 Found 741 hits with Last Name = 'frazer' and Initial = 'j'
Found 741 hits with Last Name = 'frazer' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374104
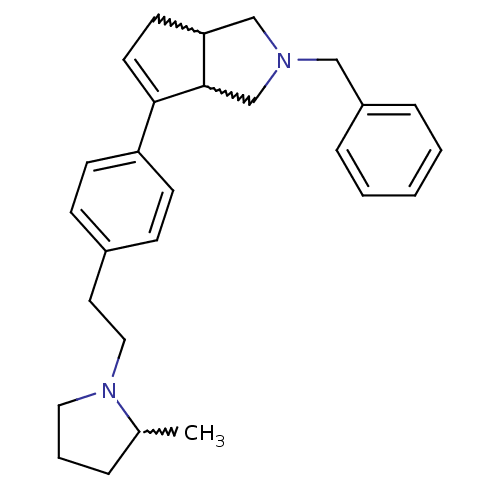
(CHEMBL255962)Show SMILES CC1CCCN1CCc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12 |w:17.18,28.30,1.0,t:16| Show InChI InChI=1S/C27H34N2/c1-21-6-5-16-29(21)17-15-22-9-11-24(12-10-22)26-14-13-25-19-28(20-27(25)26)18-23-7-3-2-4-8-23/h2-4,7-12,14,21,25,27H,5-6,13,15-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374100

(CHEMBL270011)Show SMILES C(Cc1ccc(cc1)C1=CC[C@H]2CN(Cc3ccccc3)C[C@@H]12)N1CCCC1 |t:9| Show InChI InChI=1S/C26H32N2/c1-2-6-22(7-3-1)18-28-19-24-12-13-25(26(24)20-28)23-10-8-21(9-11-23)14-17-27-15-4-5-16-27/h1-3,6-11,13,24,26H,4-5,12,14-20H2/t24-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50232355

((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...)Show SMILES C(Cc1ccc(cc1)C1=CC[C@H]2CN(CC3CC3)C[C@@H]12)N1CCCC1 |r,t:9| Show InChI InChI=1S/C23H32N2/c1-2-13-24(12-1)14-11-18-5-7-20(8-6-18)22-10-9-21-16-25(17-23(21)22)15-19-3-4-19/h5-8,10,19,21,23H,1-4,9,11-17H2/t21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
Bioorg Med Chem Lett 18: 4133-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.086
BindingDB Entry DOI: 10.7270/Q2C82942 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50232355

((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...)Show SMILES C(Cc1ccc(cc1)C1=CC[C@H]2CN(CC3CC3)C[C@@H]12)N1CCCC1 |r,t:9| Show InChI InChI=1S/C23H32N2/c1-2-13-24(12-1)14-11-18-5-7-20(8-6-18)22-10-9-21-16-25(17-23(21)22)15-19-3-4-19/h5-8,10,19,21,23H,1-4,9,11-17H2/t21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374110

(CHEMBL401954)Show SMILES C(Cc1ccc(cc1)C1=CC[C@H]2CN(C[C@@H]12)C1CCCC1)N1CCCC1 |t:9| Show InChI InChI=1S/C24H34N2/c1-2-6-22(5-1)26-17-21-11-12-23(24(21)18-26)20-9-7-19(8-10-20)13-16-25-14-3-4-15-25/h7-10,12,21-22,24H,1-6,11,13-18H2/t21-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50232355

