

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B2 bradykinin receptor (RAT) | BDBM50370083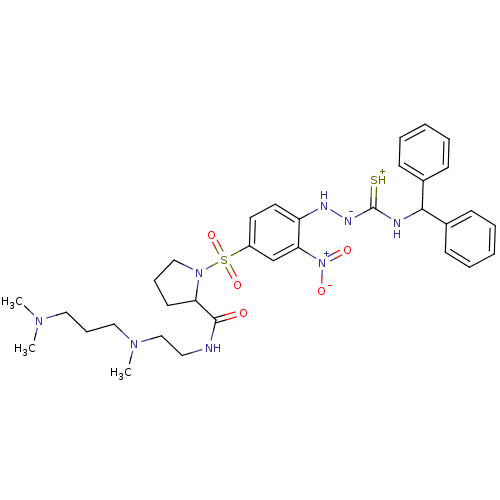 (CHEMBL1907651) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50485941 (CHEMBL2181004) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford Curated by ChEMBL | Assay Description Binding affinity to pig brain tubulin | J Med Chem 55: 11062-6 (2012) Article DOI: 10.1021/jm301151t BindingDB Entry DOI: 10.7270/Q22V2K0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50485945 (CHEMBL2181003) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0585 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford Curated by ChEMBL | Assay Description Binding affinity to pig brain tubulin | J Med Chem 55: 11062-6 (2012) Article DOI: 10.1021/jm301151t BindingDB Entry DOI: 10.7270/Q22V2K0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50485942 (CHEMBL2181002) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford Curated by ChEMBL | Assay Description Binding affinity to pig brain tubulin | J Med Chem 55: 11062-6 (2012) Article DOI: 10.1021/jm301151t BindingDB Entry DOI: 10.7270/Q22V2K0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50485950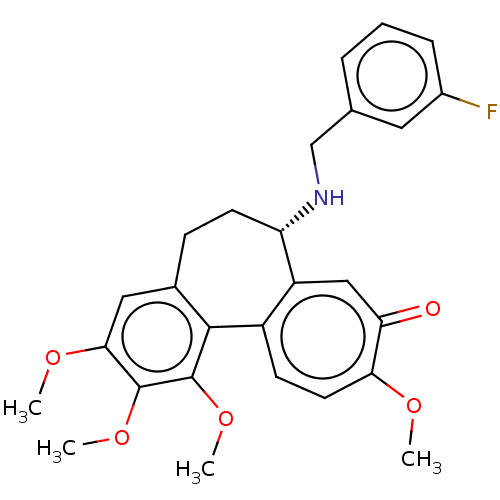 (CHEMBL2181009) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.122 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford Curated by ChEMBL | Assay Description Binding affinity to pig brain tubulin | J Med Chem 55: 11062-6 (2012) Article DOI: 10.1021/jm301151t BindingDB Entry DOI: 10.7270/Q22V2K0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50485944 (CHEMBL2181006) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford Curated by ChEMBL | Assay Description Binding affinity to pig brain tubulin | J Med Chem 55: 11062-6 (2012) Article DOI: 10.1021/jm301151t BindingDB Entry DOI: 10.7270/Q22V2K0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50485943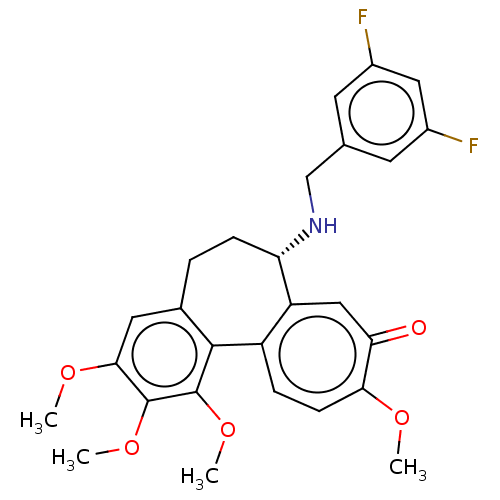 (CHEMBL2181001) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford Curated by ChEMBL | Assay Description Binding affinity to pig brain tubulin | J Med Chem 55: 11062-6 (2012) Article DOI: 10.1021/jm301151t BindingDB Entry DOI: 10.7270/Q22V2K0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50485946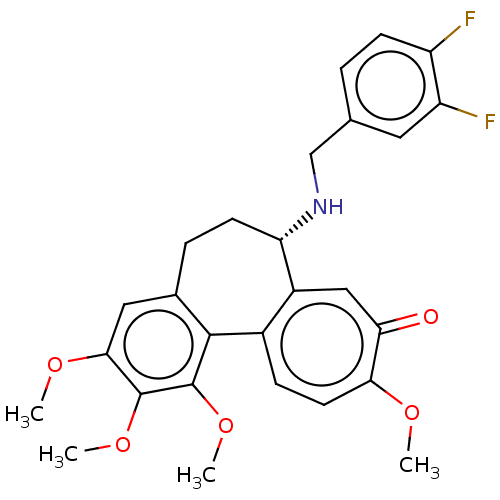 (CHEMBL2181000) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.143 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford Curated by ChEMBL | Assay Description Binding affinity to pig brain tubulin | J Med Chem 55: 11062-6 (2012) Article DOI: 10.1021/jm301151t BindingDB Entry DOI: 10.7270/Q22V2K0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50499869 (CHEMBL3742140) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]Spiroperidol from cloned rat dopamine D3 receptor expressed in HEK293 cells by liquid scintillation counting analysis | J Med Chem 58: 9179-95 (2015) Article DOI: 10.1021/acs.jmedchem.5b01031 BindingDB Entry DOI: 10.7270/Q2N019HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50485947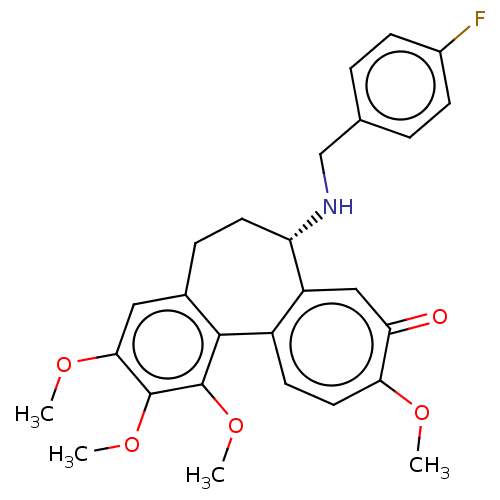 (CHEMBL2181008) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.183 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford Curated by ChEMBL | Assay Description Binding affinity to pig brain tubulin | J Med Chem 55: 11062-6 (2012) Article DOI: 10.1021/jm301151t BindingDB Entry DOI: 10.7270/Q22V2K0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50307830 ((S)-6-(propyl(2-(4-(quinolin-4-yl)piperazin-1-yl)e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.186 