

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50102711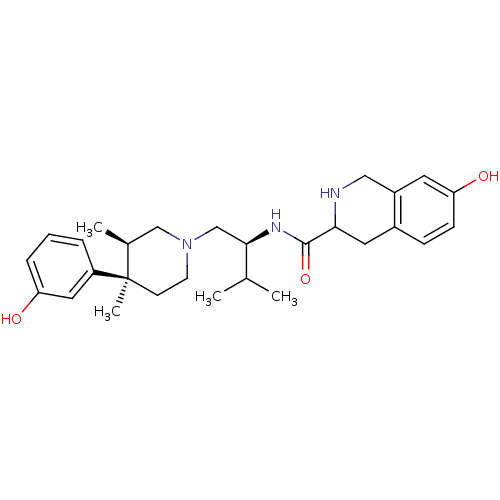 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist (U50,488) stimulated [35S]GTP-gamma-S, binding in cloned opioid receptor kappa 1 | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064515 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-o-tolyl-allyl)-pi...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (U69593, kappa-receptor), in guinea pig caudate membranes. | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50045775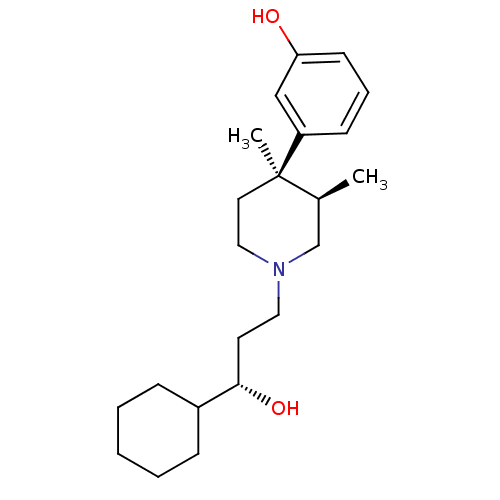 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064518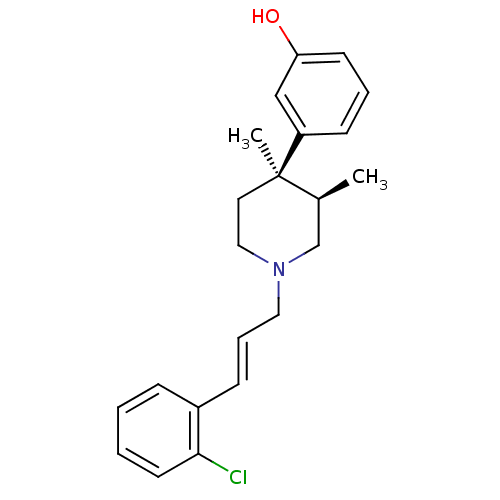 (3-{(3R,4R)-1-[(E)-3-(2-Chloro-phenyl)-allyl]-3,4-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551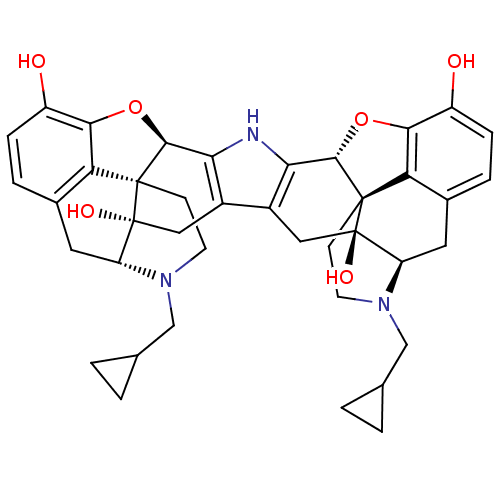 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of stimulation of [35S]GTP-gamma-S, binding produced by the selective agonist (U69593, kappa-receptor), in guinea pig caudate membranes | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor kappa 1 in Guinea pig Caudate stimulated by U69,593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50291970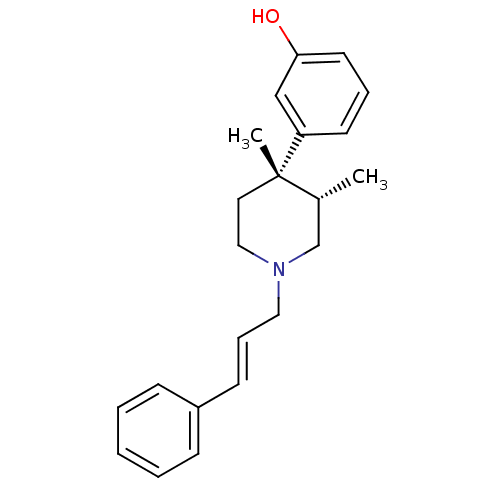 (3-[(3S,4S)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]GTP-gamma-S, binding from Opioid receptor mu 1 in Guinea pig Caudate stimulated by DAMGO | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50064521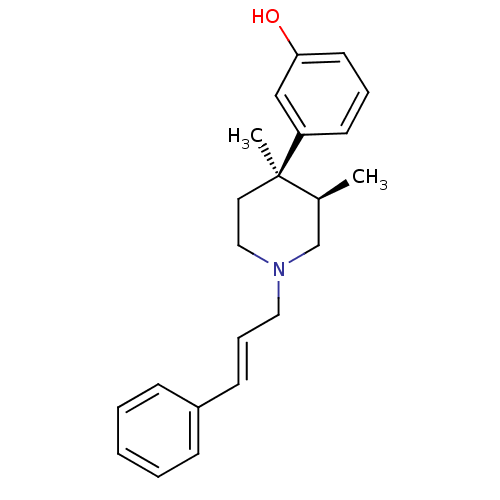 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-phenyl-allyl)-pip...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding to Opioid receptor mu 1 in Guinea pig caudate st... