 Found 305 hits with Last Name = 'felder' and Initial = 's'
Found 305 hits with Last Name = 'felder' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50215054
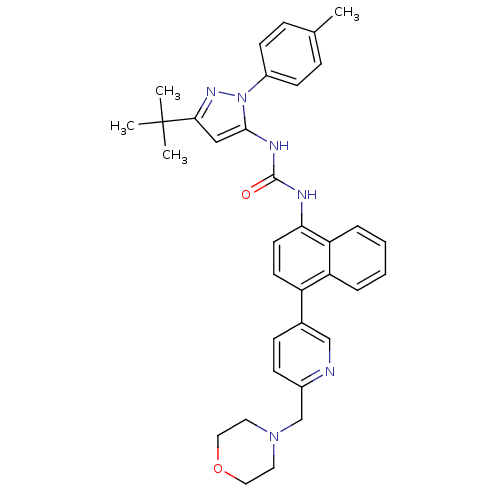
(1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(4-(6...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(-c2ccc(CN3CCOCC3)nc2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C35H38N6O2/c1-24-9-13-27(14-10-24)41-33(21-32(39-41)35(2,3)4)38-34(42)37-31-16-15-28(29-7-5-6-8-30(29)31)25-11-12-26(36-22-25)23-40-17-19-43-20-18-40/h5-16,21-22H,17-20,23H2,1-4H3,(H2,37,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Jnk2 |
Bioorg Med Chem Lett 17: 4242-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.042
BindingDB Entry DOI: 10.7270/Q27S7NHZ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of JNK2alpha2 (unknown origin) by by exchange curve binding kinetic analysis |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Competitive inhibition of androgen binding to androgen receptor (unknown origin) by invitrogen polar screen assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218689
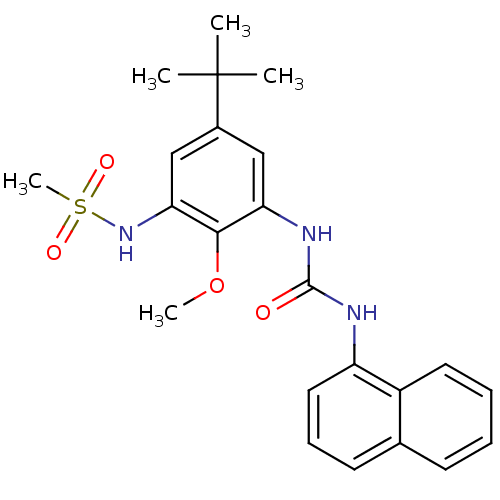
(CHEMBL245230 | N-(5-tert-butyl-2-methoxy-3-(3-naph...)Show SMILES COc1c(NC(=O)Nc2cccc3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C23H27N3O4S/c1-23(2,3)16-13-19(21(30-4)20(14-16)26-31(5,28)29)25-22(27)24-18-12-8-10-15-9-6-7-11-17(15)18/h6-14,26H,1-5H3,(H2,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 9
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against c-Jun N-terminal kinase 2-alpha 2 protein kinase |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50215054

