

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826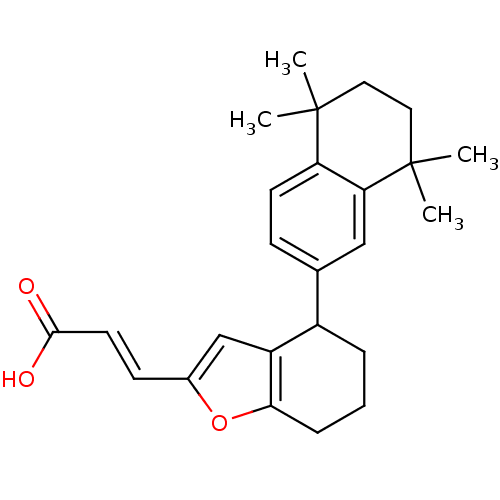 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143827 ((E)-3-[4-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143825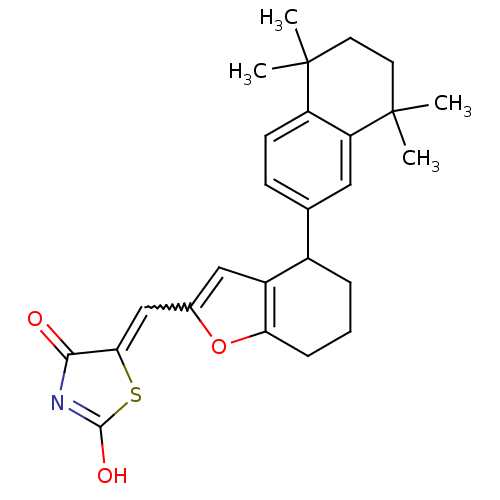 (5-[1-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-na...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143832 ((E)-3-[4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143833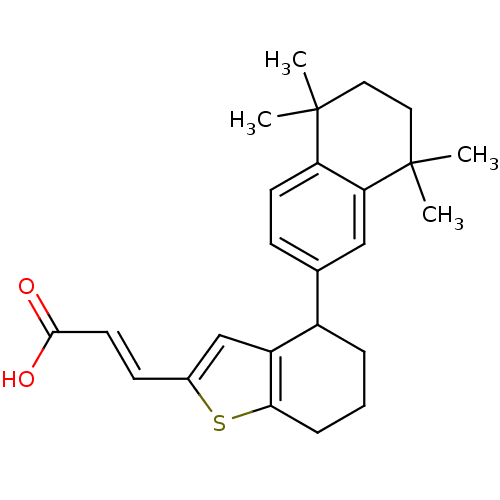 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50409928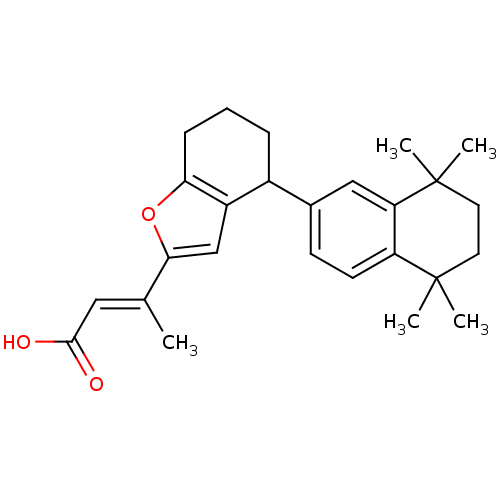 (CHEMBL2113737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50409929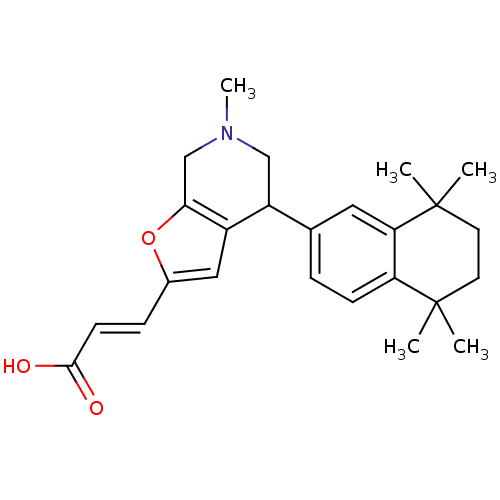 (CHEMBL2113736) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143824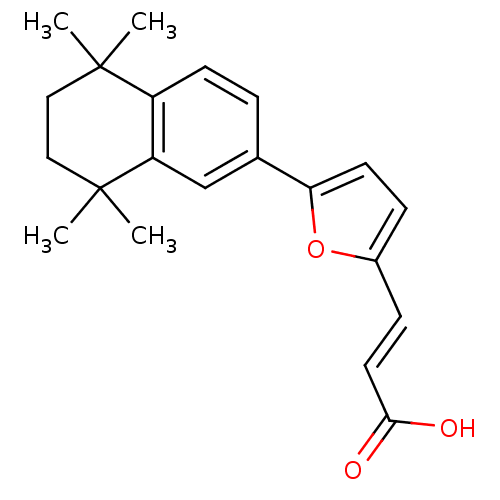 ((E)-3-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143821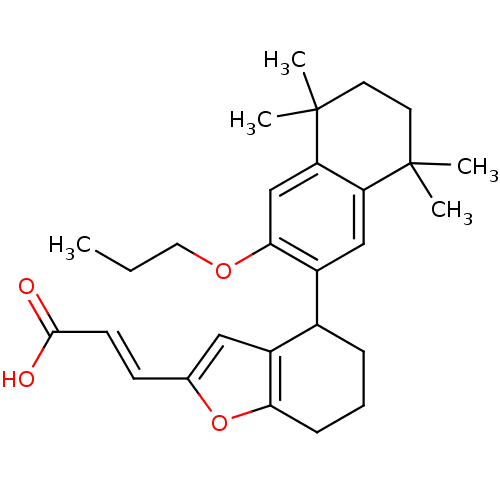 ((E)-3-[4-(5,5,8,8-Tetramethyl-3-propoxy-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143835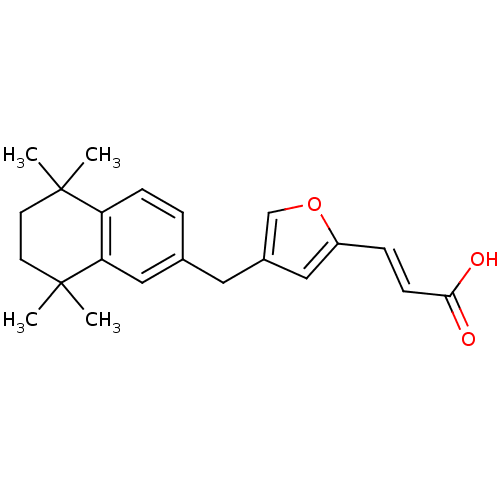 