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from cloned dopamine D3 receptor expressed in HEK cells | J Med Chem 53: 2114-25 (2010) Article DOI: 10.1021/jm901618d BindingDB Entry DOI: 10.7270/Q20K28QZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50303799 ((-)-(S)-6-(Propyl(2-(4-(quinolin-4-yl)piperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 53: 1023-37 (2010) Article DOI: 10.1021/jm901184n BindingDB Entry DOI: 10.7270/Q29023VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50485949 (CHEMBL2180999) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford Curated by ChEMBL | Assay Description Binding affinity to pig brain tubulin | J Med Chem 55: 11062-6 (2012) Article DOI: 10.1021/jm301151t BindingDB Entry DOI: 10.7270/Q22V2K0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50485948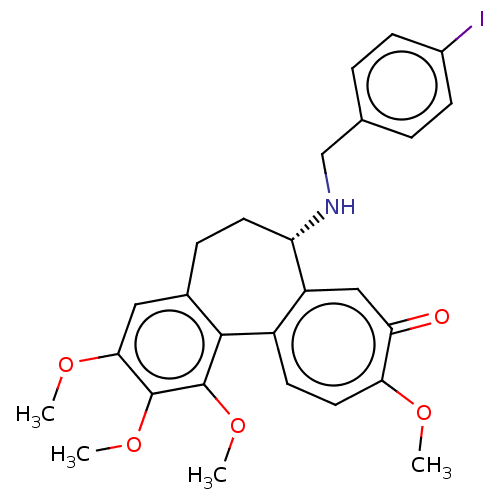 (CHEMBL2181007) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford Curated by ChEMBL | Assay Description Binding affinity to pig brain tubulin | J Med Chem 55: 11062-6 (2012) Article DOI: 10.1021/jm301151t BindingDB Entry DOI: 10.7270/Q22V2K0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50483134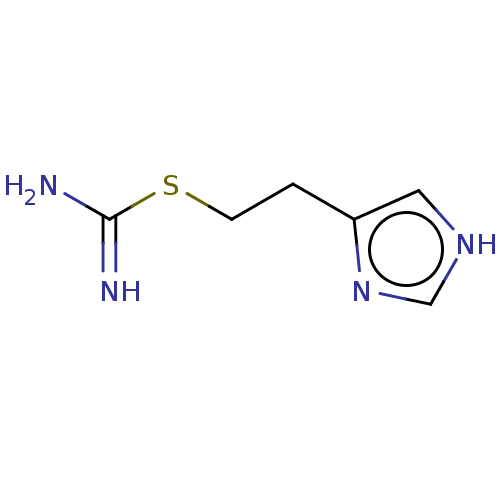 (CHEBI:64156 | Imetit) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd Curated by ChEMBL | Assay Description Displacement of [3]-RAMH from recombinant human histamine H3 receptor expressed in CHO-K1 cell membranes after 60 mins by scintillation counting | J Med Chem 62: 1203-1217 (2019) Article DOI: 10.1021/acs.jmedchem.8b01280 BindingDB Entry DOI: 10.7270/Q2XD152G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120625 (CHEMBL3618330) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50370077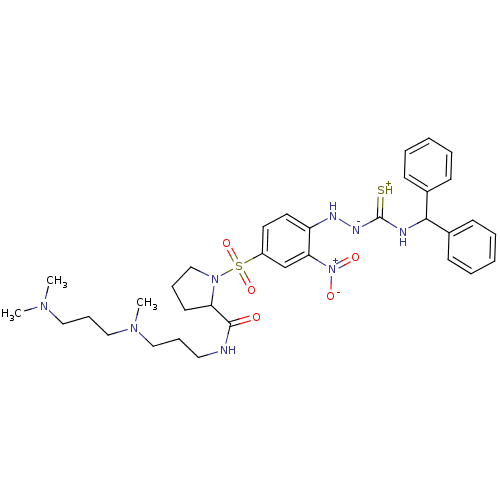 (CHEMBL1907652) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50374645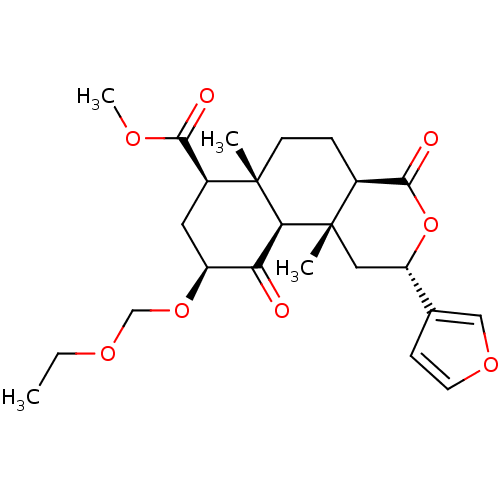 (CHEMBL272939) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem 16: 1279-86 (2008) Article DOI: 10.1016/j.bmc.2007.10.067 BindingDB Entry DOI: 10.7270/Q2ZK5HJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50241083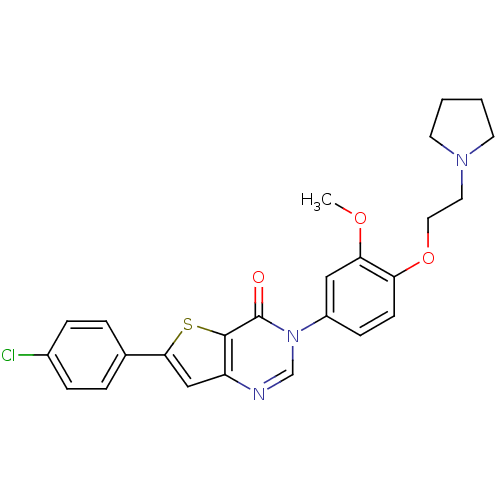 (6-(4-chlorophenyl)-3-(3-methoxy-4-(2-(pyrrolidin-1...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Antagonist activity at rat MCHR1 | Bioorg Med Chem Lett 25: 2793-9 (2015) Article DOI: 10.1016/j.bmcl.2015.05.008 BindingDB Entry DOI: 10.7270/Q29C7053 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cAMP and cAMP-inhibited cGMP 3',5'-cyclic phosphodiesterase 10A (Homo sapiens (Human)) | BDBM50594974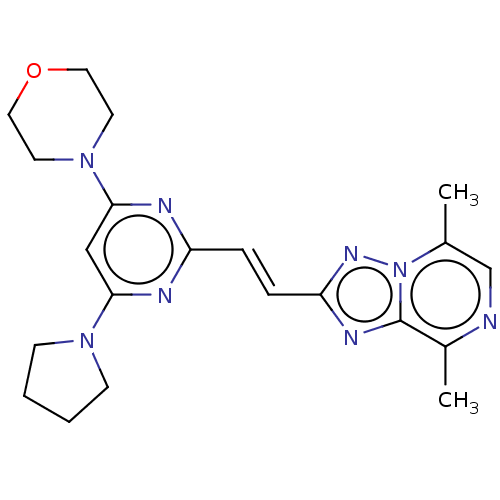 (CHEMBL5186554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.ejmech.2022.114170 BindingDB Entry DOI: 10.7270/Q2RX9H3Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tubulin beta chain (Sus scrofa) | BDBM50485940 (CHEMBL2181005) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.367 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bradford Curated by ChEMBL | Assay Description Binding affinity to pig brain tubulin | J Med Chem 55: 11062-6 (2012) Article DOI: 10.1021/jm301151t BindingDB Entry DOI: 10.7270/Q22V2K0N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50303799 ((-)-(S)-6-(Propyl(2-(4-(quinolin-4-yl)piperazin-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 53: 1023-37 (2010) Article DOI: 10.1021/jm901184n BindingDB Entry DOI: 10.7270/Q29023VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120624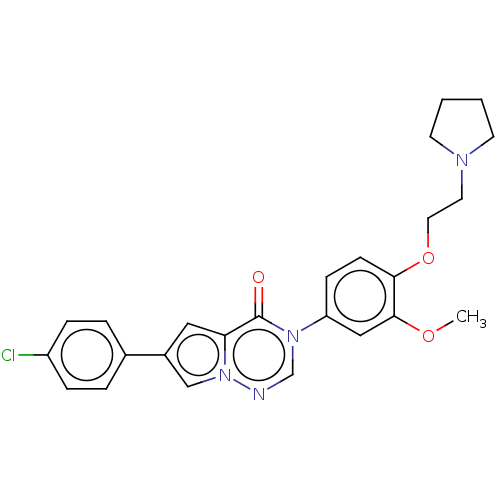 (CHEMBL3618324) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120625 (CHEMBL3618330) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-interacting