((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...)Show SMILES C(Cc1ccc(cc1)C1=CC[C@H]2CN(CC3CC3)C[C@@H]12)N1CCCC1 |r,t:9| Show InChI InChI=1S/C23H32N2/c1-2-13-24(12-1)14-11-18-5-7-20(8-6-18)22-10-9-21-16-25(17-23(21)22)15-19-3-4-19/h5-8,10,19,21,23H,1-4,9,11-17H2/t21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM472930
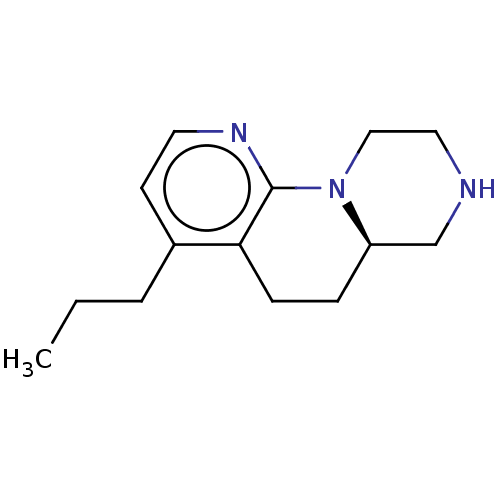
((R)-4-propyl-6,6a,7,8,9,10-hexahydro-5H- pyrazino[...)Show InChI InChI=1S/C14H21N3/c1-2-3-11-6-7-16-14-13(11)5-4-12-10-15-8-9-17(12)14/h6-7,12,15H,2-5,8-10H2,1H3/t12-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127872
BindingDB Entry DOI: 10.7270/Q2SF30VK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374102
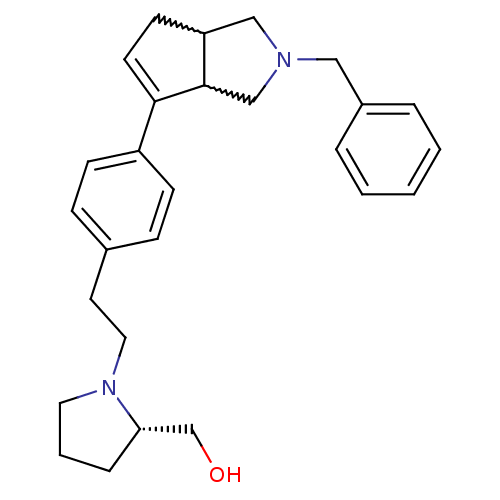
(CHEMBL402297)Show SMILES OC[C@@H]1CCCN1CCc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12 |w:18.19,29.31,t:17| Show InChI InChI=1S/C27H34N2O/c30-20-25-7-4-15-29(25)16-14-21-8-10-23(11-9-21)26-13-12-24-18-28(19-27(24)26)17-22-5-2-1-3-6-22/h1-3,5-6,8-11,13,24-25,27,30H,4,7,12,14-20H2/t24?,25-,27?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361235
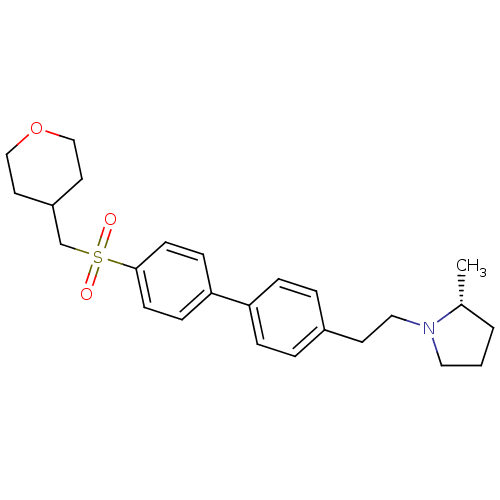
(CHEMBL1934525)Show SMILES C[C@@H]1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)CC1CCOCC1 |r| Show InChI InChI=1S/C25H33NO3S/c1-20-3-2-15-26(20)16-12-21-4-6-23(7-5-21)24-8-10-25(11-9-24)30(27,28)19-22-13-17-29-18-14-22/h4-11,20,22H,2-3,12-19H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352357
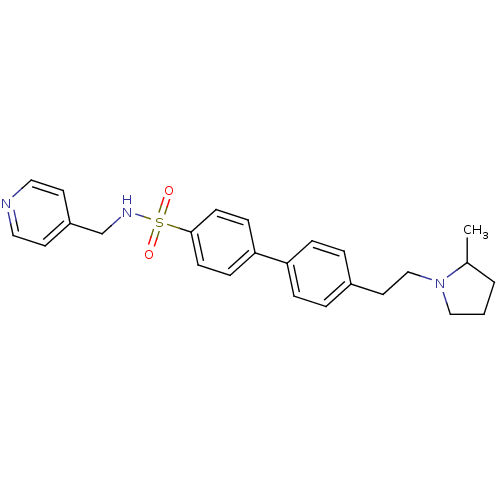
(CHEMBL558655)Show SMILES CC1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NCc1ccncc1 Show InChI InChI=1S/C25H29N3O2S/c1-20-3-2-17-28(20)18-14-21-4-6-23(7-5-21)24-8-10-25(11-9-24)31(29,30)27-19-22-12-15-26-16-13-22/h4-13,15-16,20,27H,2-3,14,17-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
J Med Chem 52: 5603-11 (2009)
Article DOI: 10.1021/jm900857n
BindingDB Entry DOI: 10.7270/Q2KW5G2F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361237
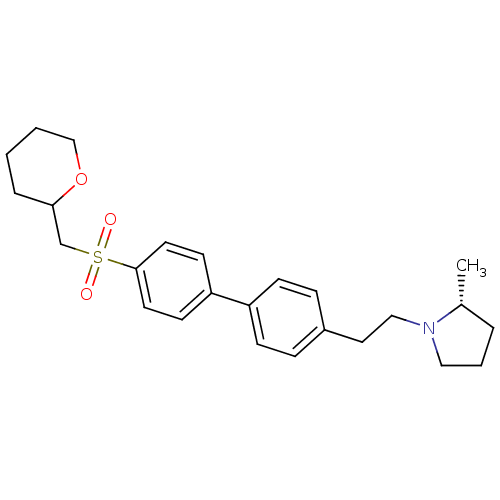
(CHEMBL1934527)Show SMILES C[C@@H]1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)CC1CCCCO1 |r| Show InChI InChI=1S/C25H33NO3S/c1-20-5-4-16-26(20)17-15-21-7-9-22(10-8-21)23-11-13-25(14-12-23)30(27,28)19-24-6-2-3-18-29-24/h7-14,20,24H,2-6,15-19H2,1H3/t20-,24?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50459718

(CHEMBL4213379 | US10836764, Compound 106)Show InChI InChI=1S/C18H21N3/c1-2-4-14(5-3-1)12-15-8-9-20-18-17(15)7-6-16-13-19-10-11-21(16)18/h1-5,8-9,16,19H,6-7,10-13H2/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127872
BindingDB Entry DOI: 10.7270/Q2SF30VK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374109

(CHEMBL255840)Show SMILES CC(C)N1C[C@@H]2CC=C([C@@H]2C1)c1ccc(CCN2CCCC2)cc1 |c:7| Show InChI InChI=1S/C22H32N2/c1-17(2)24-15-20-9-10-21(22(20)16-24)19-7-5-18(6-8-19)11-14-23-12-3-4-13-23/h5-8,10,17,20,22H,3-4,9,11-16H2,1-2H3/t20-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352358
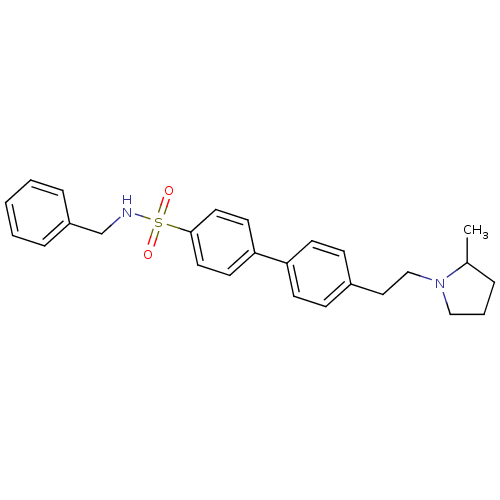
(CHEMBL558456)Show SMILES CC1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)NCc1ccccc1 Show InChI InChI=1S/C26H30N2O2S/c1-21-6-5-18-28(21)19-17-22-9-11-24(12-10-22)25-13-15-26(16-14-25)31(29,30)27-20-23-7-3-2-4-8-23/h2-4,7-16,21,27H,5-6,17-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.295 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
J Med Chem 52: 5603-11 (2009)
Article DOI: 10.1021/jm900857n
BindingDB Entry DOI: 10.7270/Q2KW5G2F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50243638