(1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(4-(6...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(-c2ccc(CN3CCOCC3)nc2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C35H38N6O2/c1-24-9-13-27(14-10-24)41-33(21-32(39-41)35(2,3)4)38-34(42)37-31-16-15-28(29-7-5-6-8-30(29)31)25-11-12-26(36-22-25)23-40-17-19-43-20-18-40/h5-16,21-22H,17-20,23H2,1-4H3,(H2,37,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of cRaf |
Bioorg Med Chem Lett 17: 4242-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.042
BindingDB Entry DOI: 10.7270/Q27S7NHZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Abl (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50114395
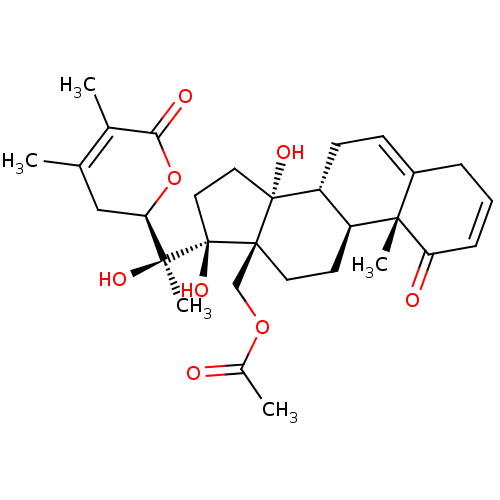
(CHEMBL3605564)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)CC[C@@]2(O)[C@]3([H])CC=C4CC=CC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:26,t:4,23| Show InChI InChI=1S/C30H40O8/c1-17-15-24(38-25(33)18(17)2)27(5,34)30(36)14-13-29(35)22-10-9-20-7-6-8-23(32)26(20,4)21(22)11-12-28(29,30)16-37-19(3)31/h6,8-9,21-22,24,34-36H,7,10-16H2,1-5H3/t21-,22+,24+,26-,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 580 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced KLK2 gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50114397
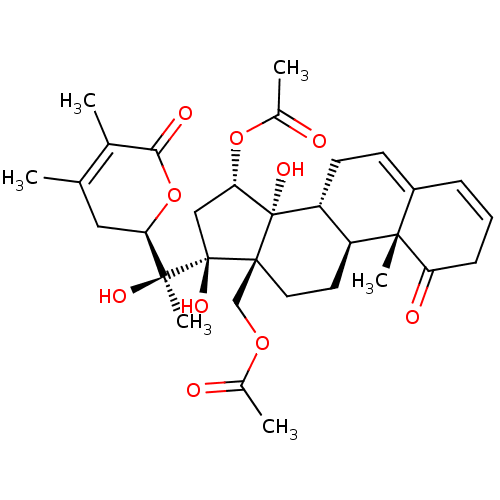
(CHEMBL3605563)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)C[C@H](OC(C)=O)[C@@]2(O)[C@]3([H])CC=C4C=CCC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:29,t:4,27| Show InChI InChI=1S/C32H42O10/c1-17-14-25(42-27(36)18(17)2)29(6,37)31(38)15-26(41-20(4)34)32(39)23-11-10-21-8-7-9-24(35)28(21,5)22(23)12-13-30(31,32)16-40-19(3)33/h7-8,10,22-23,25-26,37-39H,9,11-16H2,1-6H3/t22-,23+,25+,26-,28-,29-,30-,31+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced KLK2 gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50114395

(CHEMBL3605564)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)CC[C@@]2(O)[C@]3([H])CC=C4CC=CC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:26,t:4,23| Show InChI InChI=1S/C30H40O8/c1-17-15-24(38-25(33)18(17)2)27(5,34)30(36)14-13-29(35)22-10-9-20-7-6-8-23(32)26(20,4)21(22)11-12-28(29,30)16-37-19(3)31/h6,8-9,21-22,24,34-36H,7,10-16H2,1-5H3/t21-,22+,24+,26-,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced KLK3 gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50114395

(CHEMBL3605564)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)CC[C@@]2(O)[C@]3([H])CC=C4CC=CC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:26,t:4,23| Show InChI InChI=1S/C30H40O8/c1-17-15-24(38-25(33)18(17)2)27(5,34)30(36)14-13-29(35)22-10-9-20-7-6-8-23(32)26(20,4)21(22)11-12-28(29,30)16-37-19(3)31/h6,8-9,21-22,24,34-36H,7,10-16H2,1-5H3/t21-,22+,24+,26-,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced SPDEF gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of craf (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50215054