serine/threonine-protein kinase 1 (Homo sapiens (Human)) | BDBM50244722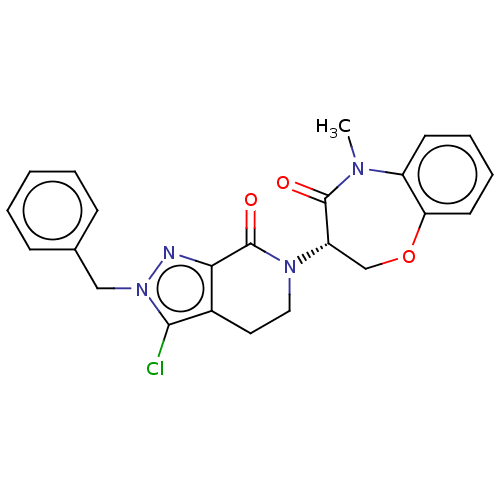 (CHEMBL4075976) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.407 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of fluorescent-labeled 3-(3-((3-(4-amino-5-(4-(3-(2-fluoro-5-(trifluoromethyl)phenyl)ureido)-phenyl)-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propy... | J Med Chem 61: 2384-2409 (2018) Article DOI: 10.1021/acs.jmedchem.7b01647 BindingDB Entry DOI: 10.7270/Q2V98BGX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50303796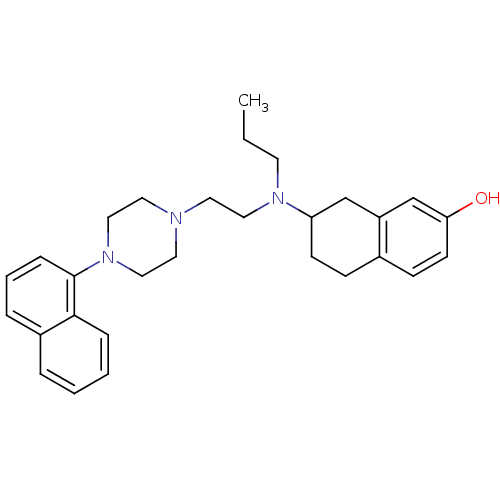 (7-((2-(4-(Naphthalen-1-yl)-piperazin-1-yl)ethyl)(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.435 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 53: 1023-37 (2010) Article DOI: 10.1021/jm901184n BindingDB Entry DOI: 10.7270/Q29023VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50303795 (7-((2-(4-(Isoquinolin-1-yl)piperazin-1-yl)ethyl)(p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.441 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 53: 1023-37 (2010) Article DOI: 10.1021/jm901184n BindingDB Entry DOI: 10.7270/Q29023VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229783 (6-[(2-(4-(2,3-dichlorophenyl)piperazin-1-yl)ethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229776 (6-[(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)(pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50229776 (6-[(2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)(pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50533790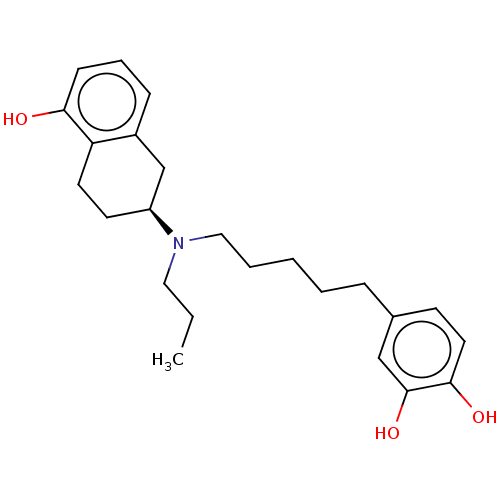 (CHEMBL4538230) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.493 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from