 Found 96 hits with Last Name = 'dieckman' and Initial = 'dk'
Found 96 hits with Last Name = 'dieckman' and Initial = 'dk' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060725

(2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...)Show SMILES Clc1ccc(OCc2c(C(=O)CN3CCC(CC3)N3CCCCC3)c3ccccc3n2CCC[C@@H]2CCCNC2)cc1 Show InChI InChI=1S/C35H47ClN4O2/c36-28-12-14-30(15-13-28)42-26-33-35(34(41)25-38-22-16-29(17-23-38)39-19-4-1-5-20-39)31-10-2-3-11-32(31)40(33)21-7-9-27-8-6-18-37-24-27/h2-3,10-15,27,29,37H,1,4-9,16-26H2/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060726
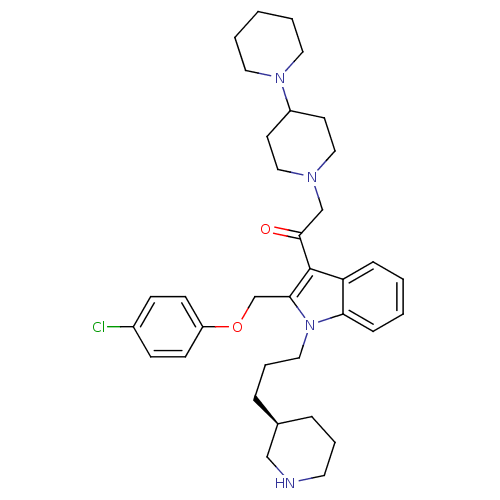
(2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...)Show SMILES Clc1ccc(OCc2c(C(=O)CN3CCC(CC3)N3CCCCC3)c3ccccc3n2CCC[C@H]2CCCNC2)cc1 Show InChI InChI=1S/C35H47ClN4O2/c36-28-12-14-30(15-13-28)42-26-33-35(34(41)25-38-22-16-29(17-23-38)39-19-4-1-5-20-39)31-10-2-3-11-32(31)40(33)21-7-9-27-8-6-18-37-24-27/h2-3,10-15,27,29,37H,1,4-9,16-26H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060725

(2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...)Show SMILES Clc1ccc(OCc2c(C(=O)CN3CCC(CC3)N3CCCCC3)c3ccccc3n2CCC[C@@H]2CCCNC2)cc1 Show InChI InChI=1S/C35H47ClN4O2/c36-28-12-14-30(15-13-28)42-26-33-35(34(41)25-38-22-16-29(17-23-38)39-19-4-1-5-20-39)31-10-2-3-11-32(31)40(33)21-7-9-27-8-6-18-37-24-27/h2-3,10-15,27,29,37H,1,4-9,16-26H2/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability of the compound to reverse Neuropeptide Y receptor type 1-induced inhibition of forskolin-induced inhibition of forskolin-stimulated cAMP |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060724

(2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...)Show SMILES Clc1ccc(OCc2c(C(=O)CN3CCC(CC3)N3CCCCC3)c3ccccc3n2CCC2CCNCC2)cc1 Show InChI InChI=1S/C34H45ClN4O2/c35-27-8-10-29(11-9-27)41-25-32-34(33(40)24-37-21-15-28(16-22-37)38-19-4-1-5-20-38)30-6-2-3-7-31(30)39(32)23-14-26-12-17-36-18-13-26/h2-3,6-11,26,28,36H,1,4-5,12-25H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060721