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864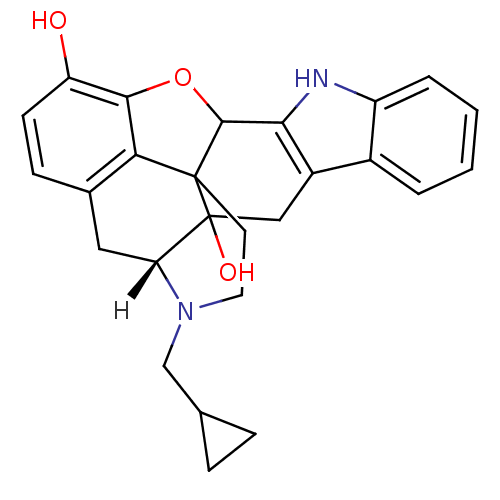 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist stimulated [35S]GTP-gamma-S, binding in guinea pig caudate membranes (10 uM SNC-80 as the agonist ligand for delta-rec... | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50026614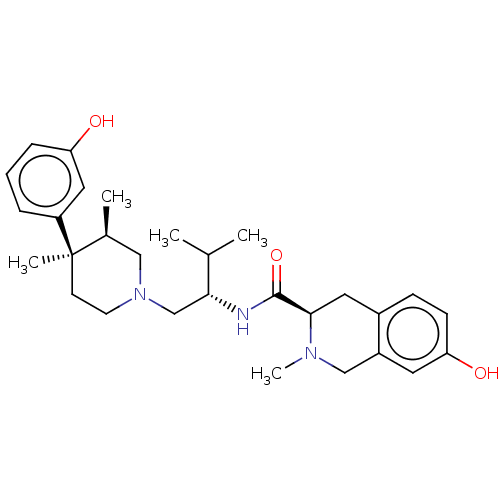 (CHEMBL575508) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description The ability of compound to inhibit [35S]GTP-delta-S binding in guinea pig caudate stimulated by SNC80 (Opioid receptor delta 1) antagonist | J Med Chem 45: 5378-83 (2002) BindingDB Entry DOI: 10.7270/Q2R21244 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Apparent antagonist activity determined by measuring the ability to inhibit stimulation of [35S]GTP-gamma-S, binding to opioid receptor delta 1 in gu... | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50037134 ((+)-trans-3-Cyclopropylmethyl-2,3,4,4aalpha,5,6,7,...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [125I]OXY from human mu opioid receptor expressed in CHO cells | J Med Chem 53: 1392-6 (2010) Article DOI: 10.1021/jm901503e BindingDB Entry DOI: 10.7270/Q2RX9C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50037134 ((+)-trans-3-Cyclopropylmethyl-2,3,4,4aalpha,5,6,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [125I]OXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 53: 1392-6 (2010) Article DOI: 10.1021/jm901503e BindingDB Entry DOI: 10.7270/Q2RX9C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50037134 ((+)-trans-3-Cyclopropylmethyl-2,3,4,4aalpha,5,6,7,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [125I]OXY from human kappa opioid receptor expressed in CHO cells | J Med Chem 53: 1392-6 (2010) Article DOI: 10.1021/jm901503e BindingDB Entry DOI: 10.7270/Q2RX9C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist stimulated [35S]GTP-gamma-S, binding in guinea pig caudate membranes (10 uM U69,593 as the agonist ligand for kappa-re... | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50064515 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-o-tolyl-allyl)-pi...