(1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(4-(6...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(-c2ccc(CN3CCOCC3)nc2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C35H38N6O2/c1-24-9-13-27(14-10-24)41-33(21-32(39-41)35(2,3)4)38-34(42)37-31-16-15-28(29-7-5-6-8-30(29)31)25-11-12-26(36-22-25)23-40-17-19-43-20-18-40/h5-16,21-22H,17-20,23H2,1-4H3,(H2,37,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Lyn |
Bioorg Med Chem Lett 17: 4242-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.042
BindingDB Entry DOI: 10.7270/Q27S7NHZ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50215054

(1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(4-(6...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(-c2ccc(CN3CCOCC3)nc2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C35H38N6O2/c1-24-9-13-27(14-10-24)41-33(21-32(39-41)35(2,3)4)38-34(42)37-31-16-15-28(29-7-5-6-8-30(29)31)25-11-12-26(36-22-25)23-40-17-19-43-20-18-40/h5-16,21-22H,17-20,23H2,1-4H3,(H2,37,38,42) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Abl |
Bioorg Med Chem Lett 17: 4242-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.042
BindingDB Entry DOI: 10.7270/Q27S7NHZ |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50215054

(1-(3-tert-butyl-1-p-tolyl-1H-pyrazol-5-yl)-3-(4-(6...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(-c2ccc(CN3CCOCC3)nc2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C35H38N6O2/c1-24-9-13-27(14-10-24)41-33(21-32(39-41)35(2,3)4)38-34(42)37-31-16-15-28(29-7-5-6-8-30(29)31)25-11-12-26(36-22-25)23-40-17-19-43-20-18-40/h5-16,21-22H,17-20,23H2,1-4H3,(H2,37,38,42) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Src |
Bioorg Med Chem Lett 17: 4242-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.042
BindingDB Entry DOI: 10.7270/Q27S7NHZ |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50114397

(CHEMBL3605563)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)C[C@H](OC(C)=O)[C@@]2(O)[C@]3([H])CC=C4C=CCC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:29,t:4,27| Show InChI InChI=1S/C32H42O10/c1-17-14-25(42-27(36)18(17)2)29(6,37)31(38)15-26(41-20(4)34)32(39)23-11-10-21-8-7-9-24(35)28(21,5)22(23)12-13-30(31,32)16-40-19(3)33/h7-8,10,22-23,25-26,37-39H,9,11-16H2,1-6H3/t22-,23+,25+,26-,28-,29-,30-,31+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced SPDEF gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against RAF proto-oncogene serine/threonine-protein kinase (c-Raf-1) |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50114397