(2-(4-Chloro-phenoxymethyl)-1-methyl-3-(2-piperidin...)Show InChI InChI=1S/C23H27ClN2O/c1-25-22-8-4-3-7-20(22)21(13-16-26-14-5-2-6-15-26)23(25)17-27-19-11-9-18(24)10-12-19/h3-4,7-12H,2,5-6,13-17H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060722
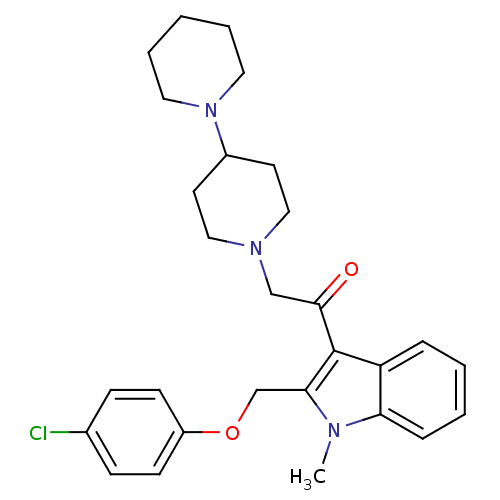
(2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...)Show SMILES Cn1c(COc2ccc(Cl)cc2)c(C(=O)CN2CCC(CC2)N2CCCCC2)c2ccccc12 Show InChI InChI=1S/C28H34ClN3O2/c1-30-25-8-4-3-7-24(25)28(26(30)20-34-23-11-9-21(29)10-12-23)27(33)19-31-17-13-22(14-18-31)32-15-5-2-6-16-32/h3-4,7-12,22H,2,5-6,13-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86754
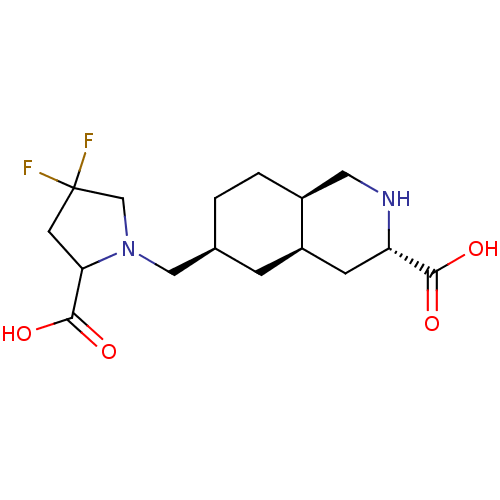
(LY 466195 | LY-466195)Show SMILES OC(=O)C1CC(F)(F)CN1C[C@H]1CC[C@H]2CN[C@@H](C[C@H]2C1)C(O)=O |r| Show InChI InChI=1S/C16H24F2N2O4/c17-16(18)5-13(15(23)24)20(8-16)7-9-1-2-10-6-19-12(14(21)22)4-11(10)3-9/h9-13,19H,1-8H2,(H,21,22)(H,23,24)/t9-,10-,11+,12-,13?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060720
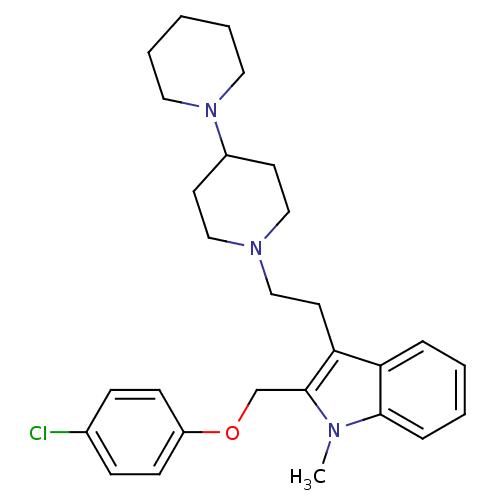
(1'-{2-[2-(4-Chloro-phenoxymethyl)-1-methyl-1H-indo...)Show SMILES Cn1c(COc2ccc(Cl)cc2)c(CCN2CCC(CC2)N2CCCCC2)c2ccccc12 Show InChI InChI=1S/C28H36ClN3O/c1-30-27-8-4-3-7-25(27)26(28(30)21-33-24-11-9-22(29)10-12-24)15-20-31-18-13-23(14-19-31)32-16-5-2-6-17-32/h3-4,7-12,23H,2,5-6,13-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060730

(1'-[2-(4-Chloro-phenoxymethyl)-1-(2-piperidin-1-yl...)Show SMILES Clc1ccc(OCc2c(CN3CCC(CC3)N3CCCCC3)c3ccccc3n2CCN2CCCCC2)cc1 Show InChI InChI=1S/C33H45ClN4O/c34-27-11-13-29(14-12-27)39-26-33-31(25-36-21-15-28(16-22-36)37-19-7-2-8-20-37)30-9-3-4-10-32(30)38(33)24-23-35-17-5-1-6-18-35/h3-4,9-14,28H,1-2,5-8,15-26H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50207594