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143831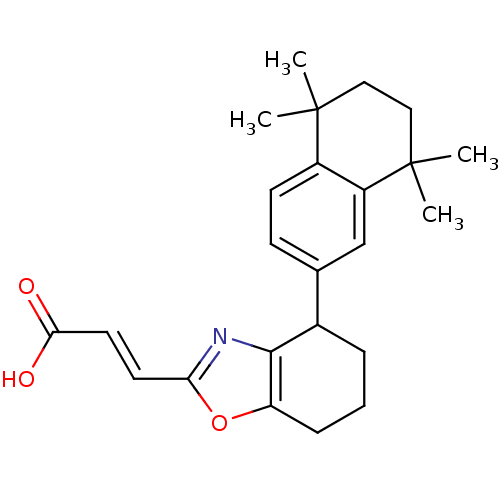 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143823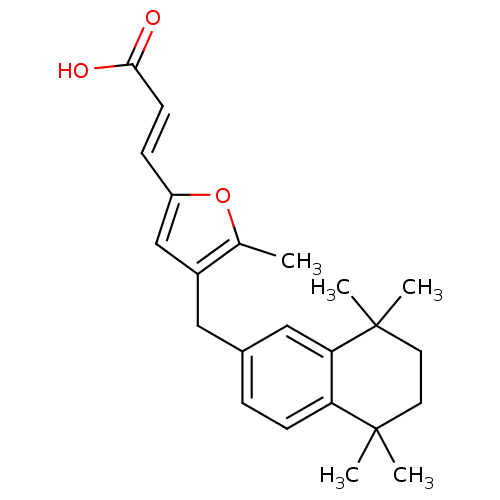 ((E)-3-[5-Methyl-4-(5,5,8,8-tetramethyl-5,6,7,8-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143828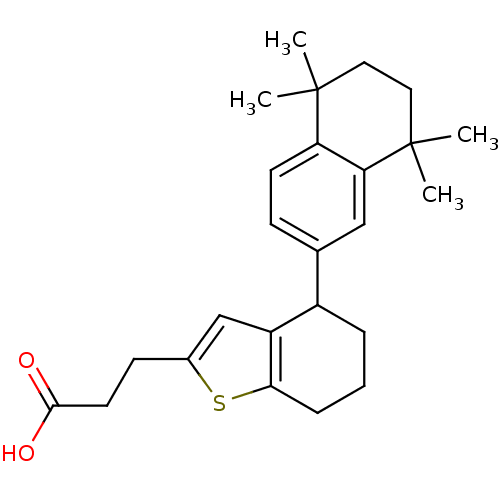 (3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143831 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143830 (4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143825 (5-[1-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-na...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143823 ((E)-3-[5-Methyl-4-(5,5,8,8-tetramethyl-5,6,7,8-tet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143827 ((E)-3-[4-(3-Methoxy-5,5,8,8-tetramethyl-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143824 ((E)-3-[5-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143833 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143828 (3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-napht...