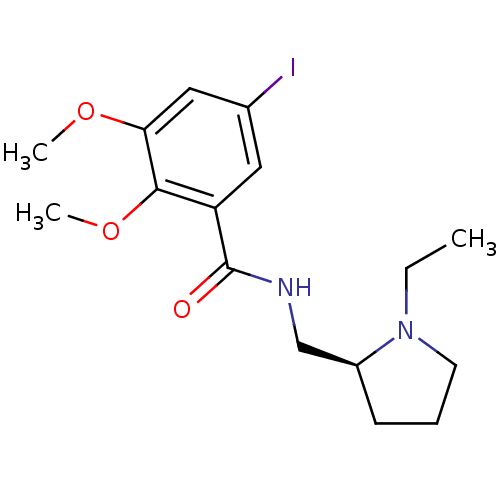 (CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by PDSP Ki Database | Synapse 15: 169-76 (1993) Article DOI: 10.1002/syn.890150302 BindingDB Entry DOI: 10.7270/Q2MS3R8Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50008782 (CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by PDSP Ki Database | Synapse 15: 169-76 (1993) Article DOI: 10.1002/syn.890150302 BindingDB Entry DOI: 10.7270/Q2MS3R8Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center Curated by PDSP Ki Database | J Pharmacol Exp Ther 252: 1108-16 (1990) BindingDB Entry DOI: 10.7270/Q2TD9VV5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center Curated by PDSP Ki Database | J Pharmacol Exp Ther 252: 1108-16 (1990) BindingDB Entry DOI: 10.7270/Q2TD9VV5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50008782 (CHEMBL44237 | EPIDEPRIDE | Epidepride;N-(1-Ethyl-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by PDSP Ki Database | Synapse 15: 169-76 (1993) Article DOI: 10.1002/syn.890150302 BindingDB Entry DOI: 10.7270/Q2MS3R8Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007518 ((S)-3-chloro-5-ethyl-N-((1-ethylpyrrolidin-2-yl)me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007509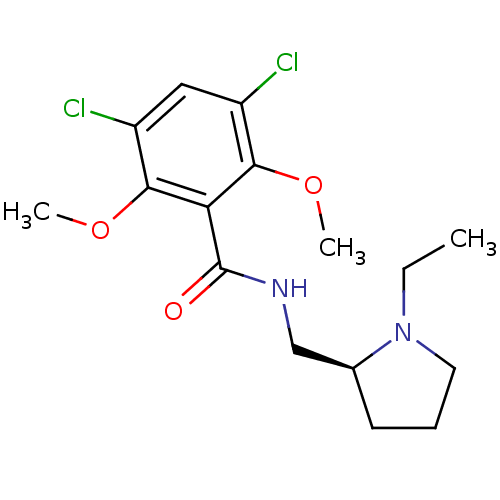 (3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor. | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007510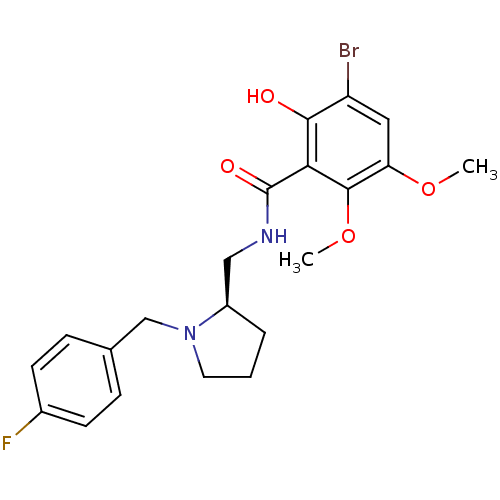 ((R) 3-Bromo-N-[1-(4-fluoro-benzyl)-pyrrolidin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal dopamine receptor D2 was determined in vitro | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50241107 (1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by PDSP Ki Database | Synapse 15: 169-76 (1993) Article DOI: 10.1002/syn.890150302 BindingDB Entry DOI: 10.7270/Q2MS3R8Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50241107 (1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by PDSP Ki Database | Synapse 15: 169-76 (1993) Article DOI: 10.1002/syn.890150302 BindingDB Entry DOI: 10.7270/Q2MS3R8Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007524 ((R) 3-Bromo-N-[1-(4-fluoro-benzyl)-pyrrolidin-2-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal dopamine receptor D2 was