(2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[nH]c(=O)c(=O)[nH]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H,14,17)(H,15,18)(H2,13,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 2 |
J Med Chem 45: 4383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2XP75N4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060723
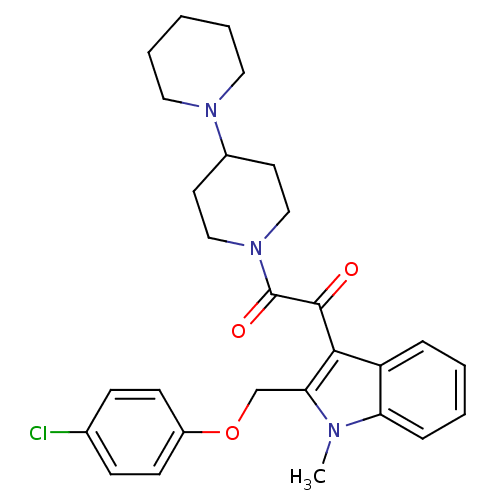
(1-[1,4']Bipiperidinyl-1'-yl-2-[2-(4-chloro-phenoxy...)Show SMILES Cn1c(COc2ccc(Cl)cc2)c(C(=O)C(=O)N2CCC(CC2)N2CCCCC2)c2ccccc12 Show InChI InChI=1S/C28H32ClN3O3/c1-30-24-8-4-3-7-23(24)26(25(30)19-35-22-11-9-20(29)10-12-22)27(33)28(34)32-17-13-21(14-18-32)31-15-5-2-6-16-31/h3-4,7-12,21H,2,5-6,13-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060729
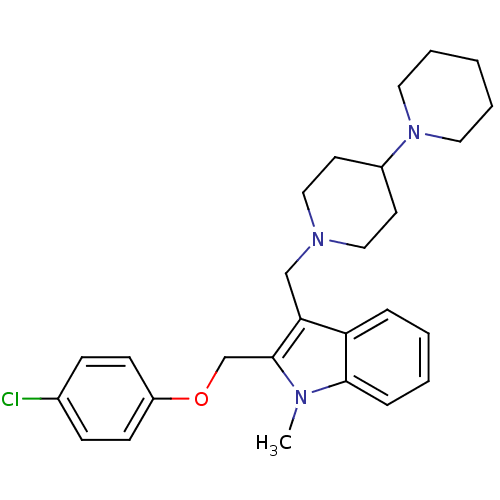
(1'-[2-(4-Chloro-phenoxymethyl)-1-methyl-1H-indol-3...)Show SMILES Cn1c(COc2ccc(Cl)cc2)c(CN2CCC(CC2)N2CCCCC2)c2ccccc12 Show InChI InChI=1S/C27H34ClN3O/c1-29-26-8-4-3-7-24(26)25(27(29)20-32-23-11-9-21(28)10-12-23)19-30-17-13-22(14-18-30)31-15-5-2-6-16-31/h3-4,7-12,22H,2,5-6,13-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 559 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Glutamate receptor 4
(Homo sapiens (Human)) | BDBM50207594

(2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[nH]c(=O)c(=O)[nH]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H,14,17)(H,15,18)(H2,13,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 4 |
J Med Chem 45: 4383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2XP75N4 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM86750
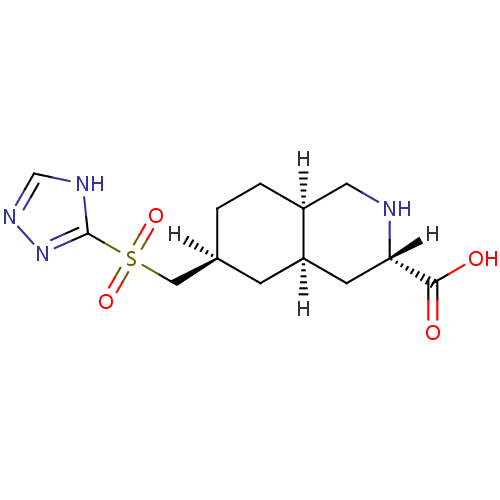
(LY 302679 | LY-302679)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](C[S](=O)(=O)c3nnc[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H20N4O4S/c18-12(19)11-4-10-3-8(1-2-9(10)5-14-11)6-22(20,21)13-15-7-16-17-13/h7-11,14H,1-6H2,(H,18,19)(H,15,16,17)/t8-,9-,10+,11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 820 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 3
(Homo sapiens (Human)) | BDBM50207594