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50409928 (CHEMBL2113737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50409929 (CHEMBL2113736) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143835 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143826 ((E)-3-[4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-n...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143832 ((E)-3-[4-(3,5,5,8,8-Pentamethyl-5,6,7,8-tetrahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor alpha (Homo sapiens (Human)) | BDBM50143821 ((E)-3-[4-(5,5,8,8-Tetramethyl-3-propoxy-5,6,7,8-te...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoic acid receptor alpha (RARalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143834 (5-[1-[5-(3-Bromo-phenyl)-furan-2-yl]-meth-(Z)-ylid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143830 (4-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydro-naphthal...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Retinoic acid receptor RXR-alpha (Homo sapiens (Human)) | BDBM50143836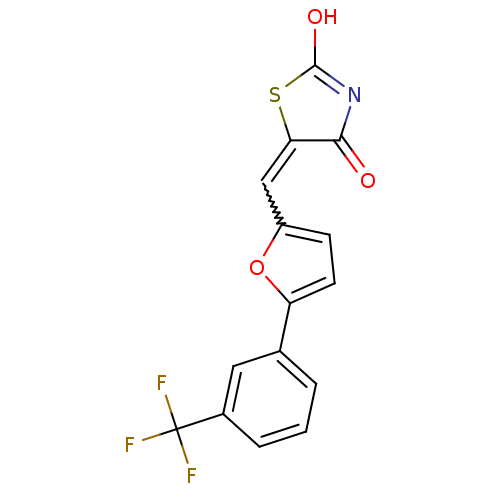 (5-[1-[5-(3-Trifluoromethyl-phenyl)-furan-2-yl]-met...