(1-((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)...)Show SMILES C[C@@H]1CCCN1CCc1ccc(cc1)C1=CC[C@H]2CN(C[C@@H]12)C(=O)Cc1ccccc1 |r,t:16| Show InChI InChI=1S/C28H34N2O/c1-21-6-5-16-29(21)17-15-22-9-11-24(12-10-22)26-14-13-25-19-30(20-27(25)26)28(31)18-23-7-3-2-4-8-23/h2-4,7-12,14,21,25,27H,5-6,13,15-20H2,1H3/t21-,25+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
Bioorg Med Chem Lett 18: 4133-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.086
BindingDB Entry DOI: 10.7270/Q2C82942 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374095
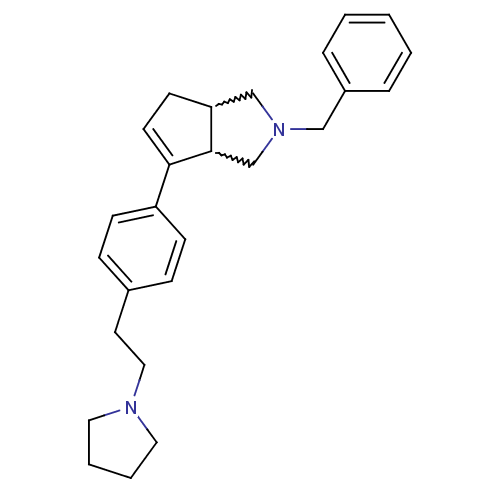
(CHEMBL255752)Show SMILES C(Cc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12)N1CCCC1 |w:11.12,22.23,t:9| Show InChI InChI=1S/C26H32N2/c1-2-6-22(7-3-1)18-28-19-24-12-13-25(26(24)20-28)23-10-8-21(9-11-23)14-17-27-15-4-5-16-27/h1-3,6-11,13,24,26H,4-5,12,14-20H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50557505
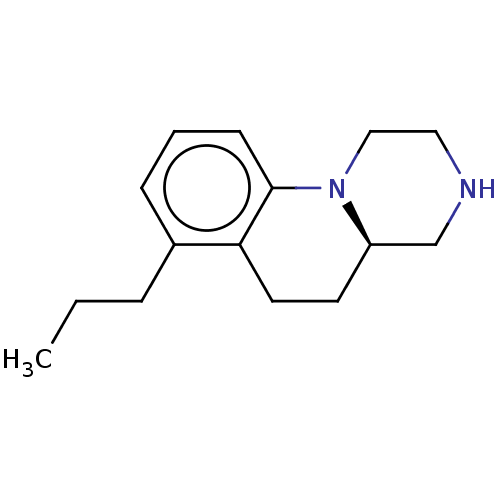
(CHEMBL4776557) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127872
BindingDB Entry DOI: 10.7270/Q2SF30VK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50365592

(CHEMBL1957805)Show SMILES Fc1ccc(CCN2CCN(CC2)C(=O)c2cccc3ccnn23)c(F)c1 Show InChI InChI=1S/C20H20F2N4O/c21-16-5-4-15(18(22)14-16)7-9-24-10-12-25(13-11-24)20(27)19-3-1-2-17-6-8-23-26(17)19/h1-6,8,14H,7,9-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-DOI from recombinant human 5HT2A receptor |
Bioorg Med Chem Lett 22: 1870-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.080
BindingDB Entry DOI: 10.7270/Q2XP75D0 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50243123

(CHEMBL488464 | cyclopentyl((3aR,6aR)-6-(4-(2-((R)-...)Show SMILES C[C@@H]1CCCN1CCc1ccc(cc1)C1=CC[C@H]2CN(C[C@@H]12)C(=O)C1CCCC1 |r,t:16| Show InChI InChI=1S/C26H36N2O/c1-19-5-4-15-27(19)16-14-20-8-10-21(11-9-20)24-13-12-23-17-28(18-25(23)24)26(29)22-6-2-3-7-22/h8-11,13,19,22-23,25H,2-7,12,14-18H2,1H3/t19-,23+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
Bioorg Med Chem Lett 18: 4133-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.086
BindingDB Entry DOI: 10.7270/Q2C82942 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374098
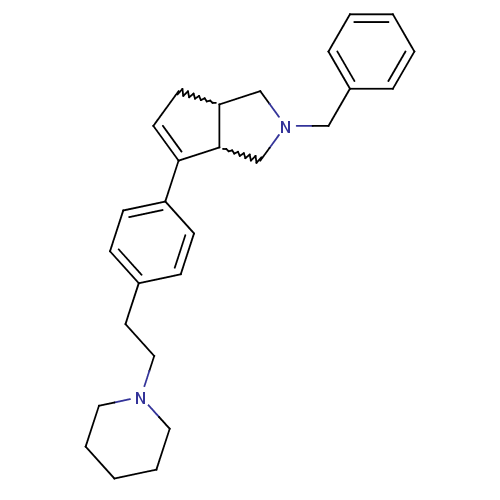
(CHEMBL256225)Show SMILES C(Cc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12)N1CCCCC1 |w:11.11,22.23,t:9| Show InChI InChI=1S/C27H34N2/c1-3-7-23(8-4-1)19-29-20-25-13-14-26(27(25)21-29)24-11-9-22(10-12-24)15-18-28-16-5-2-6-17-28/h1,3-4,7-12,14,25,27H,2,5-6,13,15-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374101
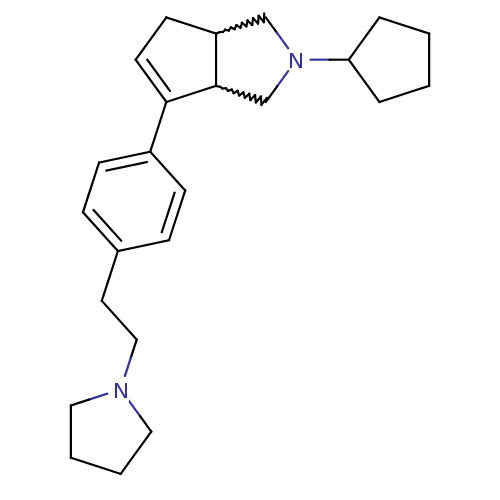
(CHEMBL410623)Show SMILES C(Cc1ccc(cc1)C1=CCC2CN(CC12)C1CCCC1)N1CCCC1 |w:11.12,15.15,t:9| Show InChI InChI=1S/C24H34N2/c1-2-6-22(5-1)26-17-21-11-12-23(24(21)18-26)20-9-7-19(8-10-20)13-16-25-14-3-4-15-25/h7-10,12,21-22,24H,1-6,11,13-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50243122

(2-methyl-1-((3aR,6aR)-6-(4-(2-((R)-2-methylpyrroli...)Show SMILES CC(C)C(=O)N1C[C@@H]2CC=C([C@@H]2C1)c1ccc(CCN2CCC[C@H]2C)cc1 |r,c:9| Show InChI InChI=1S/C24H34N2O/c1-17(2)24(27)26-15-21-10-11-22(23(21)16-26)20-8-6-19(7-9-20)12-14-25-13-4-5-18(25)3/h6-9,11,17-18,21,23H,4-5,10,12-16H2,1-3H3/t18-,21+,23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
Bioorg Med Chem Lett 18: 4133-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.086
BindingDB Entry DOI: 10.7270/Q2C82942 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50324540