(2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[nH]c(=O)c(=O)[nH]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H,14,17)(H,15,18)(H2,13,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 3 |
J Med Chem 45: 4383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2XP75N4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060731
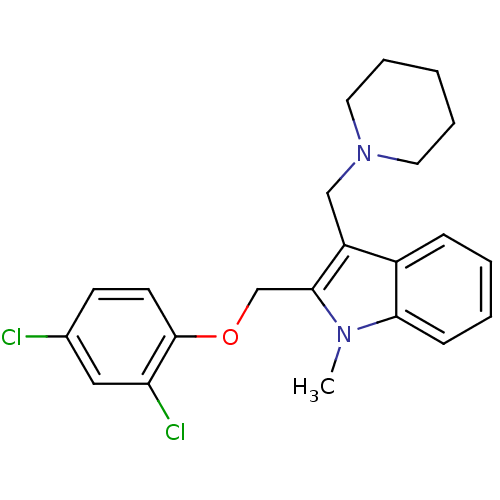
(2-(2,4-Dichloro-phenoxymethyl)-1-methyl-3-piperidi...)Show SMILES Cn1c(COc2ccc(Cl)cc2Cl)c(CN2CCCCC2)c2ccccc12 Show InChI InChI=1S/C22H24Cl2N2O/c1-25-20-8-4-3-7-17(20)18(14-26-11-5-2-6-12-26)21(25)15-27-22-10-9-16(23)13-19(22)24/h3-4,7-10,13H,2,5-6,11-12,14-15H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86749

(LY 457691)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Nc1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H22N6O2/c24-17(25)15-8-11-7-12(6-5-10(11)9-18-15)19-14-4-2-1-3-13(14)16-20-22-23-21-16/h1-4,10-12,15,18-19H,5-9H2,(H,24,25)(H,20,21,22,23)/t10-,11+,12-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Homo sapiens (Human)) | BDBM50207594

(2,3-Dihydroxy-6-nitro-benzo[f]quinoxaline-7-sulfon...)Show SMILES NS(=O)(=O)c1cccc2c1c(cc1[nH]c(=O)c(=O)[nH]c21)[N+]([O-])=O Show InChI InChI=1S/C12H8N4O6S/c13-23(21,22)8-3-1-2-5-9(8)7(16(19)20)4-6-10(5)15-12(18)11(17)14-6/h1-4H,(H,14,17)(H,15,18)(H2,13,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 1 |
J Med Chem 45: 4383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2XP75N4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86755

(LY 458545 | LY-458545)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Oc1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H21N5O3/c23-17(24)14-8-11-7-12(6-5-10(11)9-18-14)25-15-4-2-1-3-13(15)16-19-21-22-20-16/h1-4,10-12,14,18H,5-9H2,(H,23,24)(H,19,20,21,22)/t10-,11+,12-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060728

((R)-2-(2,2-diphenylacetamido)-5-guanidino-N-(4-hyd...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6](-c1ccccc1)-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C27H31N5O3/c28-27(29)30-17-7-12-23(25(34)31-18-19-13-15-22(33)16-14-19)32-26(35)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h1-6,8-11,13-16,23-24,33H,7,12,17-18H2,(H,31,34)(H,32,35)(H4,28,29,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 1
(Homo sapiens (Human)) | BDBM50060727
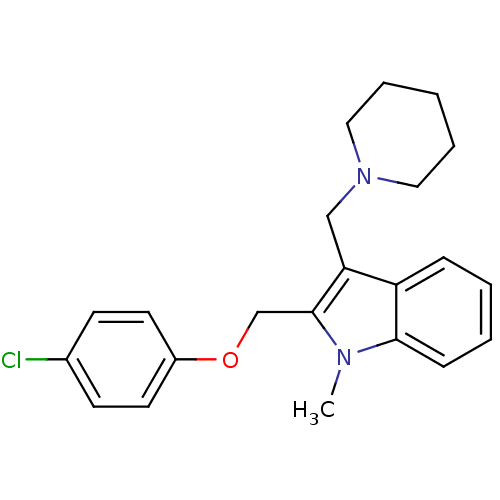
(2-(4-Chloro-phenoxymethyl)-1-methyl-3-piperidin-1-...)Show InChI InChI=1S/C22H25ClN2O/c1-24-21-8-4-3-7-19(21)20(15-25-13-5-2-6-14-25)22(24)16-26-18-11-9-17(23)10-12-18/h3-4,7-12H,2,5-6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity for Neuropeptide Y receptor type 1 expressed in AV-12 cells |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86752
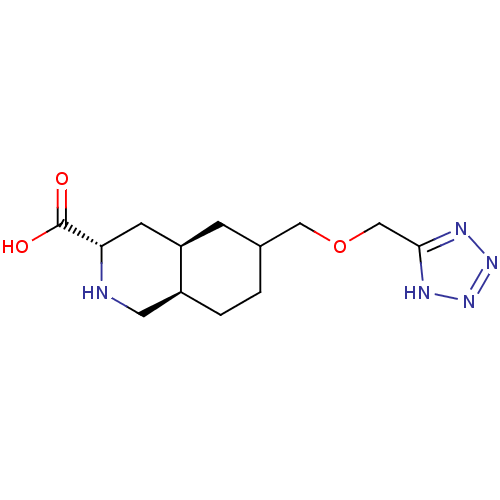
(LY 294486 | LY-294486)Show SMILES OC(=O)[C@@H]1C[C@H]2CC(COCc3nnn[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H21N5O3/c19-13(20)11-4-10-3-8(1-2-9(10)5-14-11)6-21-7-12-15-17-18-16-12/h8-11,14H,1-7H2,(H,19,20)(H,15,16,17,18)/t8?,9-,10+,11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM86751