(CHEMBL3605563)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)C[C@H](OC(C)=O)[C@@]2(O)[C@]3([H])CC=C4C=CCC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:29,t:4,27| Show InChI InChI=1S/C32H42O10/c1-17-14-25(42-27(36)18(17)2)29(6,37)31(38)15-26(41-20(4)34)32(39)23-11-10-21-8-7-9-24(35)28(21,5)22(23)12-13-30(31,32)16-40-19(3)33/h7-8,10,22-23,25-26,37-39H,9,11-16H2,1-6H3/t22-,23+,25+,26-,28-,29-,30-,31+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced KLK3 gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50112125
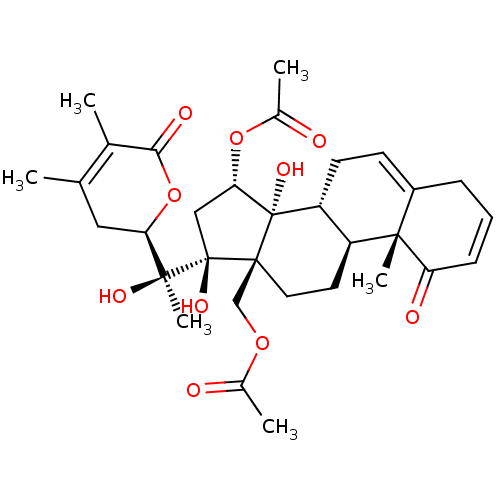
(CHEMBL3605562)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)C[C@H](OC(C)=O)[C@@]2(O)[C@]3([H])CC=C4CC=CC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:30,t:4,27| Show InChI InChI=1S/C32H42O10/c1-17-14-25(42-27(36)18(17)2)29(6,37)31(38)15-26(41-20(4)34)32(39)23-11-10-21-8-7-9-24(35)28(21,5)22(23)12-13-30(31,32)16-40-19(3)33/h7,9-10,22-23,25-26,37-39H,8,11-16H2,1-6H3/t22-,23+,25+,26-,28-,29-,30-,31+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced KLK2 gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lyn
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of Lyn (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50114392
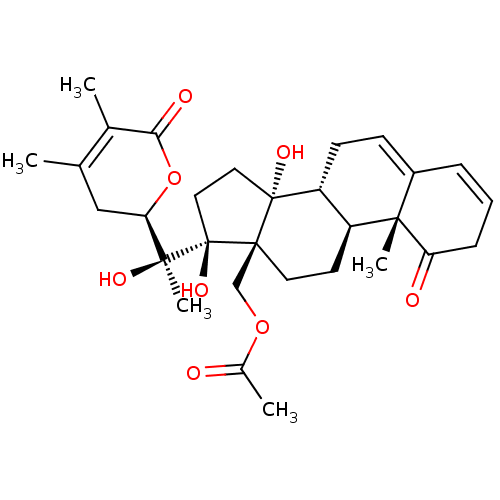
(CHEMBL3605560)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)CC[C@@]2(O)[C@]3([H])CC=C4C=CCC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:25,t:4,23| Show InChI InChI=1S/C30H40O8/c1-17-15-24(38-25(33)18(17)2)27(5,34)30(36)14-13-29(35)22-10-9-20-7-6-8-23(32)26(20,4)21(22)11-12-28(29,30)16-37-19(3)31/h6-7,9,21-22,24,34-36H,8,10-16H2,1-5H3/t21-,22+,24+,26-,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced KLK2 gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218677
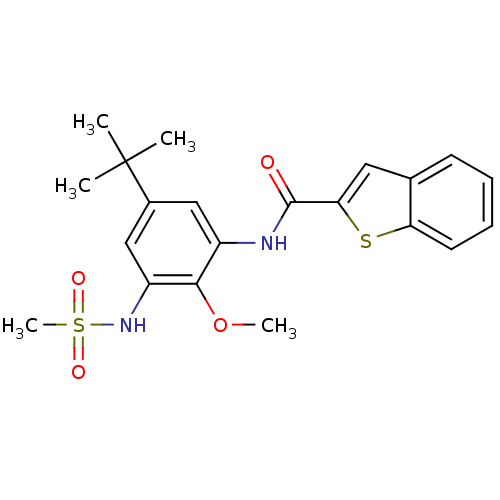
(CHEMBL242005 | N-(5-tert-butyl-2-methoxy-3-(methyl...)Show SMILES COc1c(NC(=O)c2cc3ccccc3s2)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C21H24N2O4S2/c1-21(2,3)14-11-15(19(27-4)16(12-14)23-29(5,25)26)22-20(24)18-10-13-8-6-7-9-17(13)28-18/h6-12,23H,1-5H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50112125

(CHEMBL3605562)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)C[C@H](OC(C)=O)[C@@]2(O)[C@]3([H])CC=C4CC=CC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:30,t:4,27| Show InChI InChI=1S/C32H42O10/c1-17-14-25(42-27(36)18(17)2)29(6,37)31(38)15-26(41-20(4)34)32(39)23-11-10-21-8-7-9-24(35)28(21,5)22(23)12-13-30(31,32)16-40-19(3)33/h7,9-10,22-23,25-26,37-39H,8,11-16H2,1-6H3/t22-,23+,25+,26-,28-,29-,30-,31+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced SPDEF gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 3
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of HEK (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50114392