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by U69,593 to O... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50080467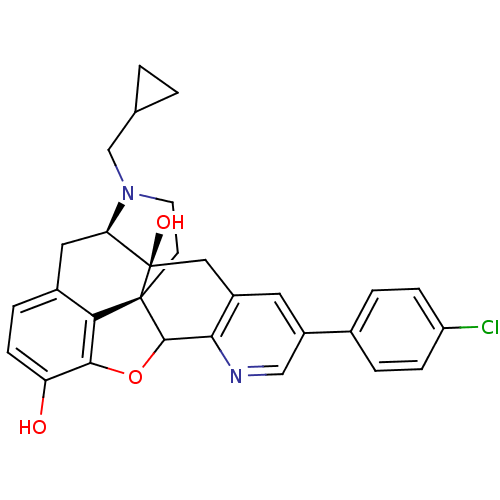 (5'-(Chlorophenyl)-17-(cyclopropylmethyl) -6,7-dide...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Apparent antagonist activity determined by measuring the ability to inhibit stimulation of [35S]GTP-gamma-S, binding to opioid receptor delta 1 in gu... | J Med Chem 47: 1400-12 (2004) Article DOI: 10.1021/jm030311v BindingDB Entry DOI: 10.7270/Q2639QGW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50027039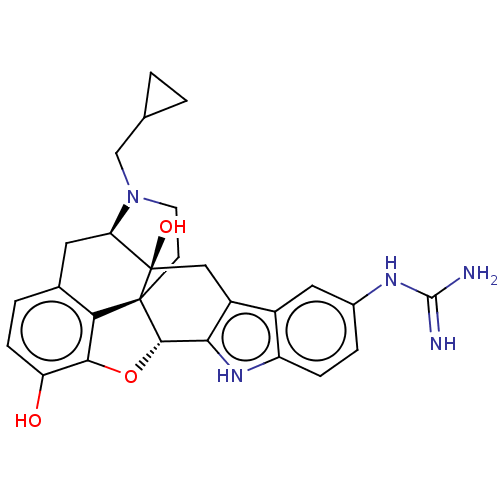 (5''-Guanidinonaltrindole | 5''-Guanidinylnaltrindo...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50217952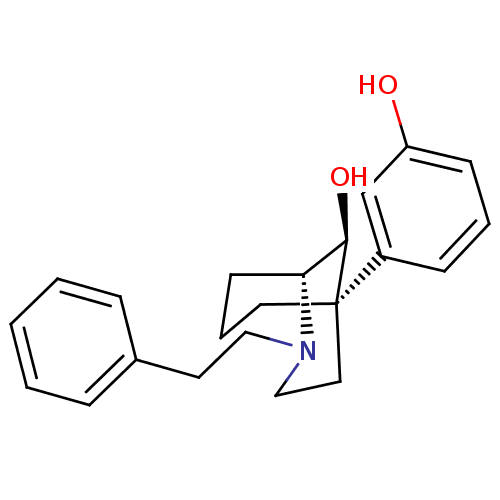 ((1R,5R,9S)-(-)-9-hydroxy-5-(3-hydroxyphenyl-2-phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human mu opioid receptor expressed in CHO cells | J Med Chem 50: 3765-76 (2007) Article DOI: 10.1021/jm061325e BindingDB Entry DOI: 10.7270/Q2D21XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50217956 ((1R,5S)-(+)-5-(3-hydroxyphenyl)-9-methylene-2-phen...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute on Drug Abuse Curated by ChEMBL | Assay Description Displacement of [125I]IOXY from human mu opioid receptor expressed in CHO cells | J Med Chem 50: 3765-76 (2007) Article DOI: 10.1021/jm061325e BindingDB Entry DOI: 10.7270/Q2D21XB2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from Opioid receptor delta 1 | Bioorg Med Chem Lett 11: 2883-5 (2001) BindingDB Entry DOI: 10.7270/Q2D79BZV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity determined on Opioid receptor kappa 1 in Guinea pig brain membranes using radioligand [3H]U-69593 | J Med Chem 41: 5188-97 (1999) Article DOI: 10.1021/jm980511k BindingDB Entry DOI: 10.7270/Q2P26ZT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50303852 ((+)-trans-3-(2-Phenylethyl)-2,3,4,4aalpha,5,6,7,7a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Displacement of [125I]OXY from human mu opioid receptor expressed in CHO cells | J Med Chem 53: 1392-6 (2010) Article DOI: 10.1021/jm901503e BindingDB Entry DOI: 10.7270/Q2RX9C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50021214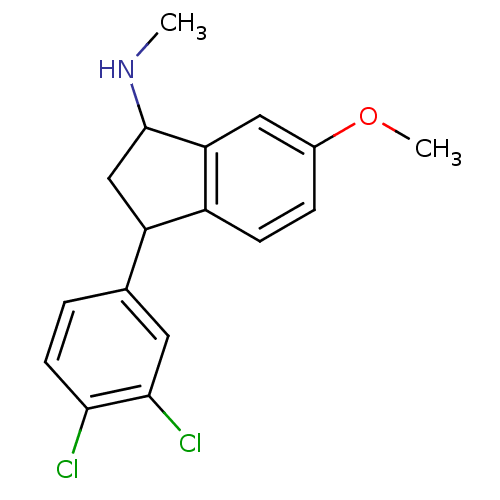 (CHEMBL300019 | CHEMBL537996 | [3-(3,4-Dichloro-phe...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50303852 ((+)-trans-3-(2-Phenylethyl)-2,3,4,4aalpha,5,6,7,7a...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Taiwan University Curated by ChEMBL | Assay Description Binding affinity to human mu opioid receptor expressed in CHO cells | J Med Chem 53: 1392-6 (2010) Article DOI: 10.1021/jm901503e BindingDB Entry DOI: 10.7270/Q2RX9C5Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by SMC-80 (Opio... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50130563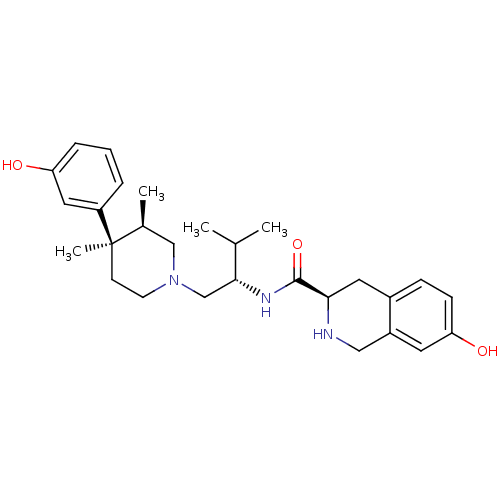 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards human cloned Opioid receptor kappa 1 using [3H]U-69593 | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]U-69593 binding to Opioid receptor kappa 1 of guinea pig brain | J Med Chem 46: 3127-37 (2003) Article DOI: 10.1021/jm030094y BindingDB Entry DOI: 10.7270/Q2319WM4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50130563 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity of compound for Opioid receptor kappa 1 was determined using [3H]U-69593 as radioligand from guinea pig caudate | J Med Chem 47: 1070-3 (2004) Article DOI: 10.1021/jm030467v BindingDB Entry DOI: 10.7270/Q2BV7HC4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50102711 (7-Hydroxy-1,2,3,4-tetrahydro-isoquinoline-3-carbox...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity to opioid receptor kappa 1 of guinea pig brain, using [3H]U-69593 as radioligand | J Med Chem 44: 2687-90 (2001) BindingDB Entry DOI: 10.7270/Q25T3M58 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [3H]-DAMGO binding to Opioid receptor mu 1 of rat brain membranes | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by U69,593 to O... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50067447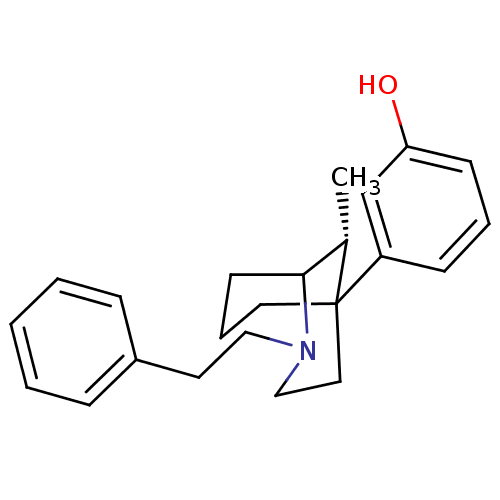 (3-((R)-9-Methyl-2-phenethyl-2-aza-bicyclo[3.3.1]no...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Inhibition of [35S]-GTP-gammaS, binding in guinea pig caudate stimulated by DAMGO (Opioid receptor mu 1) | J Med Chem 41: 4143-9 (1998) Article DOI: 10.1021/jm980290i BindingDB Entry DOI: 10.7270/Q2RB73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50064515 (3-[(3R,4R)-3,4-Dimethyl-1-((E)-3-o-tolyl-allyl)-pi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.355 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by SMC-80 (Opio... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50183549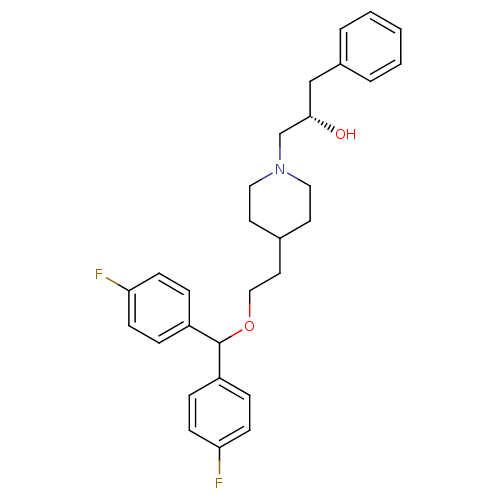 ((S)-(+)-4-[2-[bis-(4-fluorophenyl)methoxy]ethyl]-1...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from DAT | J Med Chem 49: 1766-72 (2006) Article DOI: 10.1021/jm050766f BindingDB Entry DOI: 10.7270/Q2KS6R5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Rattus norvegicus (rat)) | BDBM50021226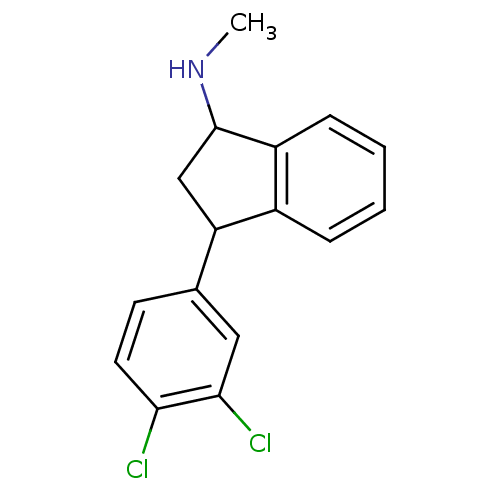 (CHEMBL296602 | Indatraline | [3-(3,4-Dichloro-phen...) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from serotonin transporter of frozen rat caudate membranes | J Med Chem 47: 2624-34 (2004) Article DOI: 10.1021/jm0305873 BindingDB Entry DOI: 10.7270/Q2MK6DF4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50123279 (19-cyclopropylmethyl-6-(1H-1-pyrrolyl)-11-oxa-8,19...