(CHEMBL14935 | LY 293558 | LY-293558)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](CCc3nnn[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H21N5O2/c19-13(20)11-6-10-5-8(1-3-9(10)7-14-11)2-4-12-15-17-18-16-12/h8-11,14H,1-7H2,(H,19,20)(H,15,16,17,18)/t8-,9+,10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM86751

(CHEMBL14935 | LY 293558 | LY-293558)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](CCc3nnn[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H21N5O2/c19-13(20)11-6-10-5-8(1-3-9(10)7-14-11)2-4-12-15-17-18-16-12/h8-11,14H,1-7H2,(H,19,20)(H,15,16,17,18)/t8-,9+,10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 2 |
J Med Chem 45: 4383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2XP75N4 |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86750

(LY 302679 | LY-302679)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](C[S](=O)(=O)c3nnc[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H20N4O4S/c18-12(19)11-4-10-3-8(1-2-9(10)5-14-11)6-22(20,21)13-15-7-16-17-13/h7-11,14H,1-6H2,(H,18,19)(H,15,16,17)/t8-,9-,10+,11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM50168962
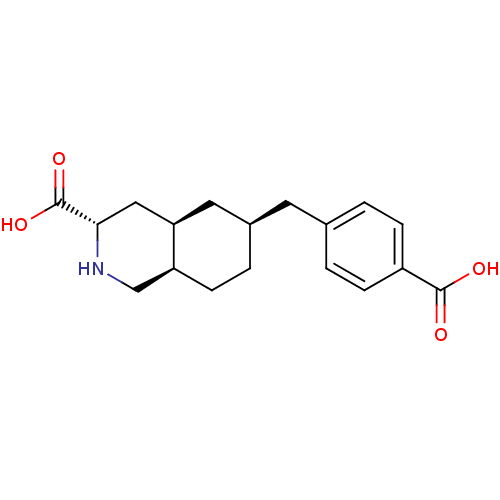
((3S,4aR,6S,8aR)-6-(4-Carboxy-benzyl)-decahydro-iso...)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](Cc3ccc(cc3)C(O)=O)CC[C@H]2CN1 Show InChI InChI=1S/C18H23NO4/c20-17(21)13-4-1-11(2-5-13)7-12-3-6-14-10-19-16(18(22)23)9-15(14)8-12/h1-2,4-5,12,14-16,19H,3,6-10H2,(H,20,21)(H,22,23)/t12-,14+,15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86751

(CHEMBL14935 | LY 293558 | LY-293558)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](CCc3nnn[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H21N5O2/c19-13(20)11-6-10-5-8(1-3-9(10)7-14-11)2-4-12-15-17-18-16-12/h8-11,14H,1-7H2,(H,19,20)(H,15,16,17,18)/t8-,9+,10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM86749

(LY 457691)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Nc1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H22N6O2/c24-17(25)15-8-11-7-12(6-5-10(11)9-18-15)19-14-4-2-1-3-13(14)16-20-22-23-21-16/h1-4,10-12,15,18-19H,5-9H2,(H,24,25)(H,20,21,22,23)/t10-,11+,12-,15-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 5
(Homo sapiens (Human)) | BDBM86756

