 Found 267 hits with Last Name = 'fraser' and Initial = 'a'
Found 267 hits with Last Name = 'fraser' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetolactate synthase catalytic subunit, mitochondrial
(Saccharomyces cerevisiae) | BDBM50424585
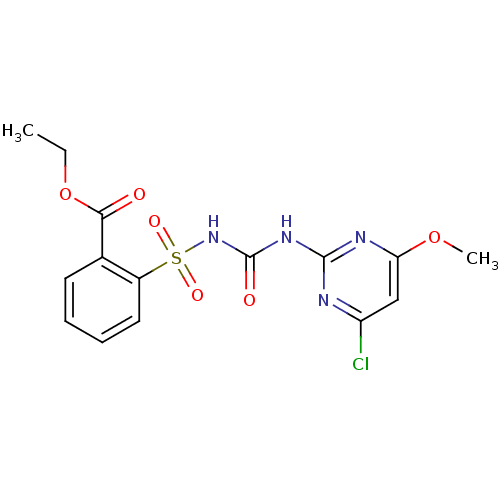
(CHEMBL1231791 | Chlorimuron Ethyl)Show SMILES CCOC(=O)c1ccccc1S(=O)(=O)NC(=O)Nc1nc(Cl)cc(OC)n1 Show InChI InChI=1S/C15H15ClN4O6S/c1-3-26-13(21)9-6-4-5-7-10(9)27(23,24)20-15(22)19-14-17-11(16)8-12(18-14)25-2/h4-8H,3H2,1-2H3,(H2,17,18,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae acetohydroxyacid synthase by colorimetric assay |
J Med Chem 56: 210-9 (2013)
Article DOI: 10.1021/jm301501k
BindingDB Entry DOI: 10.7270/Q22Z16TH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetolactate synthase catalytic subunit, mitochondrial
(Saccharomyces cerevisiae) | BDBM50424591
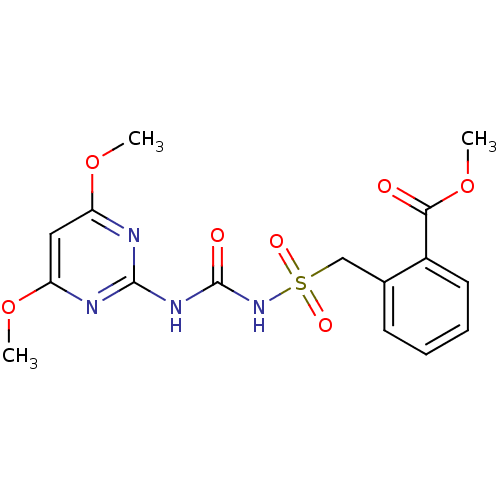
(Bensulfuron Methyl | CHEMBL2313154)Show SMILES COC(=O)c1ccccc1CS(=O)(=O)NC(=O)Nc1nc(OC)cc(OC)n1 Show InChI InChI=1S/C16H18N4O7S/c1-25-12-8-13(26-2)18-15(17-12)19-16(22)20-28(23,24)9-10-6-4-5-7-11(10)14(21)27-3/h4-8H,9H2,1-3H3,(H2,17,18,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae acetohydroxyacid synthase by colorimetric assay |
J Med Chem 56: 210-9 (2013)
Article DOI: 10.1021/jm301501k
BindingDB Entry DOI: 10.7270/Q22Z16TH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetolactate synthase, chloroplastic
(Arabidopsis thaliana) | BDBM50424585