determined in vitro | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50241107 (1-(3-(4-(5-chloro-2-oxo-2,3-dihydrobenzo[d]imidazo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by PDSP Ki Database | Synapse 15: 169-76 (1993) Article DOI: 10.1002/syn.890150302 BindingDB Entry DOI: 10.7270/Q2MS3R8Z | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028600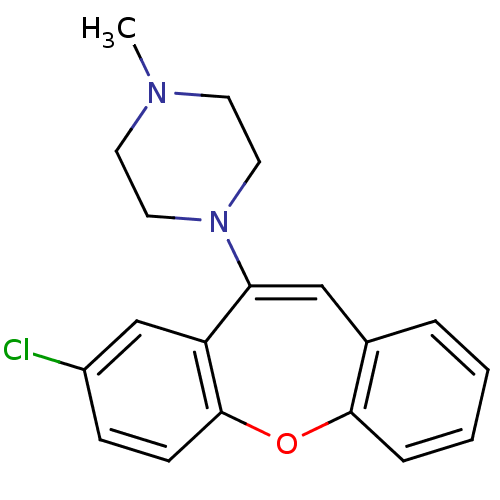 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D4 in COS7 cells | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007534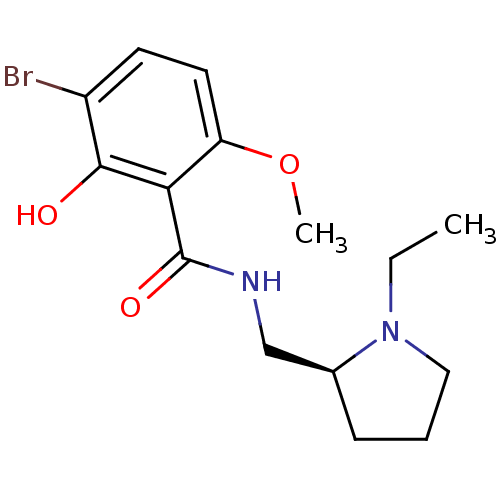 (3-Bromo-N-((S)-1-ethyl-pyrrolidin-2-ylmethyl)-2-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50007534 (3-Bromo-N-((S)-1-ethyl-pyrrolidin-2-ylmethyl)-2-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50010724 (CHEMBL305916 | N-(1-Ethyl-pyrrolidin-2-ylmethyl)-6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50453039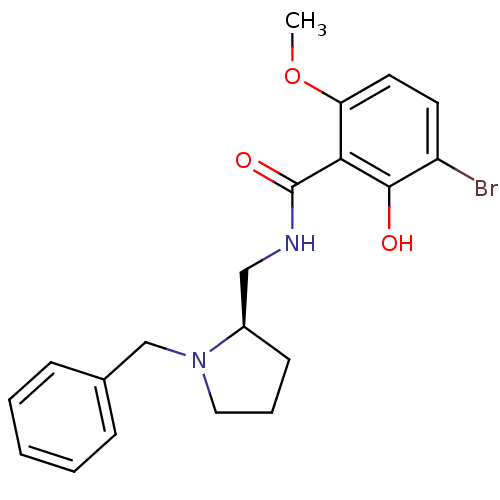 (CHEMBL2096749) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028602 (1-(2-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50028601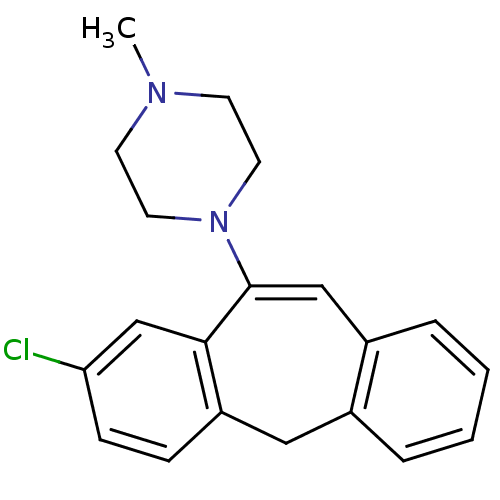 (1-(8-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-2(long) receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028601 (1-(8-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50005118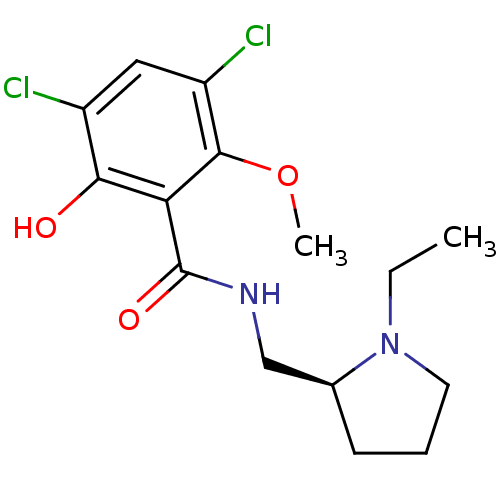 ((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]spiperone binding to rat striatal membrane Dopamine receptor D2 | J Med Chem 33: 1155-63 (1990) BindingDB Entry DOI: 10.7270/Q24X58DR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50005118 ((S)-3,5-Dichloro-N-(1-ethyl-pyrrolidin-2-ylmethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Research Centre AB Curated by ChEMBL | Assay Description Inhibition of [3H]raclopride binding to rat striatal dopamine receptor D2 | J Med Chem 34: 948-55 (1991) BindingDB Entry DOI: 10.7270/Q2GT5NS9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D2L in COS7 cells | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center Curated by PDSP Ki Database | J Pharmacol Exp Ther 252: 1108-16 (1990) BindingDB Entry DOI: 10.7270/Q2TD9VV5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028601 (1-(8-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028598 (1-Dibenzo[b,f]oxepin-10-yl-4-methyl-piperazine | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-4 receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50028598 (1-Dibenzo[b,f]oxepin-10-yl-4-methyl-piperazine | C...