(CHEMBL3605560)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)CC[C@@]2(O)[C@]3([H])CC=C4C=CCC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:25,t:4,23| Show InChI InChI=1S/C30H40O8/c1-17-15-24(38-25(33)18(17)2)27(5,34)30(36)14-13-29(35)22-10-9-20-7-6-8-23(32)26(20,4)21(22)11-12-28(29,30)16-37-19(3)31/h6-7,9,21-22,24,34-36H,8,10-16H2,1-5H3/t21-,22+,24+,26-,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced SPDEF gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50114392

(CHEMBL3605560)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)CC[C@@]2(O)[C@]3([H])CC=C4C=CCC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:25,t:4,23| Show InChI InChI=1S/C30H40O8/c1-17-15-24(38-25(33)18(17)2)27(5,34)30(36)14-13-29(35)22-10-9-20-7-6-8-23(32)26(20,4)21(22)11-12-28(29,30)16-37-19(3)31/h6-7,9,21-22,24,34-36H,8,10-16H2,1-5H3/t21-,22+,24+,26-,27-,28+,29+,30+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced KLK3 gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50112125

(CHEMBL3605562)Show SMILES [H][C@@]1(CC(C)=C(C)C(=O)O1)[C@](C)(O)[C@]1(O)C[C@H](OC(C)=O)[C@@]2(O)[C@]3([H])CC=C4CC=CC(=O)[C@]4(C)[C@@]3([H])CC[C@]12COC(C)=O |r,c:30,t:4,27| Show InChI InChI=1S/C32H42O10/c1-17-14-25(42-27(36)18(17)2)29(6,37)31(38)15-26(41-20(4)34)32(39)23-11-10-21-8-7-9-24(35)28(21,5)22(23)12-13-30(31,32)16-40-19(3)33/h7,9-10,22-23,25-26,37-39H,8,11-16H2,1-6H3/t22-,23+,25+,26-,28-,29-,30-,31+,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Inhibition of androgen receptor in human LNCAP cells assessed as inhibition of DHT-induced KLK3 gene expression by Array Plate assay |
J Med Chem 58: 6984-93 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00867
BindingDB Entry DOI: 10.7270/Q2GF0W97 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50277627
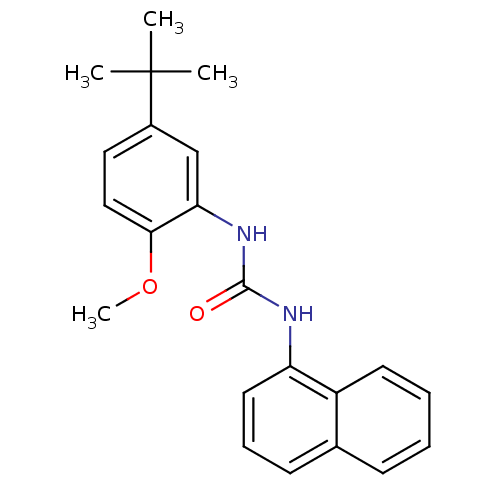
(1-(5-tert-butyl-2-methoxyphenyl)-3-(naphthalen-1-y...)Show InChI InChI=1S/C22H24N2O2/c1-22(2,3)16-12-13-20(26-4)19(14-16)24-21(25)23-18-11-7-9-15-8-5-6-10-17(15)18/h5-14H,1-4H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218695
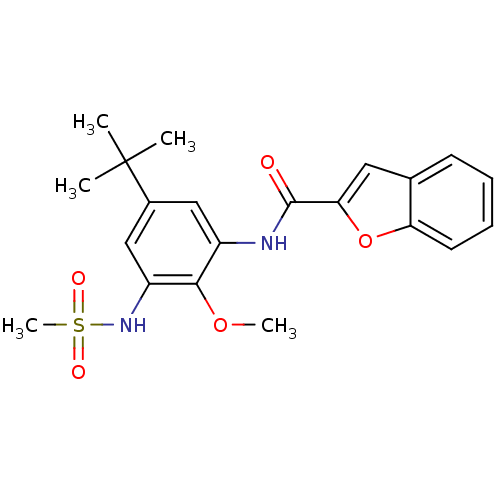
(CHEMBL390254 | N-(5-tert-butyl-2-methoxy-3-(methyl...)Show SMILES COc1c(NC(=O)c2cc3ccccc3o2)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C21H24N2O5S/c1-21(2,3)14-11-15(19(27-4)16(12-14)23-29(5,25)26)22-20(24)18-10-13-8-6-7-9-17(13)28-18/h6-12,23H,1-5H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of ECK (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of PKBalpha/Akt1 (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50277623