rat D3 dopamine receptor expressed in HEK293 cell membranes after 1 hr | Bioorg Med Chem 24: 5088-5102 (2016) Article DOI: 10.1016/j.bmc.2016.08.021 BindingDB Entry DOI: 10.7270/Q2B85CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120624 (CHEMBL3618324) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (RAT) | BDBM50120635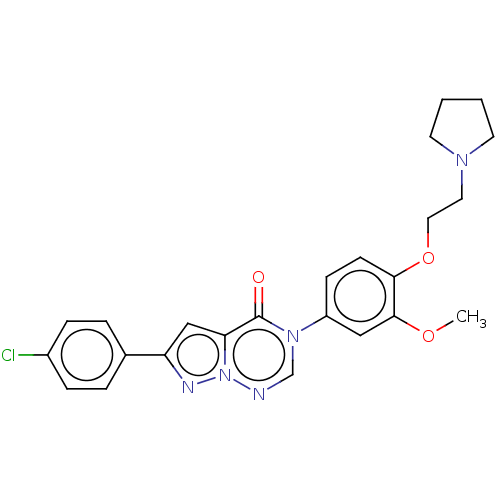 (CHEMBL3618325) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from rat MCHR1 by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50113263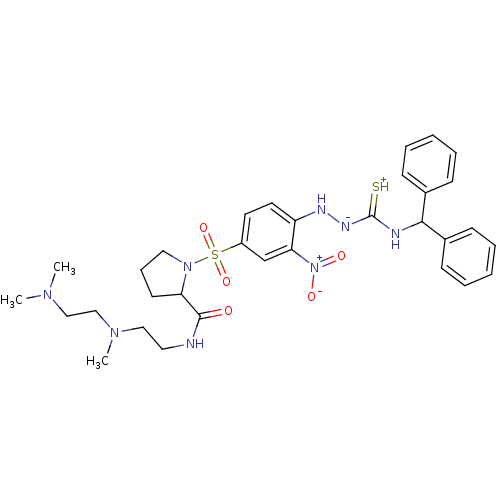 ((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409120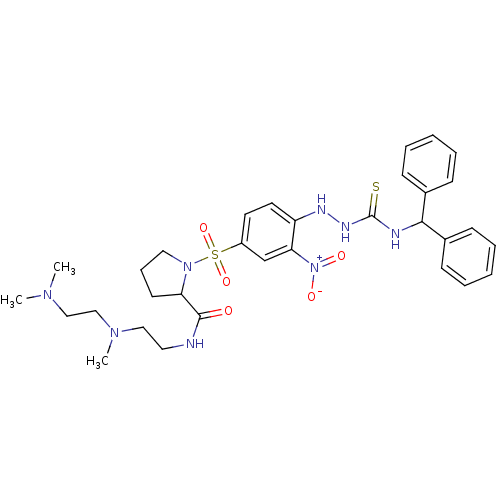 (CHEMBL2112044 | CHEMBL2112937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50294844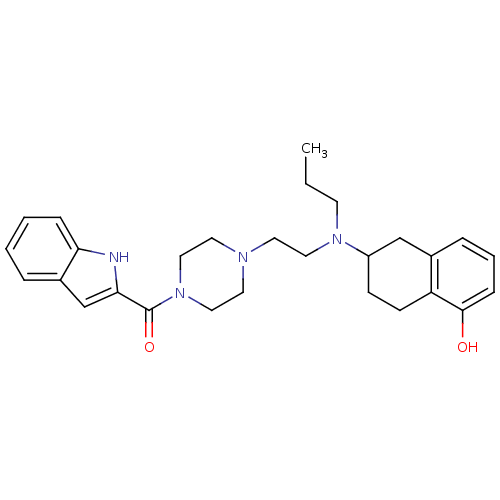 ((+)-(4-{2-[(5-Hydroxy-1,2,3,4-tetrahydro-naphthale...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from rat dopamine D3 receptor expressed in HEK293 cells | Bioorg Med Chem 17: 3923-33 (2009) Article DOI: 10.1016/j.bmc.2009.04.031 BindingDB Entry DOI: 10.7270/Q25H7G8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50294844 ((+)-(4-{2-[(5-Hydroxy-1,2,3,4-tetrahydro-naphthale...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from rat dopamine D3 receptor expressed in HEK293 cells | Bioorg Med Chem 17: 3923-33 (2009) Article DOI: 10.1016/j.bmc.2009.04.031 BindingDB Entry DOI: 10.7270/Q25H7G8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50303793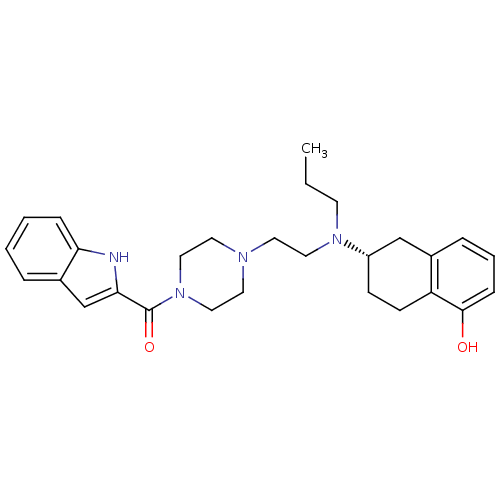 ((S)-(4-(2-((5-hydroxy-1,2,3,4-tetrahydronaphthalen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 53: 1023-37 (2010) Article DOI: 10.1021/jm901184n BindingDB Entry DOI: 10.7270/Q29023VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50294844 ((+)-(4-{2-[(5-Hydroxy-1,2,3,4-tetrahydro-naphthale...