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Research and Development Curated by ChEMBL | Assay Description Binding affinity for retinoid X receptor alpha (RXRalpha), using 9-cis-[3H]-retinoic acid | J Med Chem 47: 2010-29 (2004) Article DOI: 10.1021/jm030565g BindingDB Entry DOI: 10.7270/Q2KH0MRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50256329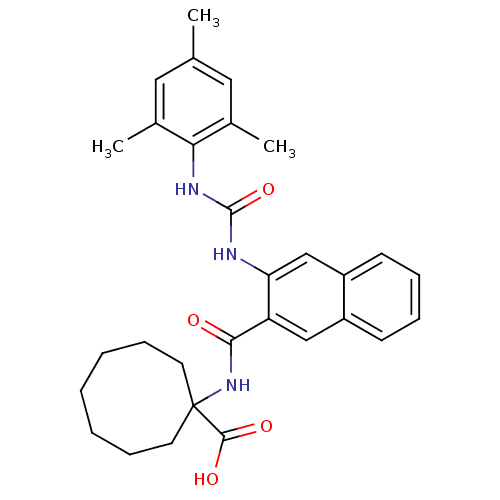 (1-(3-(3-mesitylureido)-2-naphthamido)cyclooctaneca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50255975 ((S)-2-cyclohexyl-2-(3-(3-(2,6-dichloro-4-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50255977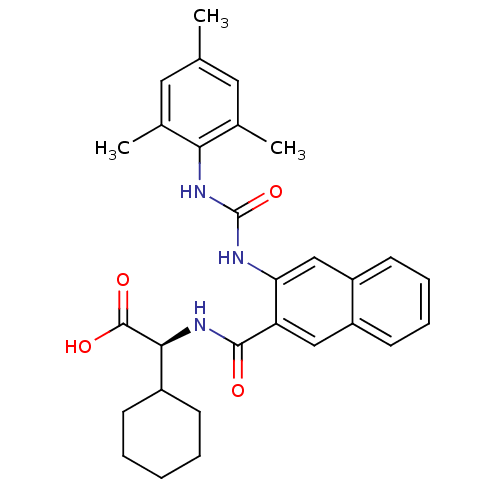 ((S)-2-cyclohexyl-2-(3-(3-mesitylureido)-2-naphtham...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50256169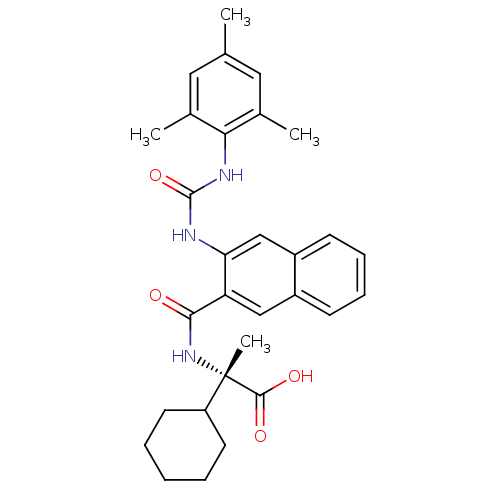 ((S)-2-cyclohexyl-2-(3-(3-mesitylureido)-2-naphtham...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50256071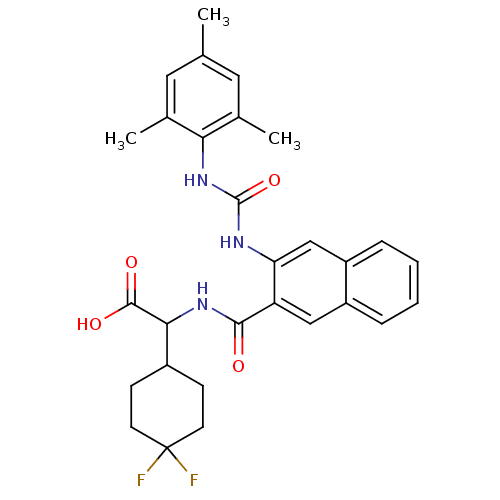 (2-(4,4-difluorocyclohexyl)-2-(3-(3-mesitylureido)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50256330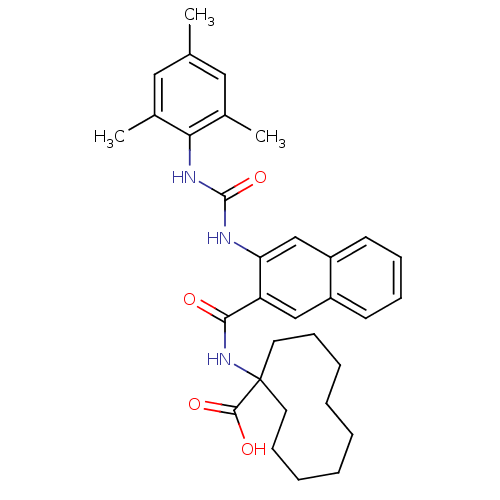 (1-(3-(3-mesitylureido)-2-naphthamido)cyclodecaneca...