(CHEMBL451523 | N-(5-tert-butyl-2-methoxy-3-(3-(4-(...)Show SMILES COc1c(NC(=O)Nc2ccc(-c3ccc(CN4CCOCC4)nc3)c3ccccc23)cc(cc1NS(C)(=O)=O)C(C)(C)C Show InChI InChI=1S/C33H39N5O5S/c1-33(2,3)23-18-29(31(42-4)30(19-23)37-44(5,40)41)36-32(39)35-28-13-12-25(26-8-6-7-9-27(26)28)22-10-11-24(34-20-22)21-38-14-16-43-17-15-38/h6-13,18-20,37H,14-17,21H2,1-5H3,(H2,35,36,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Inhibition of bRaf (unknown origin) |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50277662
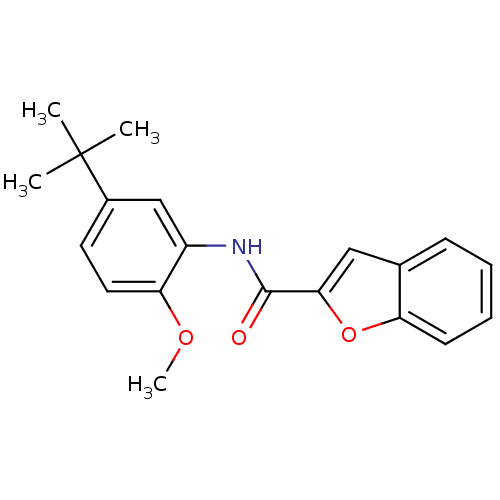
(CHEMBL484405 | N-(5-tert-butyl-2-methoxyphenyl)ben...)Show InChI InChI=1S/C20H21NO3/c1-20(2,3)14-9-10-17(23-4)15(12-14)21-19(22)18-11-13-7-5-6-8-16(13)24-18/h5-12H,1-4H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50277663

(CHEMBL519511 | N-(5-tert-butyl-2-methoxyphenyl)ben...)Show InChI InChI=1S/C20H21NO2S/c1-20(2,3)14-9-10-16(23-4)15(12-14)21-19(22)18-11-13-7-5-6-8-17(13)24-18/h5-12H,1-4H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceutical
Curated by ChEMBL
| Assay Description
Binding affinity to p38alpha (unknown origin) assessed as inhibition of ATF2 phosphorylation preincubated for 4 hrs |
Bioorg Med Chem Lett 19: 2386-91 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.104
BindingDB Entry DOI: 10.7270/Q22N525R |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against p56 Lck tyrosine kinase |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase SYK
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against Syk protein tyrosine kinase |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against I-kappa-B-kinase beta |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ZAP-70
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against Zeta-chain (TCR) associated protein kinase 70 kDa (ZAP70) |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Fyn
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against p59 Fyn tyrosine kinase |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 3
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against Extracellular signal-regulated kinase 1 (Erk-1) |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Receptor tyrosine-protein kinase erbB-2
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against HER2 kinase |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM13533