(CHEMBL1215661 | Pruvanserin)Show SMILES Fc1ccc(CCN2CCN(CC2)C(=O)c2cccc3c(c[nH]c23)C#N)cc1 Show InChI InChI=1S/C22H21FN4O/c23-18-6-4-16(5-7-18)8-9-26-10-12-27(13-11-26)22(28)20-3-1-2-19-17(14-24)15-25-21(19)20/h1-7,15,25H,8-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells after 1 hr by scintillation counting |
J Med Chem 53: 5696-706 (2010)
Article DOI: 10.1021/jm100479q
BindingDB Entry DOI: 10.7270/Q2CV4HZX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50414743
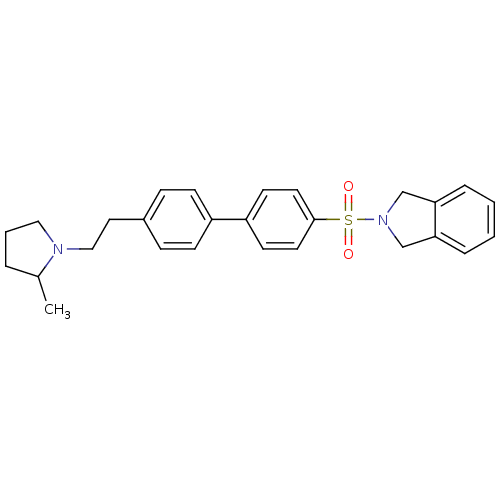
(CHEMBL564803)Show SMILES CC1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N1Cc2ccccc2C1 Show InChI InChI=1S/C27H30N2O2S/c1-21-5-4-17-28(21)18-16-22-8-10-23(11-9-22)24-12-14-27(15-13-24)32(30,31)29-19-25-6-2-3-7-26(25)20-29/h2-3,6-15,21H,4-5,16-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
J Med Chem 52: 5603-11 (2009)
Article DOI: 10.1021/jm900857n
BindingDB Entry DOI: 10.7270/Q2KW5G2F |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50459713
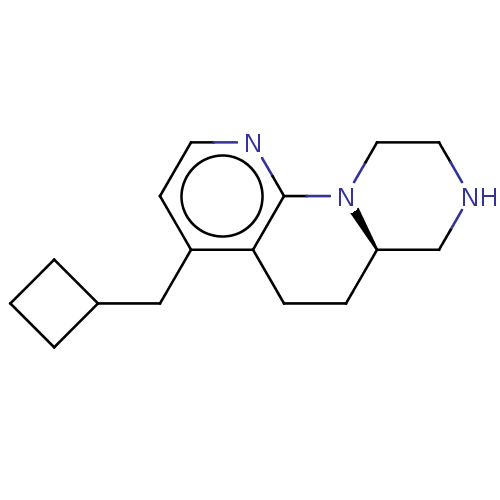
(CHEMBL4213447 | US10836764, Compound 176)Show InChI InChI=1S/C16H23N3/c1-2-12(3-1)10-13-6-7-18-16-15(13)5-4-14-11-17-8-9-19(14)16/h6-7,12,14,17H,1-5,8-11H2/t14-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127872
BindingDB Entry DOI: 10.7270/Q2SF30VK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM473021
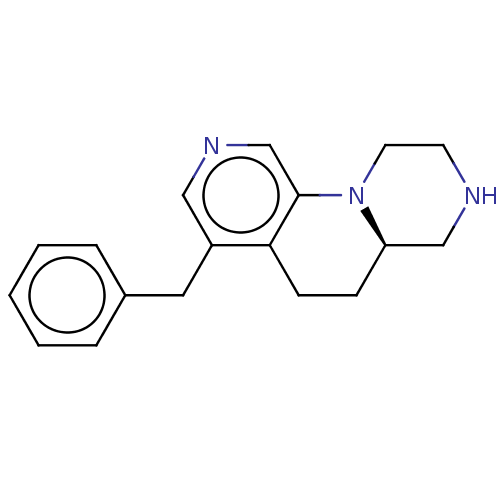
((R)-4-benzyl-6,6a,7,8,9,10-hexahydro-5H- pyrazino[...)Show InChI InChI=1S/C18H21N3/c1-2-4-14(5-3-1)10-15-11-20-13-18-17(15)7-6-16-12-19-8-9-21(16)18/h1-5,11,13,16,19H,6-10,12H2/t16-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127872
BindingDB Entry DOI: 10.7270/Q2SF30VK |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50324541

(1-[3-(4-Bromo-2-methyl-2H-pyrazol-3-yl)-4-methoxyp...)Show SMILES COc1ccc(NC(=O)Nc2ccc(F)cc2F)cc1-c1c(Br)cnn1C |(4.32,-10.34,;4.33,-11.88,;3,-12.66,;1.65,-11.89,;.32,-12.66,;.33,-14.2,;-1,-14.97,;-2.33,-14.2,;-2.34,-12.66,;-3.67,-14.97,;-5,-14.21,;-6.34,-14.98,;-7.67,-14.21,;-7.67,-12.66,;-9.01,-11.89,;-6.34,-11.89,;-5.01,-12.66,;-3.68,-11.88,;1.67,-14.97,;3,-14.2,;4.33,-14.97,;4.09,-16.48,;2.72,-17.18,;5.46,-17.18,;6.55,-16.08,;5.84,-14.71,;6.54,-13.34,)| Show InChI InChI=1S/C18H15BrF2N4O2/c1-25-17(13(19)9-22-25)12-8-11(4-6-16(12)27-2)23-18(26)24-15-5-3-10(20)7-14(15)21/h3-9H,1-2H3,(H2,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals.
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells after 1 hr by scintillation counting |
J Med Chem 53: 5696-706 (2010)
Article DOI: 10.1021/jm100479q
BindingDB Entry DOI: 10.7270/Q2CV4HZX |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374103