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50256012 ((S)-2-cyclopentyl-2-(3-(3-mesitylureido)-2-naphtha...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50243599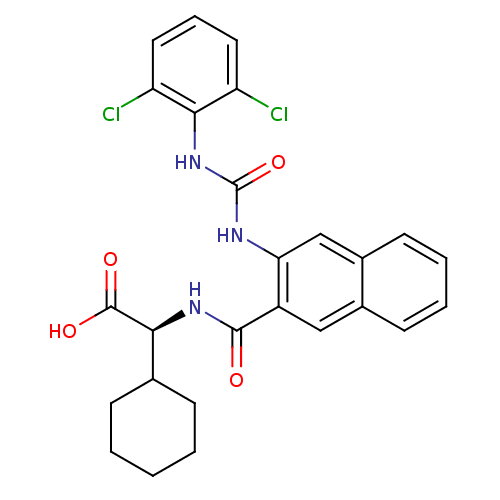 ((S)-2-cyclohexyl-2-(2-(3-(2,6-dichlorophenyl)ureid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50256393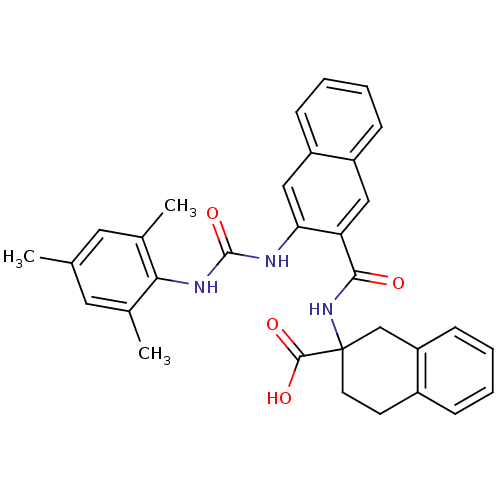 (2-(3-(3-mesitylureido)-2-naphthamido)-1,2,3,4-tetr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50256328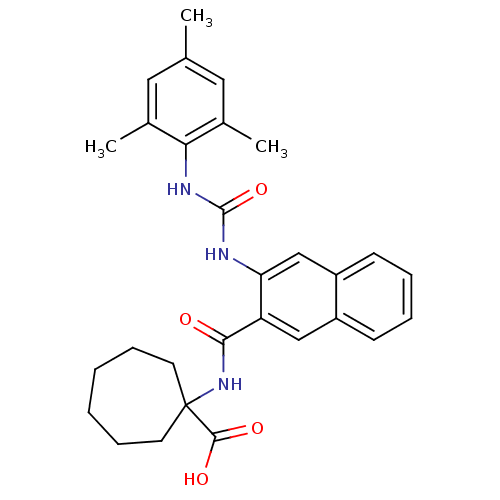 (1-(3-(3-mesitylureido)-2-naphthamido)cycloheptanec...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50256072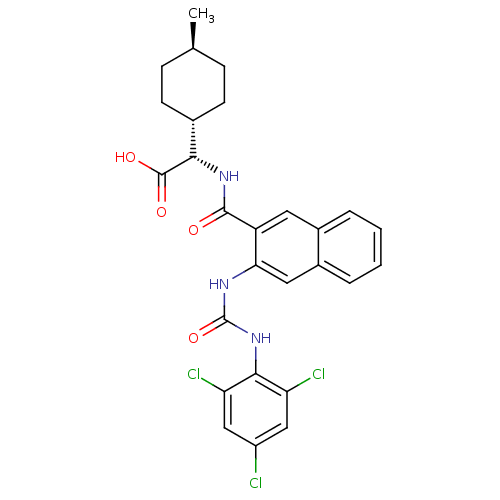 ((S)-2-((1r,4S)-4-methylcyclohexyl)-2-(3-(3-(2,4,6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50255976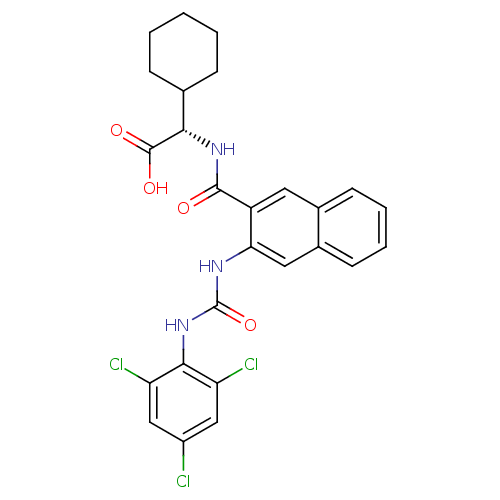 ((S)-2-cyclohexyl-2-(3-(3-(2,4,6-trichlorophenyl)ur...