(1-[2-(4-methylphenyl)-5-tert-butyl-pyrazol-3-yl]-3...)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(OCCN2CCOCC2)c2ccccc12)C(C)(C)C Show InChI InChI=1S/C31H37N5O3/c1-22-9-11-23(12-10-22)36-29(21-28(34-36)31(2,3)4)33-30(37)32-26-13-14-27(25-8-6-5-7-24(25)26)39-20-17-35-15-18-38-19-16-35/h5-14,21H,15-20H2,1-4H3,(H2,32,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Selectivity against Epidermal growth factor receptor |
J Med Chem 45: 2994-3008 (2002)
BindingDB Entry DOI: 10.7270/Q21G0KMV |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM180490

(US8772323, 52)Show SMILES CCCOc1ccc(cc1)-c1nc2ccc(cc2o1)C1=NNC(=O)CC1CC |t:22| Show InChI InChI=1S/C22H23N3O3/c1-3-11-27-17-8-5-15(6-9-17)22-23-18-10-7-16(12-19(18)28-22)21-14(4-2)13-20(26)24-25-21/h5-10,12,14H,3-4,11,13H2,1-2H3,(H,24,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | n/a | 189 | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
MIN6 cells [Miyazaki J et al. Endocrinology. 1990 July; 127(1):126-32] were stably transfected with an expression vector for human GPR119 cDNA (Acc. ... |
US Patent US8772323 (2014)
BindingDB Entry DOI: 10.7270/Q2639NHH |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM180491

(US8772323, 53)Show SMILES CCC1CC(=O)NN=C1c1ccc2nc(oc2c1)-c1ccc(OCCN2CCCCCC2)cc1 |c:7| Show InChI InChI=1S/C27H32N4O3/c1-2-19-18-25(32)29-30-26(19)21-9-12-23-24(17-21)34-27(28-23)20-7-10-22(11-8-20)33-16-15-31-13-5-3-4-6-14-31/h7-12,17,19H,2-6,13-16,18H2,1H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | n/a | 205 | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
MIN6 cells [Miyazaki J et al. Endocrinology. 1990 July; 127(1):126-32] were stably transfected with an expression vector for human GPR119 cDNA (Acc. ... |
US Patent US8772323 (2014)
BindingDB Entry DOI: 10.7270/Q2639NHH |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM180492

(US8772323, 54)Show SMILES CCC1CC(=O)NN=C1c1ccc2nc(oc2c1)-c1ccc(OCC2CCCCC2)cc1 |c:7| Show InChI InChI=1S/C26H29N3O3/c1-2-18-15-24(30)28-29-25(18)20-10-13-22-23(14-20)32-26(27-22)19-8-11-21(12-9-19)31-16-17-6-4-3-5-7-17/h8-14,17-18H,2-7,15-16H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | n/a | 145 | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
MIN6 cells [Miyazaki J et al. Endocrinology. 1990 July; 127(1):126-32] were stably transfected with an expression vector for human GPR119 cDNA (Acc. ... |
US Patent US8772323 (2014)
BindingDB Entry DOI: 10.7270/Q2639NHH |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM180493