(CHEMBL255961)Show SMILES OC[C@H]1CCCN1CCc1ccc(cc1)C1=CCC2CN(Cc3ccccc3)CC12 |w:18.19,29.31,t:17| Show InChI InChI=1S/C27H34N2O/c30-20-25-7-4-15-29(25)16-14-21-8-10-23(11-9-21)26-13-12-24-18-28(19-27(24)26)17-22-5-2-1-3-6-22/h1-3,5-6,8-11,13,24-25,27,30H,4,7,12,14-20H2/t24?,25-,27?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319419
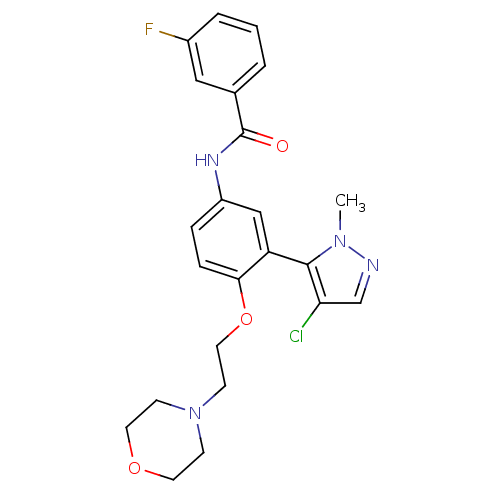
(CHEMBL1086075 | N-[3-(4-Chloro-2-methyl-2H-pyrazol...)Show SMILES Cn1ncc(Cl)c1-c1cc(NC(=O)c2cccc(F)c2)ccc1OCCN1CCOCC1 |(-5.98,-34.34,;-5.66,-35.84,;-6.69,-36.99,;-5.92,-38.32,;-4.41,-38,;-3.26,-39.03,;-4.25,-36.47,;-2.92,-35.7,;-1.59,-36.48,;-.25,-35.7,;1.09,-36.47,;2.42,-35.7,;2.42,-34.16,;3.75,-36.47,;3.75,-38,;5.08,-38.77,;6.41,-38,;6.41,-36.45,;7.74,-35.67,;5.07,-35.69,;-.26,-34.15,;-1.59,-33.39,;-2.92,-34.16,;-4.25,-33.39,;-4.25,-31.85,;-5.59,-31.08,;-5.59,-29.54,;-4.25,-28.77,;-4.25,-27.24,;-5.58,-26.46,;-6.92,-27.23,;-6.92,-28.77,)| Show InChI InChI=1S/C23H24ClFN4O3/c1-28-22(20(24)15-26-28)19-14-18(27-23(30)16-3-2-4-17(25)13-16)5-6-21(19)32-12-9-29-7-10-31-11-8-29/h2-6,13-15H,7-12H2,1H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541196