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D4 in COS7 cells | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center Curated by PDSP Ki Database | J Pharmacol Exp Ther 252: 1108-16 (1990) BindingDB Entry DOI: 10.7270/Q2TD9VV5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [3H]-spiperone binding to COS cells transfected with human dopamine D-2(long) receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50008735 ((+)-3-(tert-butyl)-(3S,4aS,13bS)-2,3,4,4a,8,9,13b,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Veterans Affairs Medical Center Curated by PDSP Ki Database | J Pharmacol Exp Ther 252: 1108-16 (1990) BindingDB Entry DOI: 10.7270/Q2TD9VV5 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of [3H]spiperone to the Dopamine receptor D2L in COS7 cells | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50036437 (4-Dibenzo[b,f]oxepin-10-yl-1-methyl-piperidine; hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor in NIH3T3 cell line membr... | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2A serotonin receptor in NIH3T3 cell line membr... | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50028600 (1-(8-Chloro-dibenzo[b,f]oxepin-10-yl)-4-methyl-pip...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Ability to inhibit the binding of iodine-125-labelled lysergic acid diethylamide([125I]-LSD) to the S-2C serotonin receptor in NIH3T3 cell line membr... | J Med Chem 38: 708-14 (1995) BindingDB Entry DOI: 10.7270/Q2PG1SCG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50028599 (1-(2-Chloro-5H-dibenzo[a,d]cyclohepten-10-yl)-4-me...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University Curated by ChEMBL | Assay Description Affinity was evaluated by inhibition of [125I]-LSD binding to NIH 3T3 cells transfected with cloned rat 5-hydroxytryptamine 2A receptor | J Med Chem 37: 2686-96 (1994) BindingDB Entry DOI: 10.7270/Q28051NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 577 total ) | Next | Last >> |