(US8772323, 55)Show SMILES CCC1CC(=O)NN=C1c1ccc2nc(oc2c1)-c1ccc(OCCN(C)C)cc1 |c:7| Show InChI InChI=1S/C23H26N4O3/c1-4-15-14-21(28)25-26-22(15)17-7-10-19-20(13-17)30-23(24-19)16-5-8-18(9-6-16)29-12-11-27(2)3/h5-10,13,15H,4,11-12,14H2,1-3H3,(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | n/a | 486 | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
MIN6 cells [Miyazaki J et al. Endocrinology. 1990 July; 127(1):126-32] were stably transfected with an expression vector for human GPR119 cDNA (Acc. ... |
US Patent US8772323 (2014)
BindingDB Entry DOI: 10.7270/Q2639NHH |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM180494
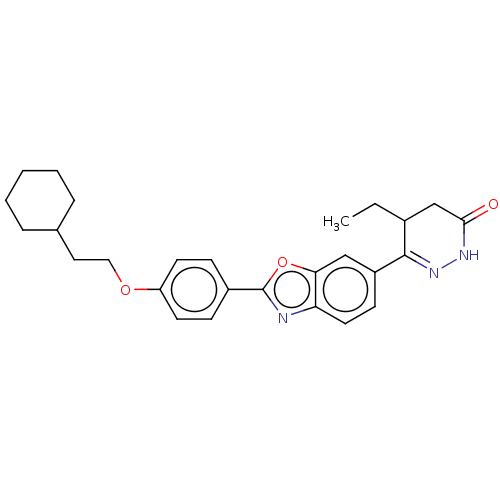
(US8772323, 56)Show SMILES CCC1CC(=O)NN=C1c1ccc2nc(oc2c1)-c1ccc(OCCC2CCCCC2)cc1 |c:7| Show InChI InChI=1S/C27H31N3O3/c1-2-19-17-25(31)29-30-26(19)21-10-13-23-24(16-21)33-27(28-23)20-8-11-22(12-9-20)32-15-14-18-6-4-3-5-7-18/h8-13,16,18-19H,2-7,14-15,17H2,1H3,(H,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | n/a | 170 | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
MIN6 cells [Miyazaki J et al. Endocrinology. 1990 July; 127(1):126-32] were stably transfected with an expression vector for human GPR119 cDNA (Acc. ... |
US Patent US8772323 (2014)
BindingDB Entry DOI: 10.7270/Q2639NHH |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM180495
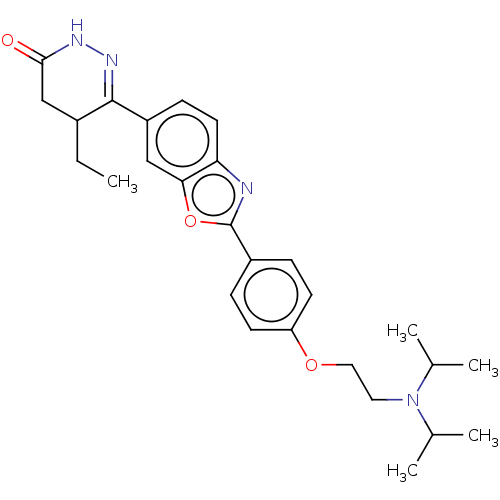
(US8772323, 57)Show SMILES CCC1CC(=O)NN=C1c1ccc2nc(oc2c1)-c1ccc(OCCN(C(C)C)C(C)C)cc1 |c:7| Show InChI InChI=1S/C27H34N4O3/c1-6-19-16-25(32)29-30-26(19)21-9-12-23-24(15-21)34-27(28-23)20-7-10-22(11-8-20)33-14-13-31(17(2)3)18(4)5/h7-12,15,17-19H,6,13-14,16H2,1-5H3,(H,29,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
MIN6 cells [Miyazaki J et al. Endocrinology. 1990 July; 127(1):126-32] were stably transfected with an expression vector for human GPR119 cDNA (Acc. ... |
US Patent US8772323 (2014)
BindingDB Entry DOI: 10.7270/Q2639NHH |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM180496

(US8772323, 58)Show SMILES CCC1CC(=O)NN=C1c1ccc2nc(oc2c1)-c1ccc(OCCN2C(C)(C)CCCC2(C)C)cc1 |c:7| Show InChI InChI=1S/C30H38N4O3/c1-6-20-19-26(35)32-33-27(20)22-10-13-24-25(18-22)37-28(31-24)21-8-11-23(12-9-21)36-17-16-34-29(2,3)14-7-15-30(34,4)5/h8-13,18,20H,6-7,14-17,19H2,1-5H3,(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | n/a | n/a | 76 | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
MIN6 cells [Miyazaki J et al. Endocrinology. 1990 July; 127(1):126-32] were stably transfected with an expression vector for human GPR119 cDNA (Acc. ... |
US Patent US8772323 (2014)
BindingDB Entry DOI: 10.7270/Q2639NHH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data