(CHEMBL4639090)Show InChI InChI=1S/C12H15BrN2/c1-8-7-15-5-4-14-6-9-2-3-10(13)11(8)12(9)15/h2-3,8,14H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361228
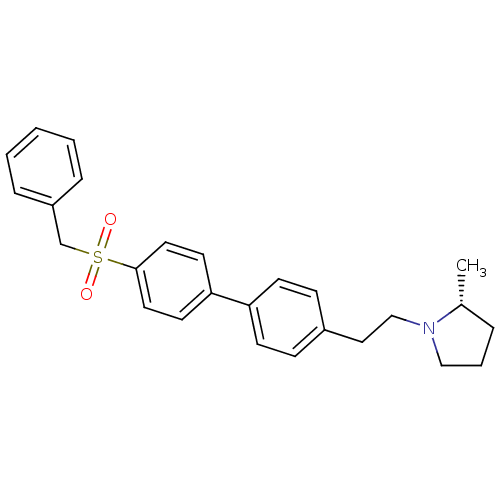
(CHEMBL1934358)Show SMILES C[C@@H]1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)Cc1ccccc1 |r| Show InChI InChI=1S/C26H29NO2S/c1-21-6-5-18-27(21)19-17-22-9-11-24(12-10-22)25-13-15-26(16-14-25)30(28,29)20-23-7-3-2-4-8-23/h2-4,7-16,21H,5-6,17-20H2,1H3/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361226
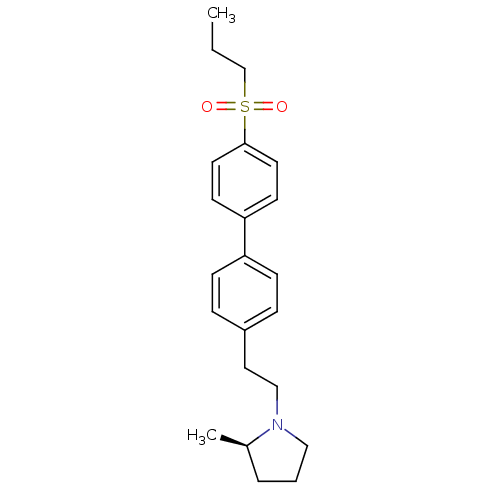
(CHEMBL1934356)Show SMILES CCCS(=O)(=O)c1ccc(cc1)-c1ccc(CCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C22H29NO2S/c1-3-17-26(24,25)22-12-10-21(11-13-22)20-8-6-19(7-9-20)14-16-23-15-4-5-18(23)2/h6-13,18H,3-5,14-17H2,1-2H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50365589
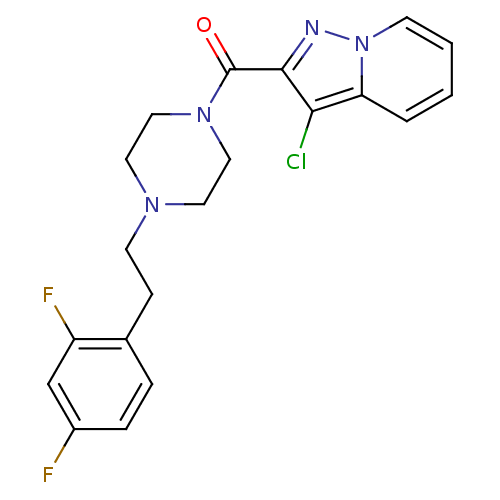
(CHEMBL1957802)Show SMILES Fc1ccc(CCN2CCN(CC2)C(=O)c2nn3ccccc3c2Cl)c(F)c1 Show InChI InChI=1S/C20H19ClF2N4O/c21-18-17-3-1-2-7-27(17)24-19(18)20(28)26-11-9-25(10-12-26)8-6-14-4-5-15(22)13-16(14)23/h1-5,7,13H,6,8-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-DOI from recombinant human 5HT2A receptor |
Bioorg Med Chem Lett 22: 1870-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.080
BindingDB Entry DOI: 10.7270/Q2XP75D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319414
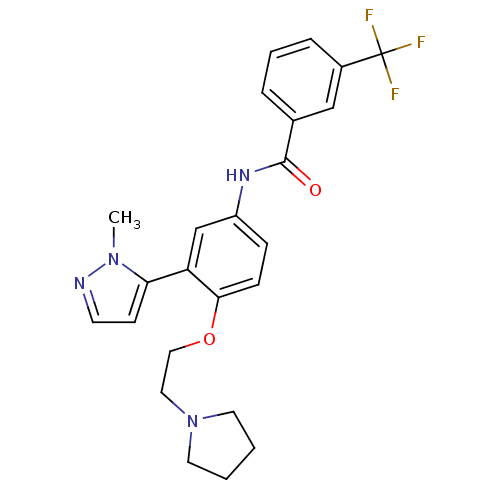
(CHEMBL1085155 | [3-(2-Methyl-2H-pyrazol-3-yl)-4-(2...)Show SMILES Cn1nccc1-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1OCCN1CCCC1 Show InChI InChI=1S/C24H25F3N4O2/c1-30-21(9-10-28-30)20-16-19(7-8-22(20)33-14-13-31-11-2-3-12-31)29-23(32)17-5-4-6-18(15-17)24(25,26)27/h4-10,15-16H,2-3,11-14H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM472931
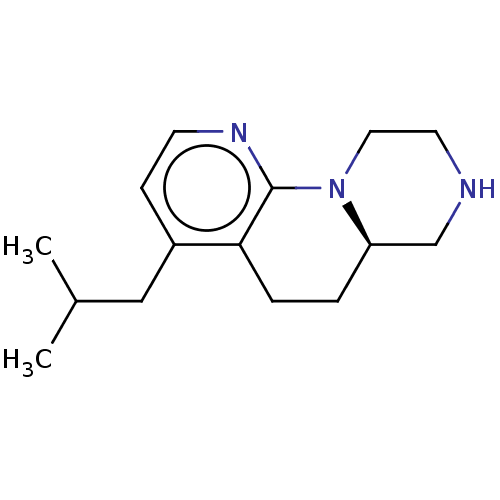
((R)-4-isobutyl-6,6a,7,8,9,10-hexahydro-5H- pyrazin...)Show InChI InChI=1S/C15H23N3/c1-11(2)9-12-5-6-17-15-14(12)4-3-13-10-16-7-8-18(13)15/h5-6,11,13,16H,3-4,7-10H2,1-2H3/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [125I]-DOI from recombinant human 5HT2CR transfected in human HEK293 cells incubated for 45 mins by radioligand binding assay |
Citation and Details
Article DOI: 10.1016/j.bmcl.2021.127872
BindingDB Entry DOI: 10.7270/Q2SF30VK |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352354
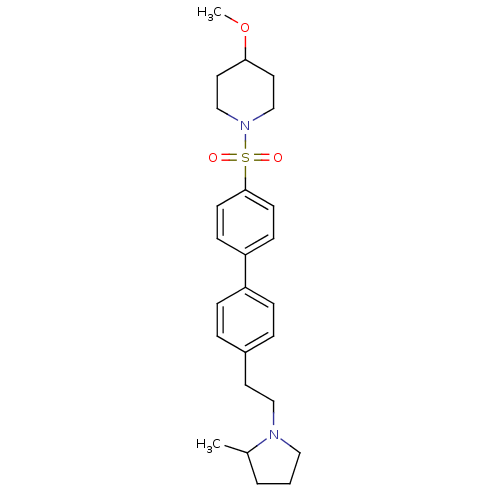
(CHEMBL554506)Show SMILES COC1CCN(CC1)S(=O)(=O)c1ccc(cc1)-c1ccc(CCN2CCCC2C)cc1 Show InChI InChI=1S/C25H34N2O3S/c1-20-4-3-16-26(20)17-13-21-5-7-22(8-6-21)23-9-11-25(12-10-23)31(28,29)27-18-14-24(30-2)15-19-27/h5-12,20,24H,3-4,13-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
J Med Chem 52: 5603-11 (2009)
Article DOI: 10.1021/jm900857n
BindingDB Entry DOI: 10.7270/Q2KW5G2F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361233
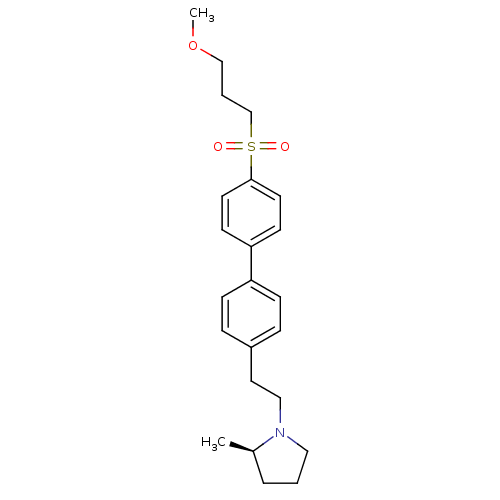
(CHEMBL1934523)Show SMILES COCCCS(=O)(=O)c1ccc(cc1)-c1ccc(CCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C23H31NO3S/c1-19-5-3-15-24(19)16-14-20-6-8-21(9-7-20)22-10-12-23(13-11-22)28(25,26)18-4-17-27-2/h6-13,19H,3-5,14-18H2,1-2H3/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50319421
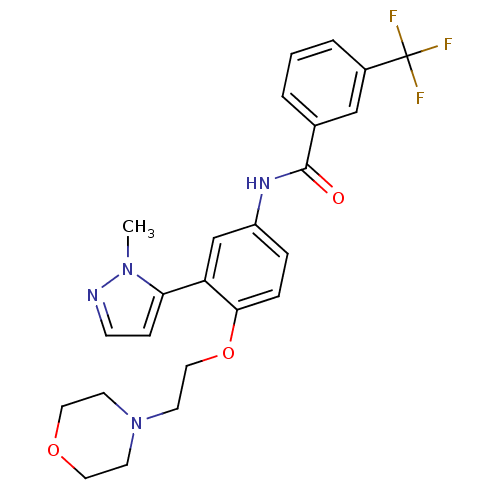
(CHEMBL1086317 | N-[3-(2-Methyl-2H-pyrazol-3-yl)-4-...)Show SMILES Cn1nccc1-c1cc(NC(=O)c2cccc(c2)C(F)(F)F)ccc1OCCN1CCOCC1 Show InChI InChI=1S/C24H25F3N4O3/c1-30-21(7-8-28-30)20-16-19(5-6-22(20)34-14-11-31-9-12-33-13-10-31)29-23(32)17-3-2-4-18(15-17)24(25,26)27/h2-8,15-16H,9-14H2,1H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]DOI from human recombinant 5HT2A receptor expressed in HEK293 cells by scintillation counting |
J Med Chem 53: 4412-21 (2010)
Article DOI: 10.1021/jm100044a
BindingDB Entry DOI: 10.7270/Q2KK9BZV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50243674
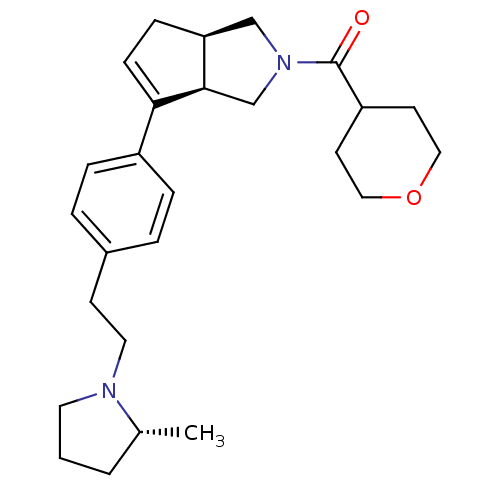
(((3aR,6aR)-6-(4-(2-((R)-2-methylpyrrolidin-1-yl)et...)Show SMILES C[C@@H]1CCCN1CCc1ccc(cc1)C1=CC[C@H]2CN(C[C@@H]12)C(=O)C1CCOCC1 |r,t:16| Show InChI InChI=1S/C26H36N2O2/c1-19-3-2-13-27(19)14-10-20-4-6-21(7-5-20)24-9-8-23-17-28(18-25(23)24)26(29)22-11-15-30-16-12-22/h4-7,9,19,22-23,25H,2-3,8,10-18H2,1H3/t19-,23+,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
Bioorg Med Chem Lett 18: 4133-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.086
BindingDB Entry DOI: 10.7270/Q2C82942 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374111