(CHEMBL1231791 | Chlorimuron Ethyl)Show SMILES CCOC(=O)c1ccccc1S(=O)(=O)NC(=O)Nc1nc(Cl)cc(OC)n1 Show InChI InChI=1S/C15H15ClN4O6S/c1-3-26-13(21)9-6-4-5-7-10(9)27(23,24)20-15(22)19-14-17-11(16)8-12(18-14)25-2/h4-8H,3H2,1-2H3,(H2,17,18,19,20,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Arabidopsis thaliana acetohydroxyacid synthase |
J Med Chem 56: 210-9 (2013)
Article DOI: 10.1021/jm301501k
BindingDB Entry DOI: 10.7270/Q22Z16TH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetolactate synthase catalytic subunit, mitochondrial
(Saccharomyces cerevisiae) | BDBM50424590
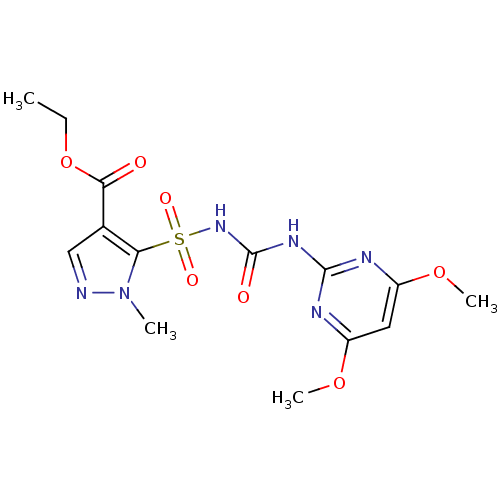
(CHEMBL2313155 | Pyrazosulfuron Ethyl)Show SMILES CCOC(=O)c1cnn(C)c1S(=O)(=O)NC(=O)Nc1nc(OC)cc(OC)n1 Show InChI InChI=1S/C14H18N6O7S/c1-5-27-12(21)8-7-15-20(2)11(8)28(23,24)19-14(22)18-13-16-9(25-3)6-10(17-13)26-4/h6-7H,5H2,1-4H3,(H2,16,17,18,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae acetohydroxyacid synthase by colorimetric assay |
J Med Chem 56: 210-9 (2013)
Article DOI: 10.1021/jm301501k
BindingDB Entry DOI: 10.7270/Q22Z16TH |
More data for this
Ligand-Target Pair | |
Acetolactate synthase catalytic subunit, mitochondrial
(Saccharomyces cerevisiae) | BDBM50424588
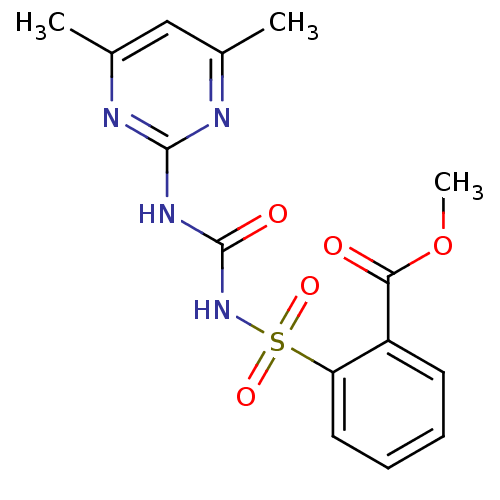
(SULFOMETURON METHYL)Show SMILES COC(=O)c1ccccc1S(=O)(=O)NC(=O)Nc1nc(C)cc(C)n1 Show InChI InChI=1S/C15H16N4O5S/c1-9-8-10(2)17-14(16-9)18-15(21)19-25(22,23)12-7-5-4-6-11(12)13(20)24-3/h4-8H,1-3H3,(H2,16,17,18,19,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae acetohydroxyacid synthase by colorimetric assay |
J Med Chem 56: 210-9 (2013)
Article DOI: 10.1021/jm301501k
BindingDB Entry DOI: 10.7270/Q22Z16TH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetolactate synthase catalytic subunit, mitochondrial
(Saccharomyces cerevisiae) | BDBM50424592
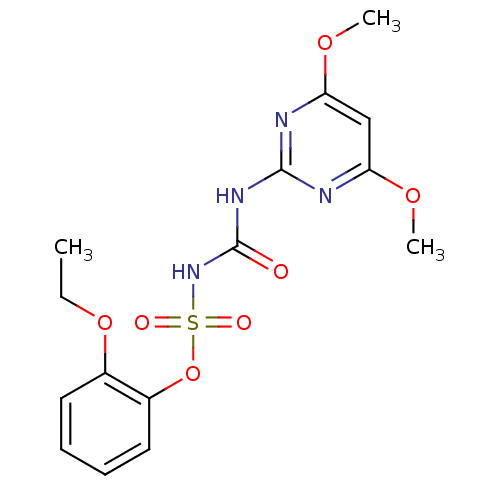
(CHEMBL2313153 | Ethoxysulfuron)Show SMILES CCOc1ccccc1OS(=O)(=O)NC(=O)Nc1nc(OC)cc(OC)n1 Show InChI InChI=1S/C15H18N4O7S/c1-4-25-10-7-5-6-8-11(10)26-27(21,22)19-15(20)18-14-16-12(23-2)9-13(17-14)24-3/h5-9H,4H2,1-3H3,(H2,16,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae acetohydroxyacid synthase by colorimetric assay |
J Med Chem 56: 210-9 (2013)
Article DOI: 10.1021/jm301501k
BindingDB Entry DOI: 10.7270/Q22Z16TH |
More data for this
Ligand-Target Pair | |
Acetolactate synthase catalytic subunit, mitochondrial
(Saccharomyces cerevisiae) | BDBM50424589
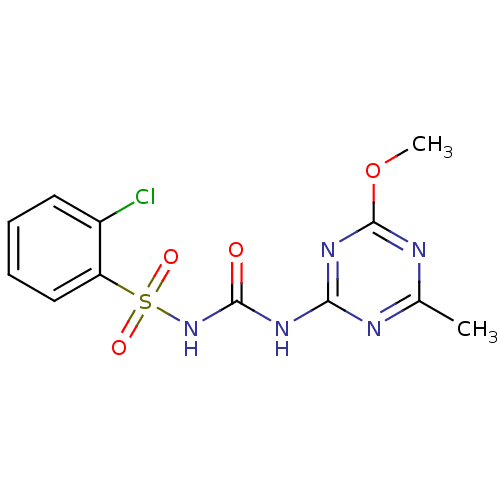
(CHEMBL1229721 | DNDI1246776)Show InChI InChI=1S/C12H12ClN5O4S/c1-7-14-10(17-12(15-7)22-2)16-11(19)18-23(20,21)9-6-4-3-5-8(9)13/h3-6H,1-2H3,(H2,14,15,16,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae acetohydroxyacid synthase by colorimetric assay |
J Med Chem 56: 210-9 (2013)
Article DOI: 10.1021/jm301501k
BindingDB Entry DOI: 10.7270/Q22Z16TH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetolactate synthase catalytic subunit, mitochondrial
(Saccharomyces cerevisiae) | BDBM50424587
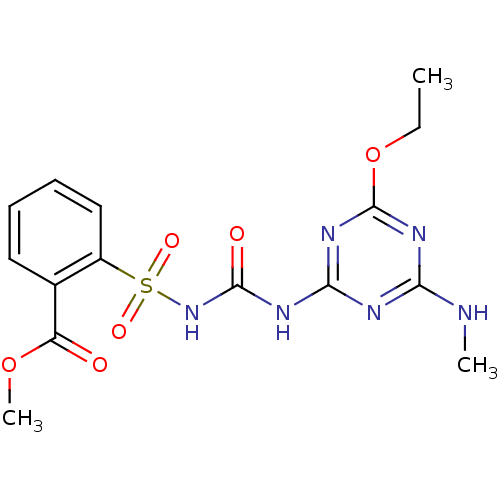
(CHEMBL1885280 | Ethametsulfuron Methyl)Show SMILES CCOc1nc(NC)nc(NC(=O)NS(=O)(=O)c2ccccc2C(=O)OC)n1 Show InChI InChI=1S/C15H18N6O6S/c1-4-27-15-19-12(16-2)17-13(20-15)18-14(23)21-28(24,25)10-8-6-5-7-9(10)11(22)26-3/h5-8H,4H2,1-3H3,(H3,16,17,18,19,20,21,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 346 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae acetohydroxyacid synthase by colorimetric assay |
J Med Chem 56: 210-9 (2013)
Article DOI: 10.1021/jm301501k
BindingDB Entry DOI: 10.7270/Q22Z16TH |
More data for this
Ligand-Target Pair | |
Acetolactate synthase catalytic subunit, mitochondrial
(Saccharomyces cerevisiae) | BDBM50424586
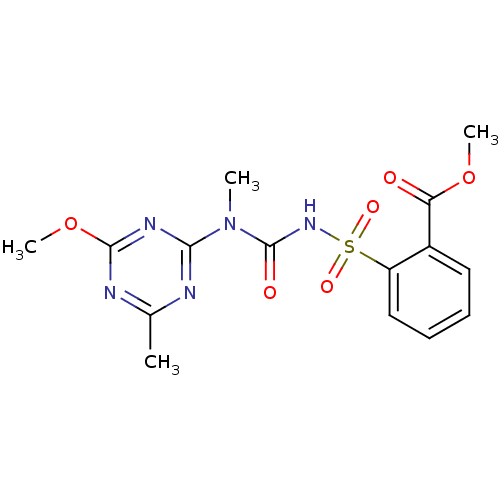
(CHEMBL1229780 | Tribenuron Methyl)Show SMILES COC(=O)c1ccccc1S(=O)(=O)NC(=O)N(C)c1nc(C)nc(OC)n1 Show InChI InChI=1S/C15H17N5O6S/c1-9-16-13(18-14(17-9)26-4)20(2)15(22)19-27(23,24)11-8-6-5-7-10(11)12(21)25-3/h5-8H,1-4H3,(H,19,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Queensland
Curated by ChEMBL
| Assay Description
Inhibition of Saccharomyces cerevisiae acetohydroxyacid synthase by colorimetric assay |
J Med Chem 56: 210-9 (2013)
Article DOI: 10.1021/jm301501k
BindingDB Entry DOI: 10.7270/Q22Z16TH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429098
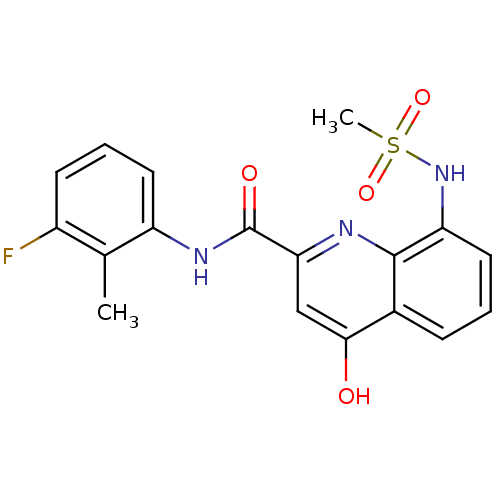
(CHEMBL2335784)Show SMILES Cc1c(F)cccc1NC(=O)c1cc(O)c2cccc(NS(C)(=O)=O)c2n1 Show InChI InChI=1S/C18H16FN3O4S/c1-10-12(19)6-4-7-13(10)21-18(24)15-9-16(23)11-5-3-8-14(17(11)20-15)22-27(2,25)26/h3-9,22H,1-2H3,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal hexahistidine-tagged Lp-PLA2 expressed in Escherichia coli using 2- thio-PAF as substrate incubated for 20... |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429094