(LY 467711 | LY-467711)Show SMILES CCC(C)OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Oc1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C21H29N5O3/c1-3-13(2)28-21(27)18-11-15-10-16(9-8-14(15)12-22-18)29-19-7-5-4-6-17(19)20-23-25-26-24-20/h4-7,13-16,18,22H,3,8-12H2,1-2H3,(H,23,24,25,26)/t13?,14-,15+,16-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM86755

(LY 458545 | LY-458545)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Oc1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C17H21N5O3/c23-17(24)14-8-11-7-12(6-5-10(11)9-18-14)25-15-4-2-1-3-13(15)16-19-21-22-20-16/h1-4,10-12,14,18H,5-9H2,(H,23,24)(H,19,20,21,22)/t10-,11+,12-,14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor ionotropic, kainate 3
(Homo sapiens (Human)) | BDBM86754

(LY 466195 | LY-466195)Show SMILES OC(=O)C1CC(F)(F)CN1C[C@H]1CC[C@H]2CN[C@@H](C[C@H]2C1)C(O)=O |r| Show InChI InChI=1S/C16H24F2N2O4/c17-16(18)5-13(15(23)24)20(8-16)7-9-1-2-10-6-19-12(14(21)22)4-11(10)3-9/h9-13,19H,1-8H2,(H,21,22)(H,23,24)/t9-,10-,11+,12-,13?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Homo sapiens (Human)) | BDBM86751

(CHEMBL14935 | LY 293558 | LY-293558)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](CCc3nnn[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H21N5O2/c19-13(20)11-6-10-5-8(1-3-9(10)7-14-11)2-4-12-15-17-18-16-12/h8-11,14H,1-7H2,(H,19,20)(H,15,16,17,18)/t8-,9+,10-,11+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Ability to displace binding of [3H]AMPA to recombinant human Ionotropic glutamate receptor AMPA 1 |
J Med Chem 45: 4383-6 (2002)
BindingDB Entry DOI: 10.7270/Q2XP75N4 |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Homo sapiens (Human)) | BDBM50168962

((3S,4aR,6S,8aR)-6-(4-Carboxy-benzyl)-decahydro-iso...)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](Cc3ccc(cc3)C(O)=O)CC[C@H]2CN1 Show InChI InChI=1S/C18H23NO4/c20-17(21)13-4-1-11(2-5-13)7-12-3-6-14-10-19-16(18(22)23)9-15(14)8-12/h1-2,4-5,12,14-16,19H,3,6-10H2,(H,20,21)(H,22,23)/t12-,14+,15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 1
(Homo sapiens (Human)) | BDBM86754

(LY 466195 | LY-466195)Show SMILES OC(=O)C1CC(F)(F)CN1C[C@H]1CC[C@H]2CN[C@@H](C[C@H]2C1)C(O)=O |r| Show InChI InChI=1S/C16H24F2N2O4/c17-16(18)5-13(15(23)24)20(8-16)7-9-1-2-10-6-19-12(14(21)22)4-11(10)3-9/h9-13,19H,1-8H2,(H,21,22)(H,23,24)/t9-,10-,11+,12-,13?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50060727

(2-(4-Chloro-phenoxymethyl)-1-methyl-3-piperidin-1-...)Show InChI InChI=1S/C22H25ClN2O/c1-24-21-8-4-3-7-19(21)20(15-25-13-5-2-6-14-25)22(24)16-26-18-11-9-17(23)10-12-18/h3-4,7-12H,2,5-6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for human Neuropeptide Y receptor type 4 |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50060724

(2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...)Show SMILES Clc1ccc(OCc2c(C(=O)CN3CCC(CC3)N3CCCCC3)c3ccccc3n2CCC2CCNCC2)cc1 Show InChI InChI=1S/C34H45ClN4O2/c35-27-8-10-29(11-9-27)41-25-32-34(33(40)24-37-21-15-28(16-22-37)38-19-4-1-5-20-38)30-6-2-3-7-31(30)39(32)23-14-26-12-17-36-18-13-26/h2-3,6-11,26,28,36H,1,4-5,12-25H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for human Neuropeptide Y receptor type 4 |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50060720

(1'-{2-[2-(4-Chloro-phenoxymethyl)-1-methyl-1H-indo...)Show SMILES Cn1c(COc2ccc(Cl)cc2)c(CCN2CCC(CC2)N2CCCCC2)c2ccccc12 Show InChI InChI=1S/C28H36ClN3O/c1-30-27-8-4-3-7-25(27)26(28(30)21-33-24-11-9-22(29)10-12-24)15-20-31-18-13-23(14-19-31)32-16-5-2-6-17-32/h3-4,7-12,23H,2,5-6,13-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for human Neuropeptide Y receptor type 4 |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50060729