(CHEMBL409899)Show SMILES C(Cc1ccc(cc1)C1=CC[C@@H]2CN(Cc3ccccc3)C[C@H]12)N1CCCC1 |t:9| Show InChI InChI=1S/C26H32N2/c1-2-6-22(7-3-1)18-28-19-24-12-13-25(26(24)20-28)23-10-8-21(9-11-23)14-17-27-15-4-5-16-27/h1-3,6-11,13,24,26H,4-5,12,14-20H2/t24-,26+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361234
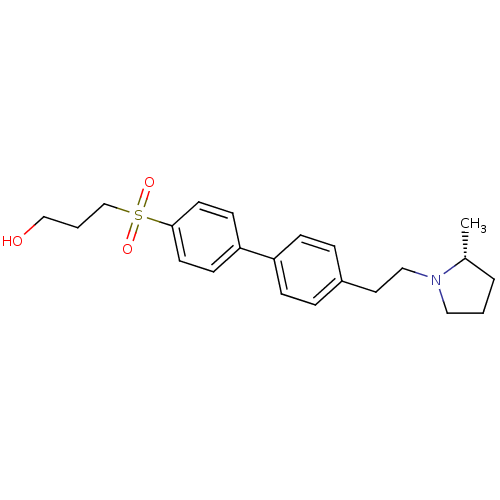
(CHEMBL1934524)Show SMILES C[C@@H]1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)CCCO |r| Show InChI InChI=1S/C22H29NO3S/c1-18-4-2-14-23(18)15-13-19-5-7-20(8-6-19)21-9-11-22(12-10-21)27(25,26)17-3-16-24/h5-12,18,24H,2-4,13-17H2,1H3/t18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541195
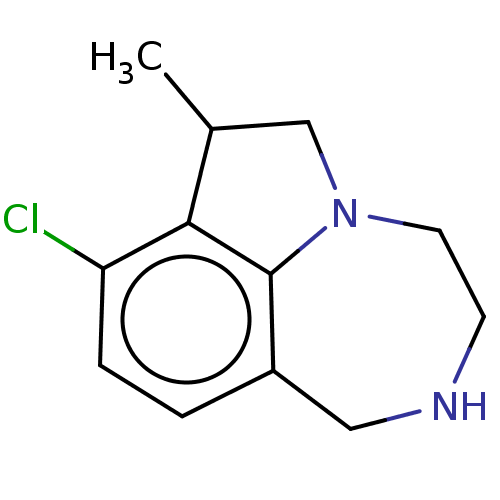
(CHEMBL4634942)Show InChI InChI=1S/C12H15ClN2/c1-8-7-15-5-4-14-6-9-2-3-10(13)11(8)12(9)15/h2-3,8,14H,4-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50365586

(CHEMBL1957801)Show SMILES Fc1ccc(CCN2CCN(CC2)C(=O)c2nn3ccccc3c2Br)c(F)c1 Show InChI InChI=1S/C20H19BrF2N4O/c21-18-17-3-1-2-7-27(17)24-19(18)20(28)26-11-9-25(10-12-26)8-6-14-4-5-15(22)13-16(14)23/h1-5,7,13H,6,8-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Displacement of [125I]-DOI from recombinant human 5HT2A receptor |
Bioorg Med Chem Lett 22: 1870-3 (2012)
Article DOI: 10.1016/j.bmcl.2012.01.080
BindingDB Entry DOI: 10.7270/Q2XP75D0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541197
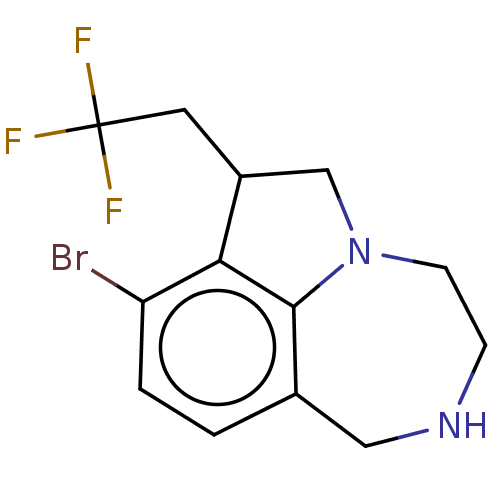
(CHEMBL4646572)Show InChI InChI=1S/C13H14BrF3N2/c14-10-2-1-8-6-18-3-4-19-7-9(5-13(15,16)17)11(10)12(8)19/h1-2,9,18H,3-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50361225
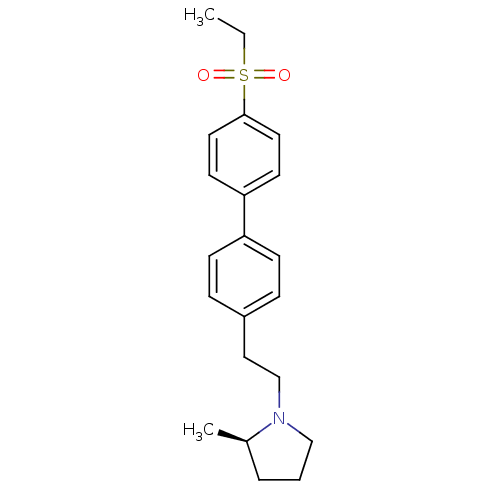
(CHEMBL1934355)Show SMILES CCS(=O)(=O)c1ccc(cc1)-c1ccc(CCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C21H27NO2S/c1-3-25(23,24)21-12-10-20(11-13-21)19-8-6-18(7-9-19)14-16-22-15-4-5-17(22)2/h6-13,17H,3-5,14-16H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]-methylhistamine from histamine H3 receptor in rat cortex membranes |
Bioorg Med Chem Lett 22: 71-5 (2011)
Article DOI: 10.1016/j.bmcl.2011.11.075
BindingDB Entry DOI: 10.7270/Q27M08C7 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50232355