(CHEMBL2335783)Show SMILES Cc1cc(F)ccc1NC(=O)c1cc(O)c2cccc(NS(C)(=O)=O)c2n1 Show InChI InChI=1S/C18H16FN3O4S/c1-10-8-11(19)6-7-13(10)21-18(24)15-9-16(23)12-4-3-5-14(17(12)20-15)22-27(2,25)26/h3-9,22H,1-2H3,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal hexahistidine-tagged Lp-PLA2 expressed in Escherichia coli using 2- thio-PAF as substrate incubated for 20... |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429101
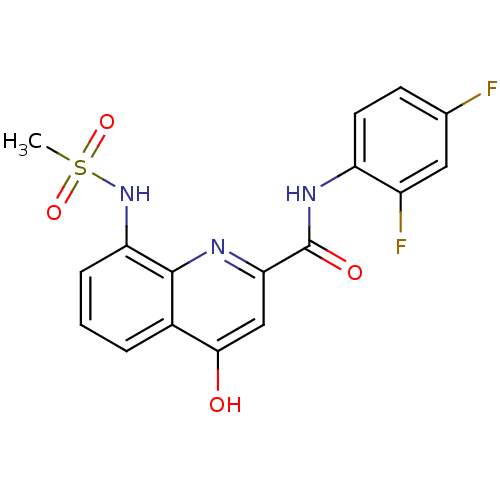
(CHEMBL2335780)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C17H13F2N3O4S/c1-27(25,26)22-13-4-2-3-10-15(23)8-14(20-16(10)13)17(24)21-12-6-5-9(18)7-11(12)19/h2-8,22H,1H3,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal hexahistidine-tagged Lp-PLA2 expressed in Escherichia coli using 2- thio-PAF as substrate incubated for 20... |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429070
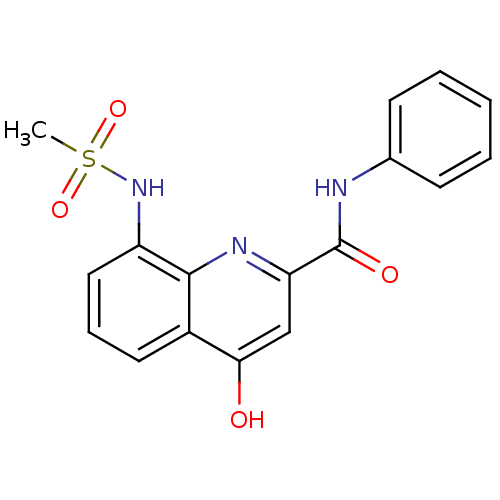
(CHEMBL2335742)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C17H15N3O4S/c1-25(23,24)20-13-9-5-8-12-15(21)10-14(19-16(12)13)17(22)18-11-6-3-2-4-7-11/h2-10,20H,1H3,(H,18,22)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal hexahistidine-tagged Lp-PLA2 expressed in Escherichia coli using 2- thio-PAF as substrate incubated for 20... |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296807