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Antagonist activity on agonist stimulated [35S]GTP-gamma-S, binding in guinea pig caudate membranes (10 uM SNC-80 as the agonist ligand for delta-rec... | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity to delta opioid receptor was measured using [3H]DADLE | Bioorg Med Chem Lett 13: 529-32 (2003) BindingDB Entry DOI: 10.7270/Q27S7P9P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor delta 1 was determined by inhibition of binding of [3H]DADLE (1.3-2.0 nM) to rat brain membranes | J Med Chem 42: 3527-38 (1999) Article DOI: 10.1021/jm990039i BindingDB Entry DOI: 10.7270/Q2QR4XSQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398759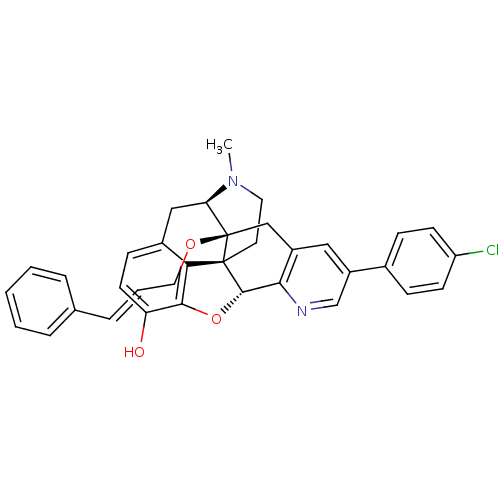 (CHEMBL2179655) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50118598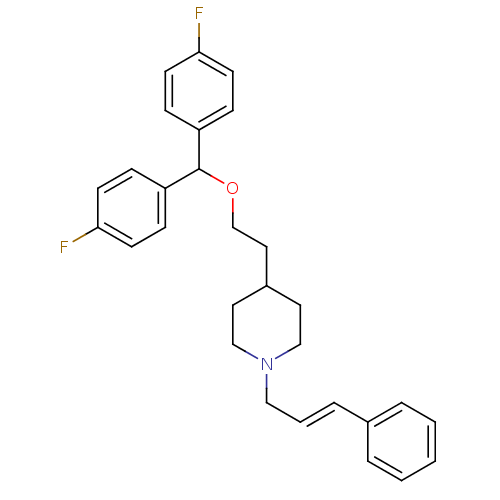 (4-{2-[Bis-(4-fluoro-phenyl)-methoxy]-ethyl}-1-(3-p...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Binding affinity of the compound towards dopamine transporter (DAT) by using [125I]-RTI-55 radioligand | J Med Chem 45: 4371-4 (2002) BindingDB Entry DOI: 10.7270/Q2RF5TCP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50183539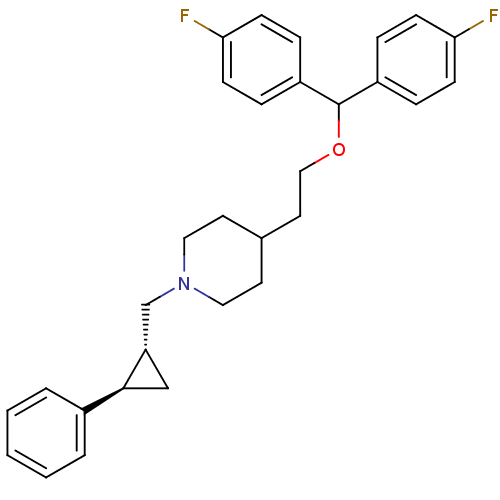 (CHEMBL381256 | CHEMBL429492 | trans-(R,R)-4-[2-[bi...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK Curated by ChEMBL | Assay Description Displacement of [125I]RTI-55 from DAT | J Med Chem 49: 1766-72 (2006) Article DOI: 10.1021/jm050766f BindingDB Entry DOI: 10.7270/Q2KS6R5F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50056543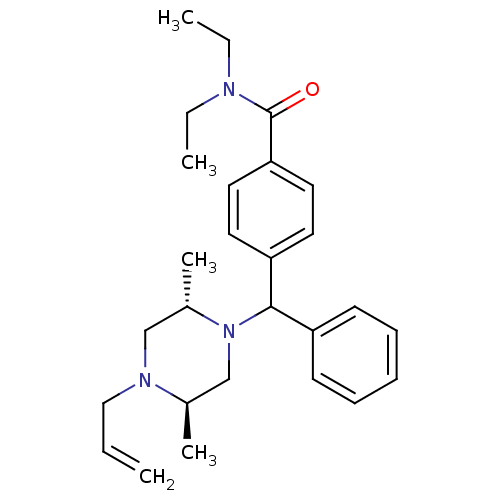 (4-[((1R,2S,5R)-4-Allyl-2,5-dimethyl-piperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description In vitro binding affinity to Opioid receptor delta 1 in rat brain membranes by [3H]DADLE (Tyr-D-Ala-Gly-Phe-D-Leu) displacement. | J Med Chem 42: 5455-63 (2000) BindingDB Entry DOI: 10.7270/Q2QJ7J0X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50398758 (CHEMBL2179656) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Southern Research Institute Curated by ChEMBL | Assay Description Agonist activity at human mu opioid receptor expressed in CHO