(1'-[2-(4-Chloro-phenoxymethyl)-1-methyl-1H-indol-3...)Show SMILES Cn1c(COc2ccc(Cl)cc2)c(CN2CCC(CC2)N2CCCCC2)c2ccccc12 Show InChI InChI=1S/C27H34ClN3O/c1-29-26-8-4-3-7-24(26)25(27(29)20-32-23-11-9-21(28)10-12-23)19-30-17-13-22(14-18-30)31-15-5-2-6-16-31/h3-4,7-12,22H,2,5-6,13-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for human Neuropeptide Y receptor type 2 |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50060726

(2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...)Show SMILES Clc1ccc(OCc2c(C(=O)CN3CCC(CC3)N3CCCCC3)c3ccccc3n2CCC[C@H]2CCCNC2)cc1 Show InChI InChI=1S/C35H47ClN4O2/c36-28-12-14-30(15-13-28)42-26-33-35(34(41)25-38-22-16-29(17-23-38)39-19-4-1-5-20-39)31-10-2-3-11-32(31)40(33)21-7-9-27-8-6-18-37-24-27/h2-3,10-15,27,29,37H,1,4-9,16-26H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for human Neuropeptide Y receptor type 2 |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50060726

(2-[1,4']Bipiperidinyl-1'-yl-1-[2-(4-chloro-phenoxy...)Show SMILES Clc1ccc(OCc2c(C(=O)CN3CCC(CC3)N3CCCCC3)c3ccccc3n2CCC[C@H]2CCCNC2)cc1 Show InChI InChI=1S/C35H47ClN4O2/c36-28-12-14-30(15-13-28)42-26-33-35(34(41)25-38-22-16-29(17-23-38)39-19-4-1-5-20-39)31-10-2-3-11-32(31)40(33)21-7-9-27-8-6-18-37-24-27/h2-3,10-15,27,29,37H,1,4-9,16-26H2/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for human Neuropeptide Y receptor type 4 |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 4
(Homo sapiens (Human)) | BDBM50060728

((R)-2-(2,2-diphenylacetamido)-5-guanidino-N-(4-hyd...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@@H](-[#7]-[#6](=O)-[#6](-c1ccccc1)-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(-[#8])cc1 |r| Show InChI InChI=1S/C27H31N5O3/c28-27(29)30-17-7-12-23(25(34)31-18-19-13-15-22(33)16-14-19)32-26(35)24(20-8-3-1-4-9-20)21-10-5-2-6-11-21/h1-6,8-11,13-16,23-24,33H,7,12,17-18H2,(H,31,34)(H,32,35)(H4,28,29,30)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for human Neuropeptide Y receptor type 4 |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50060729

(1'-[2-(4-Chloro-phenoxymethyl)-1-methyl-1H-indol-3...)Show SMILES Cn1c(COc2ccc(Cl)cc2)c(CN2CCC(CC2)N2CCCCC2)c2ccccc12 Show InChI InChI=1S/C27H34ClN3O/c1-29-26-8-4-3-7-24(26)25(27(29)20-32-23-11-9-21(28)10-12-23)19-30-17-13-22(14-18-30)31-15-5-2-6-16-31/h3-4,7-12,22H,2,5-6,13-20H2,1H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for human Neuropeptide Y receptor type 5 |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 2
(Homo sapiens (Human)) | BDBM50060727

(2-(4-Chloro-phenoxymethyl)-1-methyl-3-piperidin-1-...)Show InChI InChI=1S/C22H25ClN2O/c1-24-21-8-4-3-7-19(21)20(15-25-13-5-2-6-14-25)22(24)16-26-18-11-9-17(23)10-12-18/h3-4,7-12H,2,5-6,13-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for human Neuropeptide Y receptor type 2 |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Neuropeptide Y receptor type 5
(Homo sapiens (Human)) | BDBM50060727