(5-ethyl-2-(2-(4-methylpiperazin-1-yl)pyridin-3-yl)...)Show InChI InChI=1S/C20H22N4O2/c1-3-14-6-4-8-16-17(14)20(25)26-19(22-16)15-7-5-9-21-18(15)24-12-10-23(2)11-13-24/h4-9H,3,10-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296805
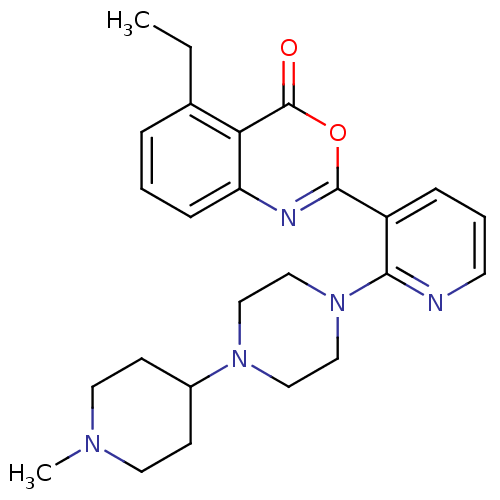
(5-ethyl-2-(2-(4-(1-methylpiperidin-4-yl)piperazin-...)Show SMILES CCc1cccc2nc(oc(=O)c12)-c1cccnc1N1CCN(CC1)C1CCN(C)CC1 Show InChI InChI=1S/C25H31N5O2/c1-3-18-6-4-8-21-22(18)25(31)32-24(27-21)20-7-5-11-26-23(20)30-16-14-29(15-17-30)19-9-12-28(2)13-10-19/h4-8,11,19H,3,9-10,12-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296806

(1-(3-(5-ethyl-4-oxo-4H-benzo[d][1,3]oxazin-2-yl)py...)Show SMILES CCc1cccc2nc(oc(=O)c12)-c1cccnc1N1CCC(CC1)C(O)=O Show InChI InChI=1S/C21H21N3O4/c1-2-13-5-3-7-16-17(13)21(27)28-19(23-16)15-6-4-10-22-18(15)24-11-8-14(9-12-24)20(25)26/h3-7,10,14H,2,8-9,11-12H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429098

(CHEMBL2335784)Show SMILES Cc1c(F)cccc1NC(=O)c1cc(O)c2cccc(NS(C)(=O)=O)c2n1 Show InChI InChI=1S/C18H16FN3O4S/c1-10-12(19)6-4-7-13(10)21-18(24)15-9-16(23)11-5-3-8-14(17(11)20-15)22-27(2,25)26/h3-9,22H,1-2H3,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429070

(CHEMBL2335742)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C17H15N3O4S/c1-25(23,24)20-13-9-5-8-12-15(21)10-14(19-16(12)13)17(22)18-11-6-3-2-4-7-11/h2-10,20H,1H3,(H,18,22)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr in presence o... |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429094

(CHEMBL2335783)Show SMILES Cc1cc(F)ccc1NC(=O)c1cc(O)c2cccc(NS(C)(=O)=O)c2n1 Show InChI InChI=1S/C18H16FN3O4S/c1-10-8-11(19)6-7-13(10)21-18(24)15-9-16(23)12-4-3-5-14(17(12)20-15)22-27(2,25)26/h3-9,22H,1-2H3,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429101

(CHEMBL2335780)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)Nc1ccc(F)cc1F Show InChI InChI=1S/C17H13F2N3O4S/c1-27(25,26)22-13-4-2-3-10-15(23)8-14(20-16(10)13)17(24)21-12-6-5-9(18)7-11(12)19/h2-8,22H,1H3,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429114
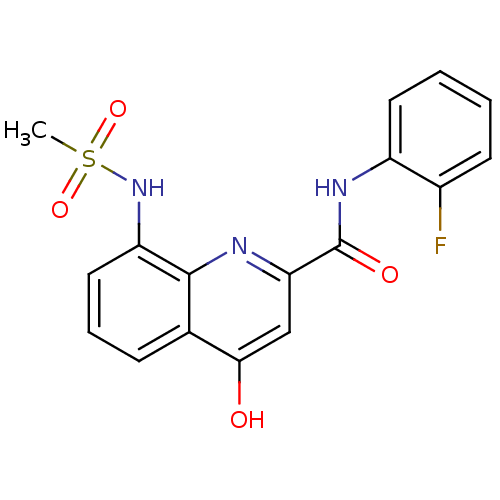
(CHEMBL2335769)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)Nc1ccccc1F Show InChI InChI=1S/C17H14FN3O4S/c1-26(24,25)21-13-8-4-5-10-15(22)9-14(19-16(10)13)17(23)20-12-7-3-2-6-11(12)18/h2-9,21H,1H3,(H,19,22)(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429113
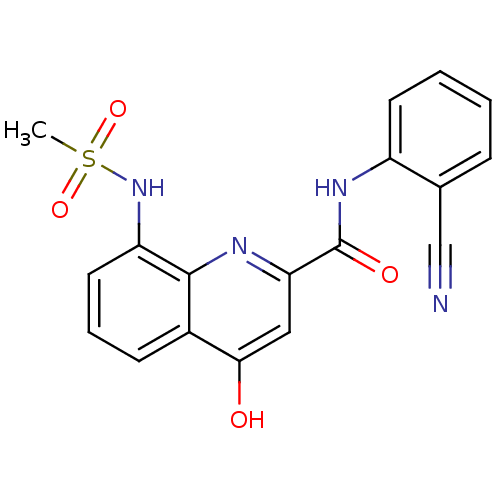
(CHEMBL2335770)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)Nc1ccccc1C#N Show InChI InChI=1S/C18H14N4O4S/c1-27(25,26)22-14-8-4-6-12-16(23)9-15(20-17(12)14)18(24)21-13-7-3-2-5-11(13)10-19/h2-9,22H,1H3,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429070