cells assessed as [35S]GTPgammaS binding by scintillation counting analysis | J Med Chem 55: 8350-63 (2012) Article DOI: 10.1021/jm300686p BindingDB Entry DOI: 10.7270/Q2N87BX5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50297503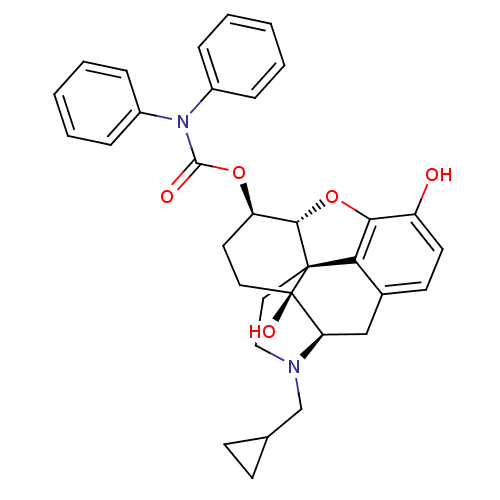 (17-(cyclopropylmethyl)-3,14-dihydroxy-6beta-(N,N-d...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of New England Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cells by liquid scintillation counting | Bioorg Med Chem Lett 19: 2811-4 (2009) Article DOI: 10.1016/j.bmcl.2009.03.095 BindingDB Entry DOI: 10.7270/Q29G5MW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212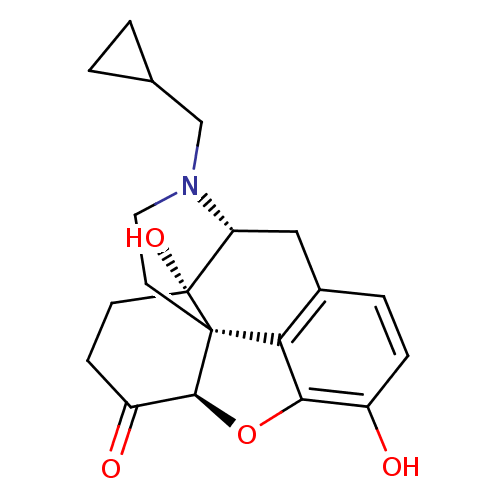 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity against Opioid receptor mu 1 using [3H]naloxone as radioligand. | J Med Chem 41: 4143-9 (1998) Article DOI: 10.1021/jm980290i BindingDB Entry DOI: 10.7270/Q2RB73Q6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50064518 (3-{(3R,4R)-1-[(E)-3-(2-Chloro-phenyl)-allyl]-3,4-d...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.567 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description The compound was tested for antagonist activity by selective inhibition of [35S]-GTP-gammaS, binding in Guinea pig caudate stimulated by U69,593 to O... | J Med Chem 41: 1980-90 (1998) Article DOI: 10.1021/jm980063g BindingDB Entry DOI: 10.7270/Q2K35SSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (RAT) | BDBM50366775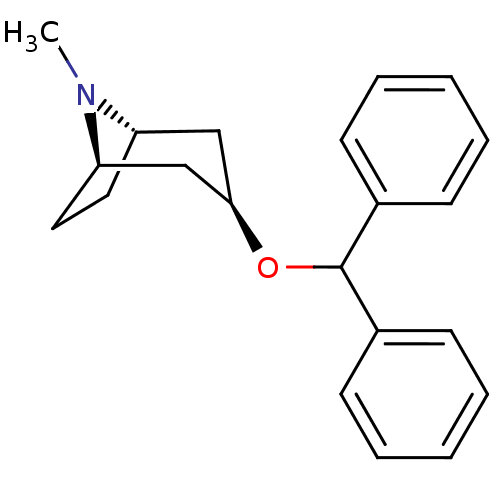 (BENZTROPINE | Benzatropine) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Diabetes and Digestive and Kidney Diseases Curated by ChEMBL | Assay Description Binding affinity against Muscarinic receptor from rat brain membranes using [3H]pirenzepine | J Med Chem 44: 3937-45 (2001) BindingDB Entry DOI: 10.7270/Q26Q1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3127 total ) | Next | Last >> |