(2-(4-Chloro-phenoxymethyl)-1-methyl-3-piperidin-1-...)Show InChI InChI=1S/C22H25ClN2O/c1-24-21-8-4-3-7-19(21)20(15-25-13-5-2-6-14-25)22(24)16-26-18-11-9-17(23)10-12-18/h3-4,7-12H,2,5-6,13-16H2,1H3 | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Affinity for human Neuropeptide Y receptor type 5 |
J Med Chem 40: 3712-4 (1997)
Article DOI: 10.1021/jm970512x
BindingDB Entry DOI: 10.7270/Q2HQ3Z1P |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM86752

(LY 294486 | LY-294486)Show SMILES OC(=O)[C@@H]1C[C@H]2CC(COCc3nnn[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H21N5O3/c19-13(20)11-4-10-3-8(1-2-9(10)5-14-11)6-21-7-12-15-17-18-16-12/h8-11,14H,1-7H2,(H,19,20)(H,15,16,17,18)/t8?,9-,10+,11-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM50168962

((3S,4aR,6S,8aR)-6-(4-Carboxy-benzyl)-decahydro-iso...)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](Cc3ccc(cc3)C(O)=O)CC[C@H]2CN1 Show InChI InChI=1S/C18H23NO4/c20-17(21)13-4-1-11(2-5-13)7-12-3-6-14-10-19-16(18(22)23)9-15(14)8-12/h1-2,4-5,12,14-16,19H,3,6-10H2,(H,20,21)(H,22,23)/t12-,14+,15-,16+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM86754

(LY 466195 | LY-466195)Show SMILES OC(=O)C1CC(F)(F)CN1C[C@H]1CC[C@H]2CN[C@@H](C[C@H]2C1)C(O)=O |r| Show InChI InChI=1S/C16H24F2N2O4/c17-16(18)5-13(15(23)24)20(8-16)7-9-1-2-10-6-19-12(14(21)22)4-11(10)3-9/h9-13,19H,1-8H2,(H,21,22)(H,23,24)/t9-,10-,11+,12-,13?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM86756

(LY 467711 | LY-467711)Show SMILES CCC(C)OC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Oc1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C21H29N5O3/c1-3-13(2)28-21(27)18-11-15-10-16(9-8-14(15)12-22-18)29-19-7-5-4-6-17(19)20-23-25-26-24-20/h4-7,13-16,18,22H,3,8-12H2,1-2H3,(H,23,24,25,26)/t13?,14-,15+,16-,18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 2
(Homo sapiens (Human)) | BDBM86753
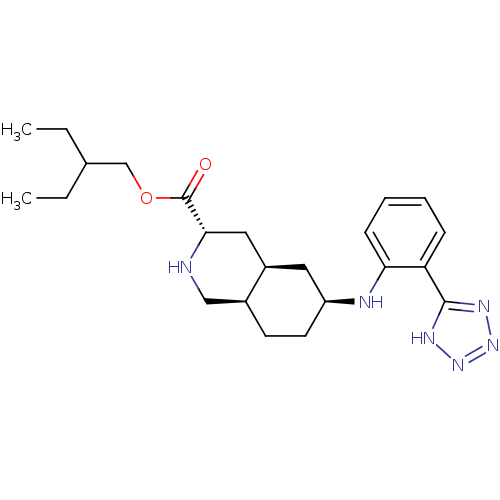
(LY 525327 | LY-525327)Show SMILES CCC(CC)COC(=O)[C@@H]1C[C@H]2C[C@H](CC[C@H]2CN1)Nc1ccccc1-c1nnn[nH]1 |r| Show InChI InChI=1S/C23H34N6O2/c1-3-15(4-2)14-31-23(30)21-12-17-11-18(10-9-16(17)13-24-21)25-20-8-6-5-7-19(20)22-26-28-29-27-22/h5-8,15-18,21,24-25H,3-4,9-14H2,1-2H3,(H,26,27,28,29)/t16-,17+,18-,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |
Glutamate receptor 4
(Homo sapiens (Human)) | BDBM86751

(CHEMBL14935 | LY 293558 | LY-293558)Show SMILES OC(=O)[C@@H]1C[C@H]2C[C@@H](CCc3nnn[nH]3)CC[C@H]2CN1 Show InChI InChI=1S/C13H21N5O2/c19-13(20)11-6-10-5-8(1-3-9(10)7-14-11)2-4-12-15-17-18-16-12/h8-11,14H,1-7H2,(H,19,20)(H,15,16,17,18)/t8-,9+,10-,11+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 318: 772-81 (2006)
Article DOI: 10.1124/jpet.106.101428
BindingDB Entry DOI: 10.7270/Q2NP230D |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data