(CHEMBL2335742)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C17H15N3O4S/c1-25(23,24)20-13-9-5-8-12-15(21)10-14(19-16(12)13)17(22)18-11-6-3-2-4-7-11/h2-10,20H,1H3,(H,18,22)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal hexahistidine-tagged Lp-PLA2 expressed in Escherichia coli using 2- thio-PAF as substrate incubated for 20... |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429112

(CHEMBL2335760)Show SMILES Cc1ccccc1NC(=O)c1cc(O)c2cccc(NS(C)(=O)=O)c2n1 Show InChI InChI=1S/C18H17N3O4S/c1-11-6-3-4-8-13(11)20-18(23)15-10-16(22)12-7-5-9-14(17(12)19-15)21-26(2,24)25/h3-10,21H,1-2H3,(H,19,22)(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296826
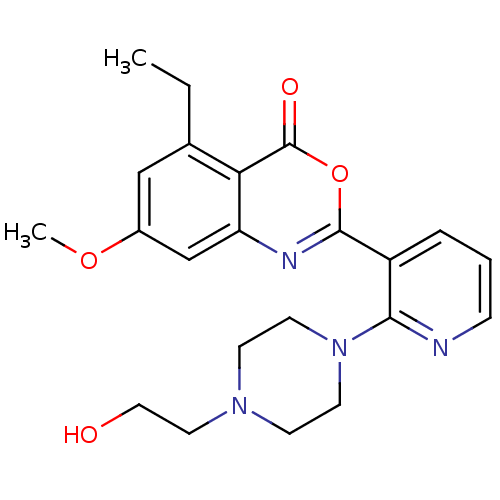
(5-ethyl-2-(2-(4-(2-hydroxyethyl)piperazin-1-yl)pyr...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCN(CCO)CC1 Show InChI InChI=1S/C22H26N4O4/c1-3-15-13-16(29-2)14-18-19(15)22(28)30-21(24-18)17-5-4-6-23-20(17)26-9-7-25(8-10-26)11-12-27/h4-6,13-14,27H,3,7-12H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296830
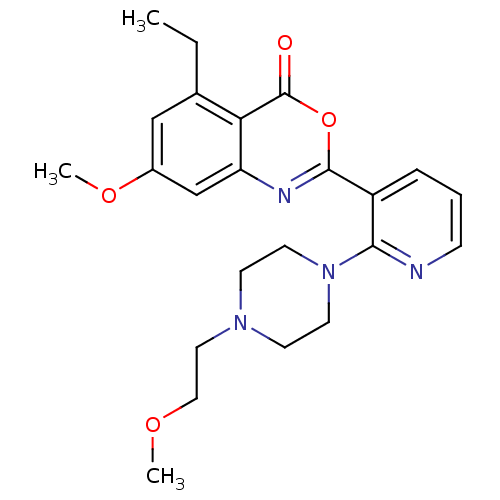
(5-ethyl-7-methoxy-2-(2-(4-(2-methoxyethyl)piperazi...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCN(CCOC)CC1 Show InChI InChI=1S/C23H28N4O4/c1-4-16-14-17(30-3)15-19-20(16)23(28)31-22(25-19)18-6-5-7-24-21(18)27-10-8-26(9-11-27)12-13-29-2/h5-7,14-15H,4,8-13H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50363386
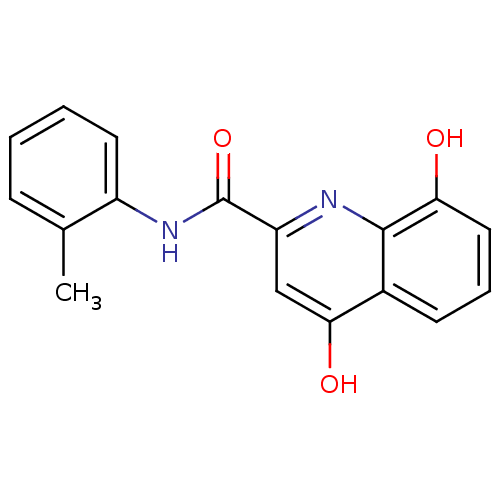
(CHEMBL1945889)Show InChI InChI=1S/C17H14N2O3/c1-10-5-2-3-7-12(10)19-17(22)13-9-15(21)11-6-4-8-14(20)16(11)18-13/h2-9,20H,1H3,(H,18,21)(H,19,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma lipoprotein-associated phospholipase A2 using 2-thio-PAF as substrate preincubated for 20 mins measured 1 hr post substrat... |
Bioorg Med Chem Lett 22: 868-71 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.045
BindingDB Entry DOI: 10.7270/Q2XG9RMD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296834
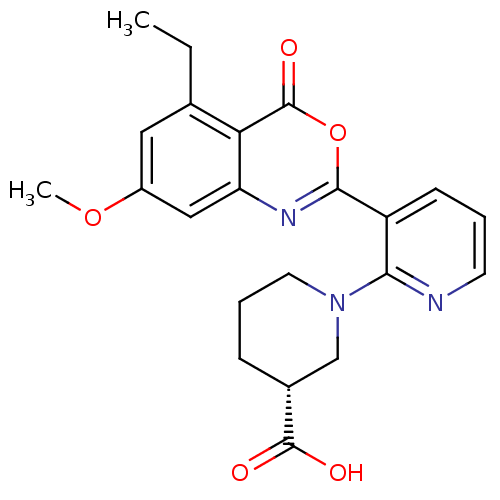
((R)-1-(3-(5-ethyl-7-methoxy-4-oxo-4H-benzo[d][1,3]...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCC[C@H](C1)C(O)=O |r| Show InChI InChI=1S/C22H23N3O5/c1-3-13-10-15(29-2)11-17-18(13)22(28)30-20(24-17)16-7-4-8-23-19(16)25-9-5-6-14(12-25)21(26)27/h4,7-8,10-11,14H,3,5-6,9,12H2,1-2H3,(H,26,27)/t14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50363379
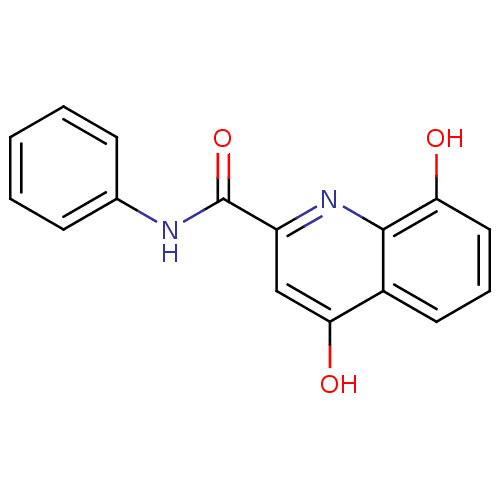
(CHEMBL1945882)Show InChI InChI=1S/C16H12N2O3/c19-13-8-4-7-11-14(20)9-12(18-15(11)13)16(21)17-10-5-2-1-3-6-10/h1-9,19H,(H,17,21)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma lipoprotein-associated phospholipase A2 using 2-thio-PAF as substrate preincubated for 20 mins measured 1 hr post substrat... |
Bioorg Med Chem Lett 22: 868-71 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.045
BindingDB Entry DOI: 10.7270/Q2XG9RMD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296804