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM27725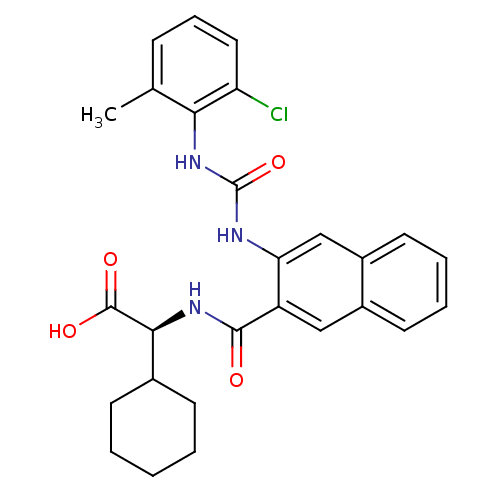 ((2S)-2-[(3-{[(2-chloro-6-methylphenyl)carbamoyl]am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50256392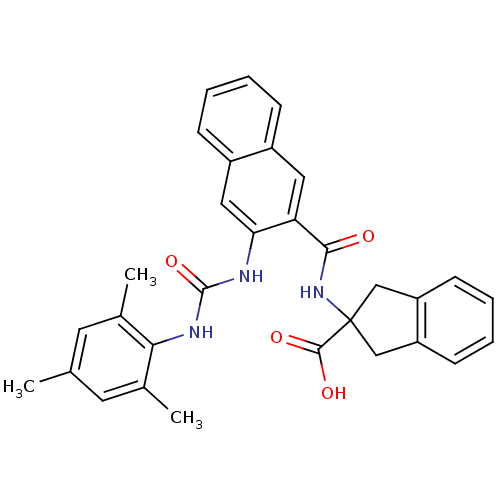 (2-(3-(3-mesitylureido)-2-naphthamido)-2,3-dihydro-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50256116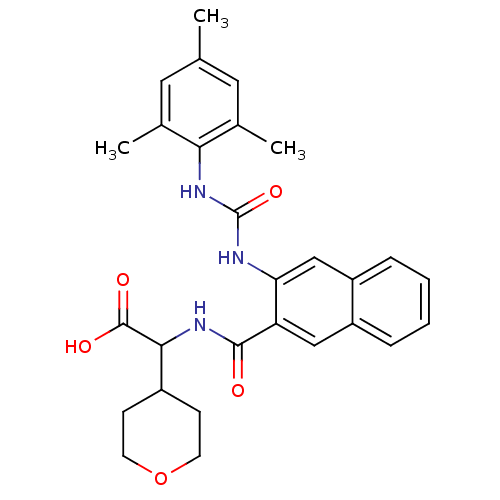 (2-(3-(3-mesitylureido)-2-naphthamido)-2-(tetrahydr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50256168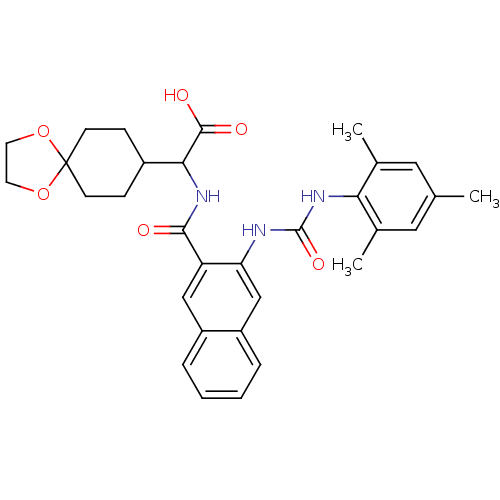 (2-(3-(3-mesitylureido)-2-naphthamido)-2-(1,4-dioxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen phosphorylase, liver form (Homo sapiens (Human)) | BDBM50243601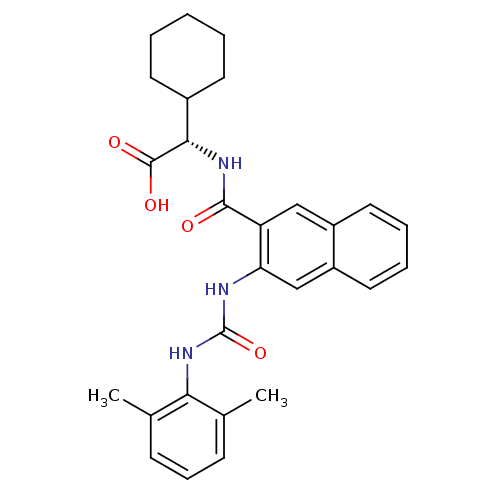 ((S)-2-cyclohexyl-2-(2-(3-(2,6-dimethylphenyl)ureid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human liver glycogen phosphorylase A by fluorescence intensity endpoint assay in presence of glucose | Bioorg Med Chem Lett 19: 976-80 (2009) Article DOI: 10.1016/j.bmcl.2008.11.085 BindingDB Entry DOI: 10.7270/Q26T0MGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 193 total ) | Next | Last >> |