((3aR,6aR)-2-(cyclopropylmethyl)-6-(4-(2-(pyrrolidi...)Show SMILES C(Cc1ccc(cc1)C1=CC[C@H]2CN(CC3CC3)C[C@@H]12)N1CCCC1 |r,t:9| Show InChI InChI=1S/C23H32N2/c1-2-13-24(12-1)14-11-18-5-7-20(8-6-18)22-10-9-21-16-25(17-23(21)22)15-19-3-4-19/h5-8,10,19,21,23H,1-4,9,11-17H2/t21-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor expressed in CHOK1 cells by [3H]R(-)-alpha-methylhistamine displacement assay |
Bioorg Med Chem Lett 18: 4133-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.05.086
BindingDB Entry DOI: 10.7270/Q2C82942 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50374107
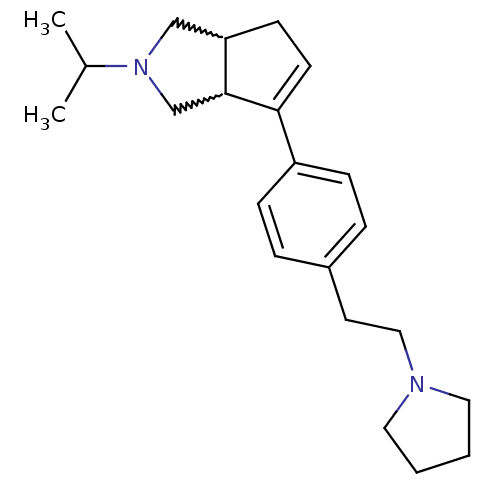
(CHEMBL270440)Show SMILES CC(C)N1CC2CC=C(C2C1)c1ccc(CCN2CCCC2)cc1 |w:5.4,9.10,c:7| Show InChI InChI=1S/C22H32N2/c1-17(2)24-15-20-9-10-21(22(20)16-24)19-7-5-18(6-8-19)11-14-23-12-3-4-13-23/h5-8,10,17,20,22H,3-4,9,11-16H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex |
Bioorg Med Chem Lett 18: 1490-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.12.059
BindingDB Entry DOI: 10.7270/Q2Q81DZS |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2C
(Homo sapiens (Human)) | BDBM50541204

(CHEMBL4636977)Show InChI InChI=1S/C13H17BrN2/c1-13(2)8-16-6-5-15-7-9-3-4-10(14)11(13)12(9)16/h3-4,15H,5-8H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [125I]-1,4-Iodanyl-2,5-dimethoxyphenyl)propan-2amine from 5HT2C receptor (unknown origin) |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2019.126929
BindingDB Entry DOI: 10.7270/Q28W3HT5 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50352355
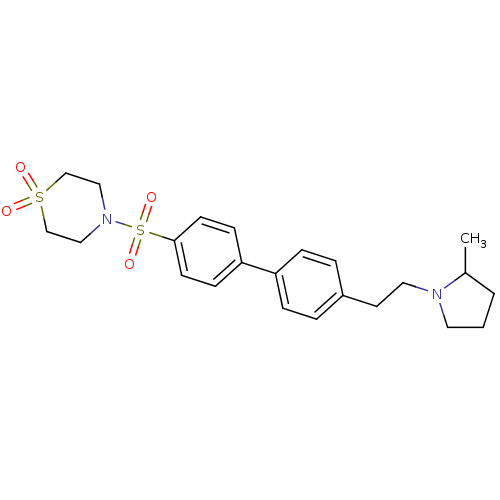
(CHEMBL560140)Show SMILES CC1CCCN1CCc1ccc(cc1)-c1ccc(cc1)S(=O)(=O)N1CCS(=O)(=O)CC1 Show InChI InChI=1S/C23H30N2O4S2/c1-19-3-2-13-24(19)14-12-20-4-6-21(7-5-20)22-8-10-23(11-9-22)31(28,29)25-15-17-30(26,27)18-16-25/h4-11,19H,2-3,12-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.933 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of N-[3H]methylhistamine from histamine H3 receptor in rat cortex membrane |
J Med Chem 52: 5603-11 (2009)
Article DOI: 10.1021/jm900857n
BindingDB Entry DOI: 10.7270/Q2KW5G2F |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50297366
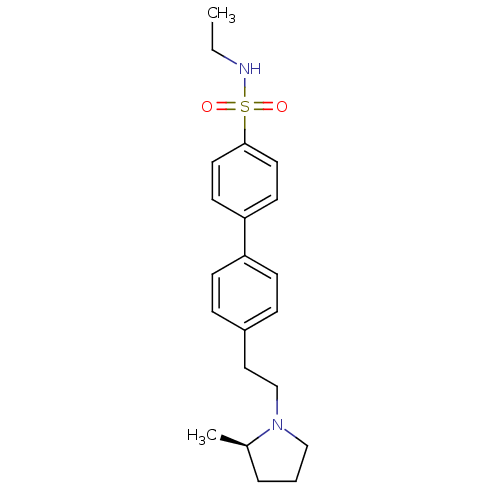
(4'-[2-((R)-2-Methyl-pyrrolidin-1-yl)-ethyl]-biphen...)Show SMILES CCNS(=O)(=O)c1ccc(cc1)-c1ccc(CCN2CCC[C@H]2C)cc1 |r| Show InChI InChI=1S/C21H28N2O2S/c1-3-22-26(24,25)21-12-10-20(11-13-21)19-8-6-18(7-9-19)14-16-23-15-4-5-17(23)2/h6-13,17,22H,3-5,14-16H2,1-2H3/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Arena Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inverse agonist activity against human histamine H3 receptor by [35S]GTPgamma binding assay |
J Med Chem 52: 5603-11 (2009)
Article DOI: 10.1021/jm900857n
BindingDB Entry DOI: 10.7270/Q2KW5G2F |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data