(5-ethyl-7-methoxy-2-(2-(4-methylpiperazin-1-yl)pyr...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCN(C)CC1 Show InChI InChI=1S/C21H24N4O3/c1-4-14-12-15(27-3)13-17-18(14)21(26)28-20(23-17)16-6-5-7-22-19(16)25-10-8-24(2)9-11-25/h5-7,12-13H,4,8-11H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296824
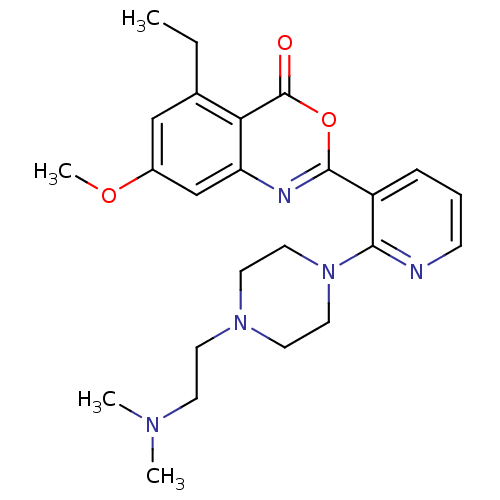
(2-(2-(4-(2-(dimethylamino)ethyl)piperazin-1-yl)pyr...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCN(CCN(C)C)CC1 Show InChI InChI=1S/C24H31N5O3/c1-5-17-15-18(31-4)16-20-21(17)24(30)32-23(26-20)19-7-6-8-25-22(19)29-13-11-28(12-14-29)10-9-27(2)3/h6-8,15-16H,5,9-14H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296828

(5-ethyl-2-(2-(4-isobutylpiperazin-1-yl)pyridin-3-y...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCN(CC(C)C)CC1 Show InChI InChI=1S/C24H30N4O3/c1-5-17-13-18(30-4)14-20-21(17)24(29)31-23(26-20)19-7-6-8-25-22(19)28-11-9-27(10-12-28)15-16(2)3/h6-8,13-14,16H,5,9-12,15H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429111

(CHEMBL2335786)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)Nc1ccc(F)cc1O Show InChI InChI=1S/C17H14FN3O5S/c1-27(25,26)21-12-4-2-3-10-14(22)8-13(19-16(10)12)17(24)20-11-6-5-9(18)7-15(11)23/h2-8,21,23H,1H3,(H,19,22)(H,20,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296827
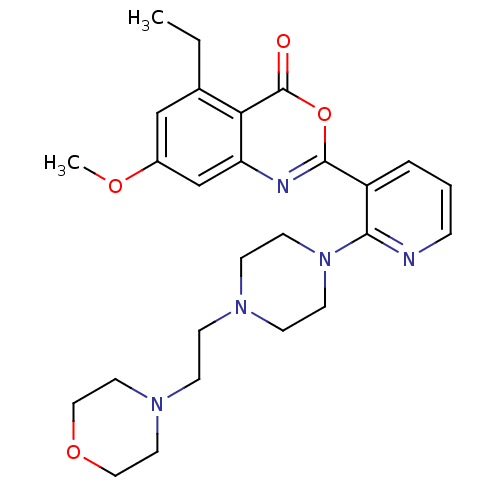
(5-ethyl-7-methoxy-2-(2-(4-(2-morpholinoethyl)piper...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCN(CCN2CCOCC2)CC1 Show InChI InChI=1S/C26H33N5O4/c1-3-19-17-20(33-2)18-22-23(19)26(32)35-25(28-22)21-5-4-6-27-24(21)31-11-9-29(10-12-31)7-8-30-13-15-34-16-14-30/h4-6,17-18H,3,7-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429110
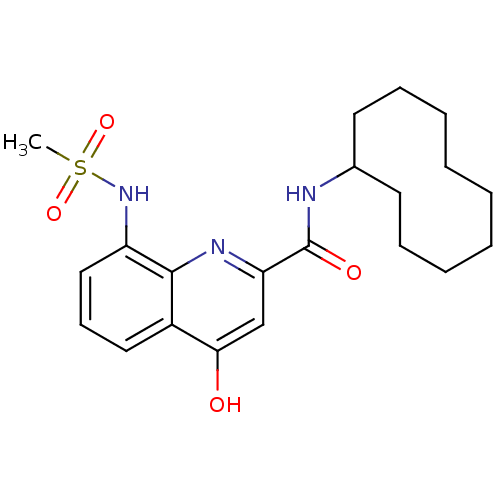
(CHEMBL2335749)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)NC1CCCCCCCCC1 Show InChI InChI=1S/C21H29N3O4S/c1-29(27,28)24-17-13-9-12-16-19(25)14-18(23-20(16)17)21(26)22-15-10-7-5-3-2-4-6-8-11-15/h9,12-15,24H,2-8,10-11H2,1H3,(H,22,26)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296835