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from rat dopamine D3 receptor expressed in HEK293 cells | Bioorg Med Chem 17: 3923-33 (2009) Article DOI: 10.1016/j.bmc.2009.04.031 BindingDB Entry DOI: 10.7270/Q25H7G8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50409120 (CHEMBL2112044 | CHEMBL2112937) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes | J Med Chem 43: 769-71 (2000) BindingDB Entry DOI: 10.7270/Q2XD10W5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (RAT) | BDBM50113263 ((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences Curated by ChEMBL | Assay Description Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes | J Med Chem 45: 2160-72 (2002) BindingDB Entry DOI: 10.7270/Q2X067SG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM82279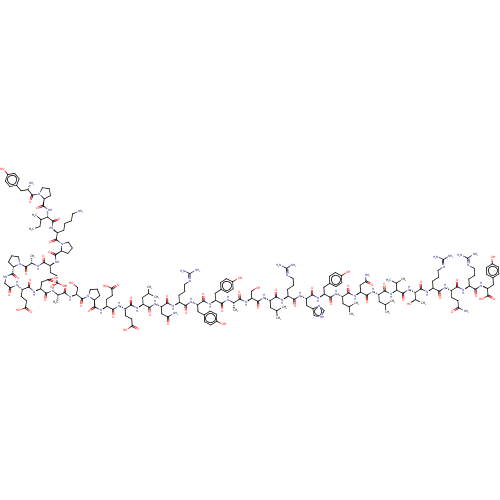 (CAS_118997-30-1 | PYY, human) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Curated by PDSP Ki Database | J Biol Chem 270: 22661-4 (1995) Article DOI: 10.1074/jbc.270.39.22661 BindingDB Entry DOI: 10.7270/Q2TT4PGD | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50374634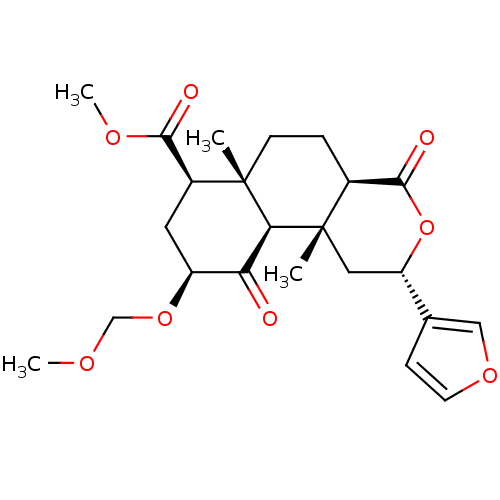 (CHEMBL258098) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McLean Hospital Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cells | Bioorg Med Chem 16: 1279-86 (2008) Article DOI: 10.1016/j.bmc.2007.10.067 BindingDB Entry DOI: 10.7270/Q2ZK5HJF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(3) dopamine receptor (Rattus norvegicus (Rat)) | BDBM50533801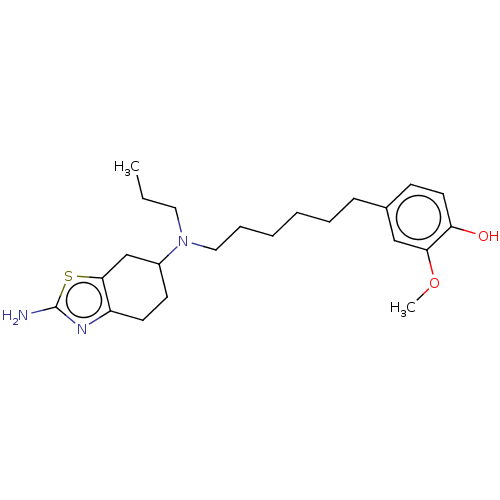 (CHEMBL4535160) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.675 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from rat D3 dopamine receptor expressed in HEK293 cell membranes