(2-(4-(3-(5-ethyl-7-methoxy-4-oxo-4H-benzo[d][1,3]o...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCN(CC(O)=O)CC1 Show InChI InChI=1S/C22H24N4O5/c1-3-14-11-15(30-2)12-17-19(14)22(29)31-21(24-17)16-5-4-6-23-20(16)26-9-7-25(8-10-26)13-18(27)28/h4-6,11-12H,3,7-10,13H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50363389
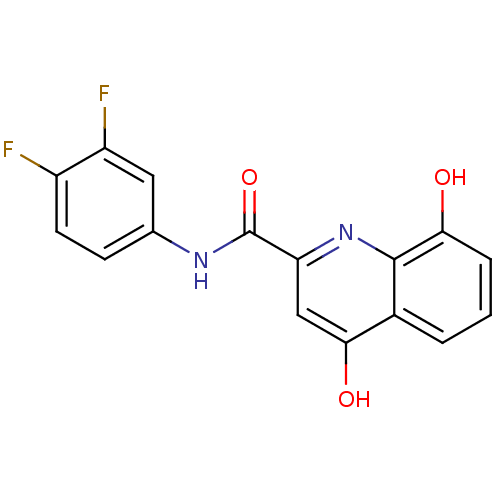
(CHEMBL1946063)Show InChI InChI=1S/C16H10F2N2O3/c17-10-5-4-8(6-11(10)18)19-16(23)12-7-14(22)9-2-1-3-13(21)15(9)20-12/h1-7,21H,(H,19,23)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma lipoprotein-associated phospholipase A2 using 2-thio-PAF as substrate preincubated for 20 mins measured 1 hr post substrat... |
Bioorg Med Chem Lett 22: 868-71 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.045
BindingDB Entry DOI: 10.7270/Q2XG9RMD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50363389

(CHEMBL1946063)Show InChI InChI=1S/C16H10F2N2O3/c17-10-5-4-8(6-11(10)18)19-16(23)12-7-14(22)9-2-1-3-13(21)15(9)20-12/h1-7,21H,(H,19,23)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma lipoprotein-associated phospholipase A2 using 2-thio-PAF as substrate preincubated for 20 mins measured 1 hr post substrat... |
Bioorg Med Chem Lett 22: 868-71 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.045
BindingDB Entry DOI: 10.7270/Q2XG9RMD |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296825

(3-(4-(3-(5-ethyl-7-methoxy-4-oxo-4H-benzo[d][1,3]o...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCN(CCC#N)CC1 Show InChI InChI=1S/C23H25N5O3/c1-3-16-14-17(30-2)15-19-20(16)23(29)31-22(26-19)18-6-4-8-25-21(18)28-12-10-27(11-13-28)9-5-7-24/h4,6,8,14-15H,3,5,9-13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296833
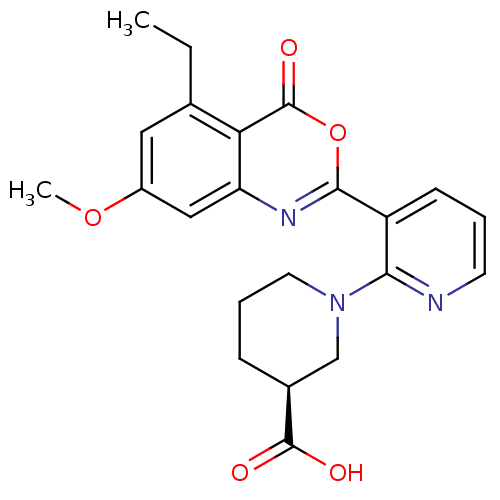
((S)-1-(3-(5-ethyl-7-methoxy-4-oxo-4H-benzo[d][1,3]...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCC[C@@H](C1)C(O)=O |r| Show InChI InChI=1S/C22H23N3O5/c1-3-13-10-15(29-2)11-17-18(13)22(28)30-20(24-17)16-7-4-8-23-19(16)25-9-5-6-14(12-25)21(26)27/h4,7-8,10-11,14H,3,5-6,9,12H2,1-2H3,(H,26,27)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429108
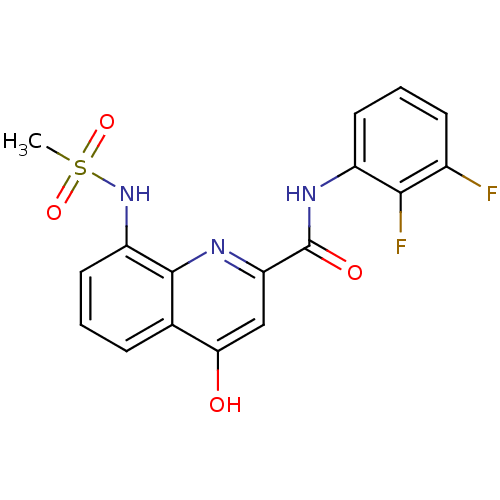
(CHEMBL2335779)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)Nc1cccc(F)c1F Show InChI InChI=1S/C17H13F2N3O4S/c1-27(25,26)22-12-7-2-4-9-14(23)8-13(20-16(9)12)17(24)21-11-6-3-5-10(18)15(11)19/h2-8,22H,1H3,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429109

(CHEMBL2335768)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)Nc1ccc(F)cc1 Show InChI InChI=1S/C17H14FN3O4S/c1-26(24,25)21-13-4-2-3-12-15(22)9-14(20-16(12)13)17(23)19-11-7-5-10(18)6-8-11/h2-9,21H,1H3,(H,19,23)(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429070

(CHEMBL2335742)Show SMILES CS(=O)(=O)Nc1cccc2c(O)cc(nc12)C(=O)Nc1ccccc1 Show InChI InChI=1S/C17H15N3O4S/c1-25(23,24)20-13-9-5-8-12-15(21)10-14(19-16(12)13)17(22)18-11-6-3-2-4-7-11/h2-10,20H,1H3,(H,18,22)(H,19,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296829

(2-(2-(4-butylpiperazin-1-yl)pyridin-3-yl)-5-ethyl-...)Show SMILES CCCCN1CCN(CC1)c1ncccc1-c1nc2cc(OC)cc(CC)c2c(=O)o1 Show InChI InChI=1S/C24H30N4O3/c1-4-6-10-27-11-13-28(14-12-27)22-19(8-7-9-25-22)23-26-20-16-18(30-3)15-17(5-2)21(20)24(29)31-23/h7-9,15-16H,4-6,10-14H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50363379

(CHEMBL1945882)Show InChI InChI=1S/C16H12N2O3/c19-13-8-4-7-11-14(20)9-12(18-15(11)13)16(21)17-10-5-2-1-3-6-10/h1-9,19H,(H,17,21)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma lipoprotein-associated phospholipase A2 using 2-thio-PAF as substrate preincubated for 20 mins measured 1 hr post substrat... |
Bioorg Med Chem Lett 22: 868-71 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.045
BindingDB Entry DOI: 10.7270/Q2XG9RMD |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50429107
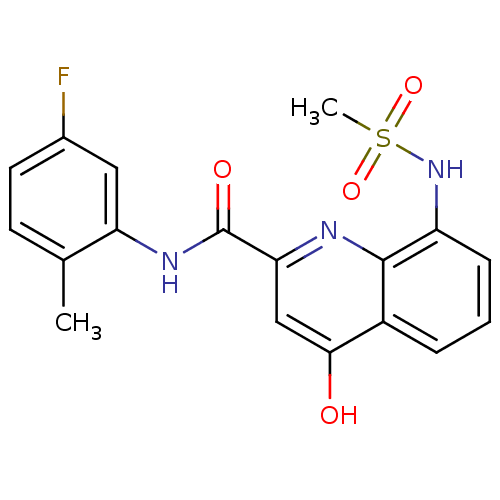
(CHEMBL2335782)Show SMILES Cc1ccc(F)cc1NC(=O)c1cc(O)c2cccc(NS(C)(=O)=O)c2n1 Show InChI InChI=1S/C18H16FN3O4S/c1-10-6-7-11(19)8-14(10)21-18(24)15-9-16(23)12-4-3-5-13(17(12)20-15)22-27(2,25)26/h3-9,22H,1-2H3,(H,20,23)(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma Lp-PLA2 using 2- thio-PAF as substrate incubated for 20 mins prior to substrate addition measured after 1 hr |
Bioorg Med Chem Lett 23: 1553-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.11.048
BindingDB Entry DOI: 10.7270/Q2571DCV |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296802

(5-ethyl-7-methoxy-2-(2-(4-(1-methylpiperidin-4-yl)...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCN(CC1)C1CCN(C)CC1 Show InChI InChI=1S/C26H33N5O3/c1-4-18-16-20(33-3)17-22-23(18)26(32)34-25(28-22)21-6-5-9-27-24(21)31-14-12-30(13-15-31)19-7-10-29(2)11-8-19/h5-6,9,16-17,19H,4,7-8,10-15H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296803

(1-(3-(5-ethyl-7-methoxy-4-oxo-4H-benzo[d][1,3]oxaz...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCC(CC1)C(O)=O Show InChI InChI=1S/C22H23N3O5/c1-3-13-11-15(29-2)12-17-18(13)22(28)30-20(24-17)16-5-4-8-23-19(16)25-9-6-14(7-10-25)21(26)27/h4-5,8,11-12,14H,3,6-7,9-10H2,1-2H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50296831
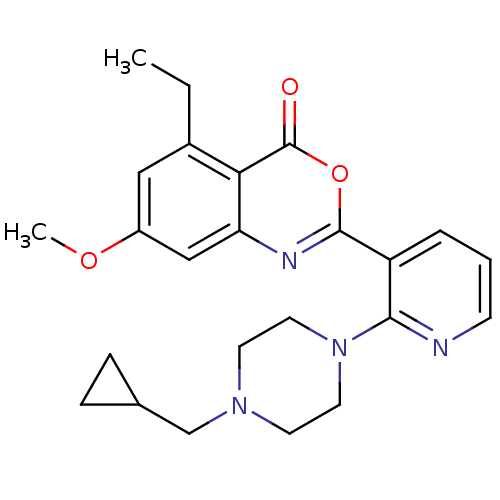
(2-(2-(4-(cyclopropylmethyl)piperazin-1-yl)pyridin-...)Show SMILES CCc1cc(OC)cc2nc(oc(=O)c12)-c1cccnc1N1CCN(CC2CC2)CC1 Show InChI InChI=1S/C24H28N4O3/c1-3-17-13-18(30-2)14-20-21(17)24(29)31-23(26-20)19-5-4-8-25-22(19)28-11-9-27(10-12-28)15-16-6-7-16/h4-5,8,13-14,16H,3,6-7,9-12,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc
Curated by ChEMBL
| Assay Description
Inhibition of human sputum neutrophil elastase by fluorescence based assay |
Bioorg Med Chem Lett 19: 4743-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.053
BindingDB Entry DOI: 10.7270/Q2M32VT3 |
More data for this
Ligand-Target Pair | |
Platelet-activating factor acetylhydrolase
(Homo sapiens (Human)) | BDBM50363387

(CHEMBL1946061)Show InChI InChI=1S/C16H10F2N2O3/c17-8-4-5-11(10(18)6-8)20-16(23)12-7-14(22)9-2-1-3-13(21)15(9)19-12/h1-7,21H,(H,19,22)(H,20,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
ActivX Biosciences, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human plasma lipoprotein-associated phospholipase A2 using 2-thio-PAF as substrate preincubated for 20 mins measured 1 hr post substrat... |
Bioorg Med Chem Lett 22: 868-71 (2012)
Article DOI: 10.1016/j.bmcl.2011.12.045
BindingDB Entry DOI: 10.7270/Q2XG9RMD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data