after 1 hr | Bioorg Med Chem 24: 5088-5102 (2016) Article DOI: 10.1016/j.bmc.2016.08.021 BindingDB Entry DOI: 10.7270/Q2B85CNF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50303797 (7-((2-(4-(Naphthalen-2-yl)piperazin-1-yl)ethyl)(pr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.685 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human dopamine D3 receptor expressed in HEK293 cells | J Med Chem 53: 1023-37 (2010) Article DOI: 10.1021/jm901184n BindingDB Entry DOI: 10.7270/Q29023VM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Melanin-concentrating hormone receptor 1 (Homo sapiens (Human)) | BDBM50120635 (CHEMBL3618325) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Displacement of [125I][Phe13,Tyr19]-MCH from human MCHR1 expressed in HEK293 cells by scintillation counting analysis | Bioorg Med Chem Lett 25: 4412-8 (2015) Article DOI: 10.1016/j.bmcl.2015.09.018 BindingDB Entry DOI: 10.7270/Q2JD4ZMW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109927 ((+)-7-[(4-(4-phenylpiperazin-1-yl)butyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109927 ((+)-7-[(4-(4-phenylpiperazin-1-yl)butyl)(propyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109931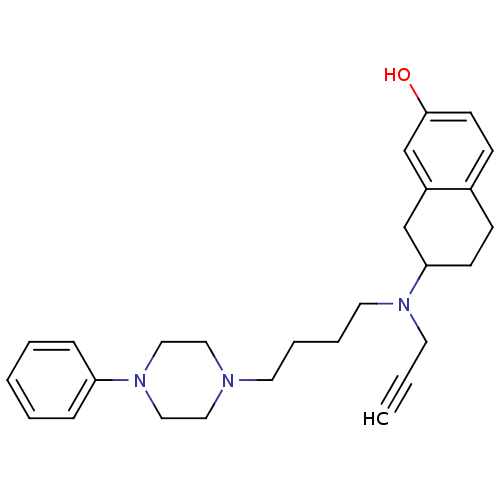 ((+)-7-{[4-(4-phenylpiperazin-1-yl)butyl]prop-2-yny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50109931 ((+)-7-{[4-(4-phenylpiperazin-1-yl)butyl]prop-2-yny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Wayne State University Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from human cloned dopamine D3 receptor expressed in HEK293 cells | J Med Chem 51: 101-17 (2008) Article DOI: 10.1021/jm070860r BindingDB Entry DOI: 10.7270/Q2VQ32FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 23000 total ) | Next | Last >> |