

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194141 (CHEMBL3938686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194190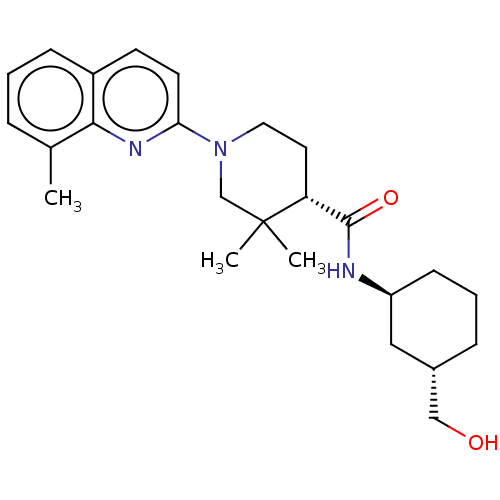 (CHEMBL3956184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194190 (CHEMBL3956184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233277 (CHEMBL4085873) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233278 (CHEMBL4064335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233281 (CHEMBL4084995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233283 (CHEMBL4102262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233285 (CHEMBL4101413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233283 (CHEMBL4102262) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194141 (CHEMBL3938686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194138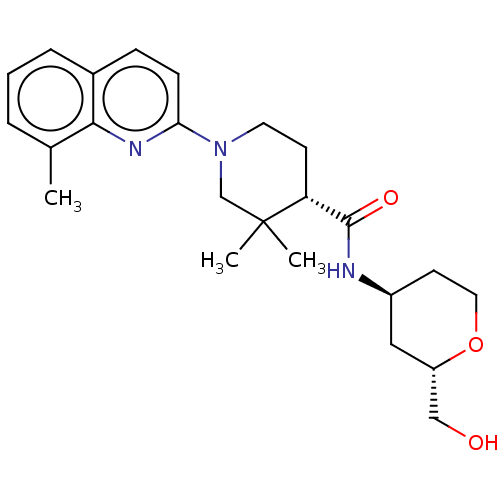 (CHEMBL3928608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194141 (CHEMBL3938686) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233274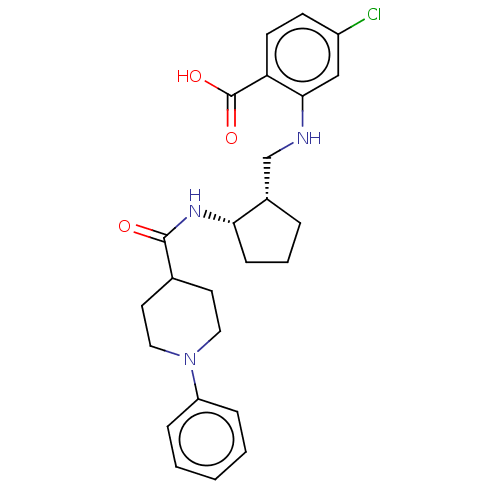 (CHEMBL4067045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233276 (CHEMBL4078000) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233280 (CHEMBL4063350) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233284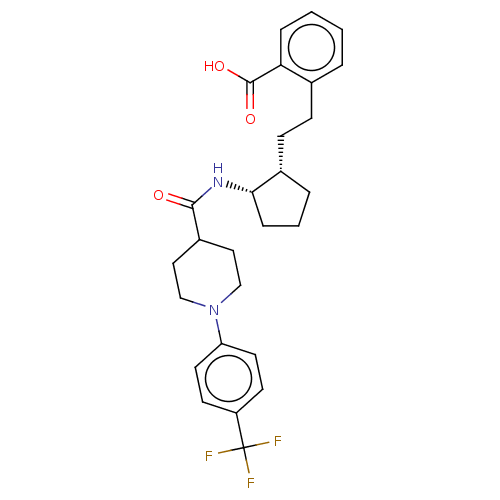 (CHEMBL4071976) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194190 (CHEMBL3956184) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194140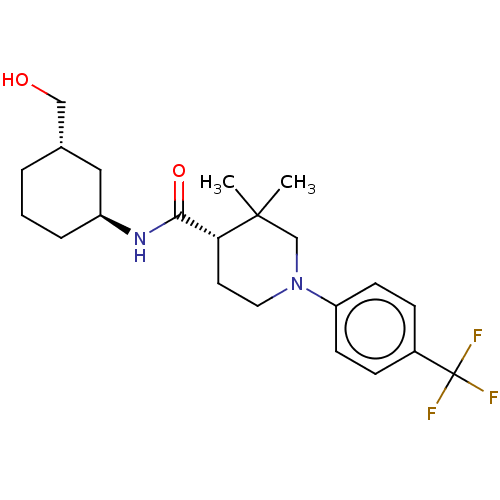 (CHEMBL3947494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233275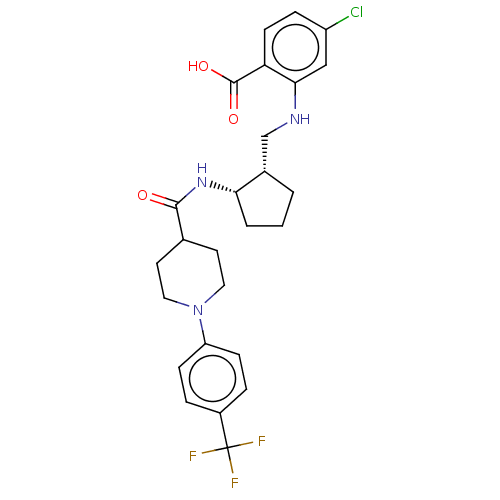 (CHEMBL4094518) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233277 (CHEMBL4085873) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233275 (CHEMBL4094518) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human A549 cells assessed as reduction in IL-1beta-induced PGE2 production preincubated for 30 mins followed by IL-1beta addi... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194137 (CHEMBL3922684) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233275 (CHEMBL4094518) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194138 (CHEMBL3928608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194140 (CHEMBL3947494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441819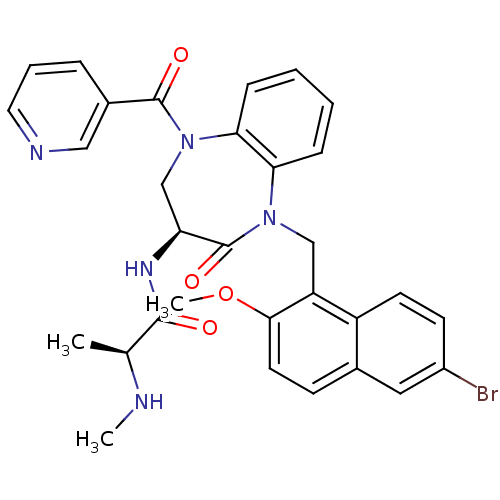 (CHEMBL2436209 | US10053431, 89b) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233284 (CHEMBL4071976) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194193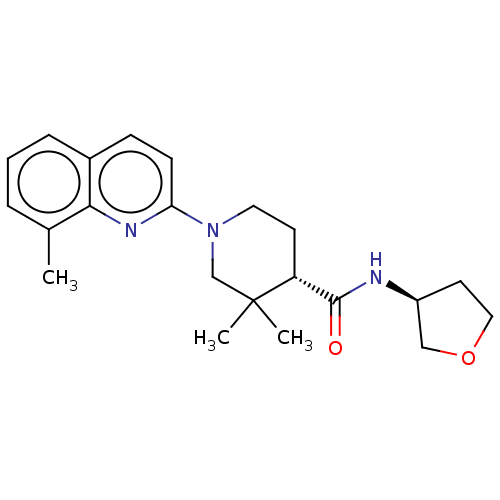 (CHEMBL3926051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441818 (CHEMBL2436213 | US10053431, 75d) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441831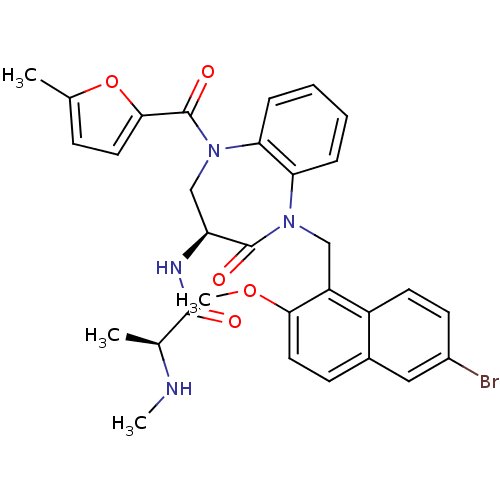 (CHEMBL2436212 | US10053431, 91c) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441816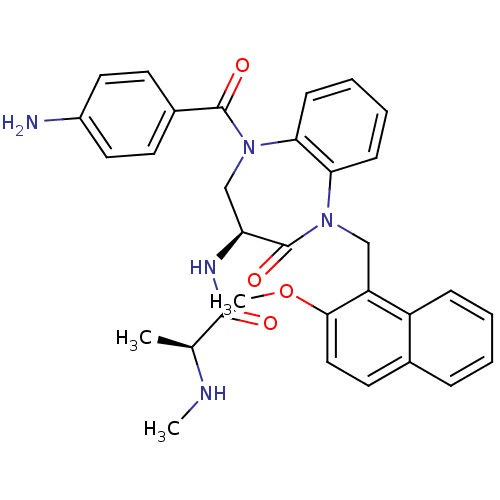 (CHEMBL2436208 | US10053431, 41) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441832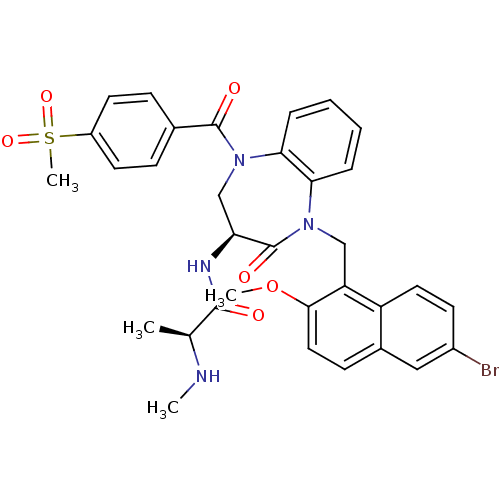 (CHEMBL2436205 | US10053431, 90d) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441833 (CHEMBL2436334 | US10053431, 75b) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441834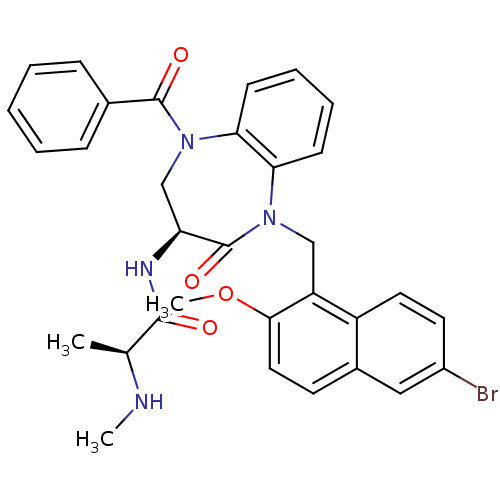 (CHEMBL2436330 | US10053431, 91a) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233279 (CHEMBL4092750) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES1 expressed in HEK293 microsomes assessed as reduction in PGE2 production using PGH2 as substrate after 2.5 mins by LC-MS an... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233285 (CHEMBL4101413) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233278 (CHEMBL4064335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194138 (CHEMBL3928608) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS stimulation ... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194192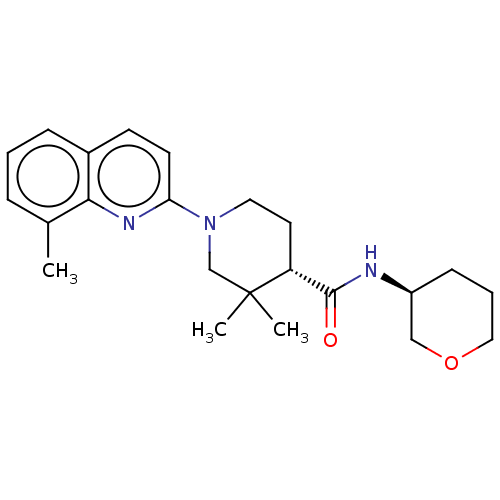 (CHEMBL3910746) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human mPGES-1 expressed in 293E cells assessed as reduction in conversion of PGH2 to PGE2 after 1.5 min by LC/MS analysis | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441830 (CHEMBL2436210 | US10053431, 85) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441829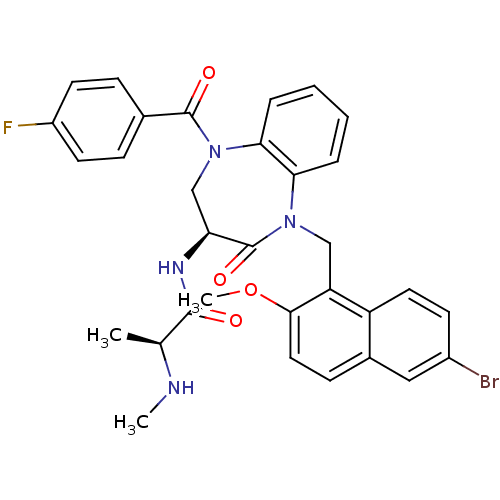 (CHEMBL2436331 | US10053431, 75a) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233276 (CHEMBL4078000) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441827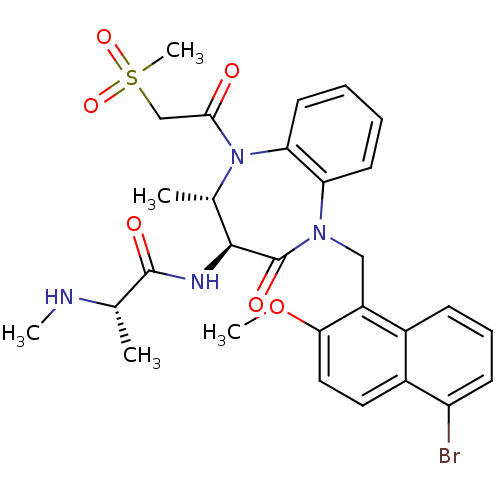 (CHEMBL2436217 | US9422331, 11) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441810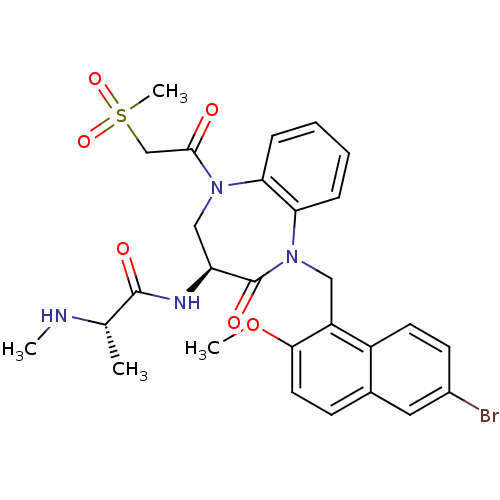 (CHEMBL2436215 | US10053431, 76b) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441828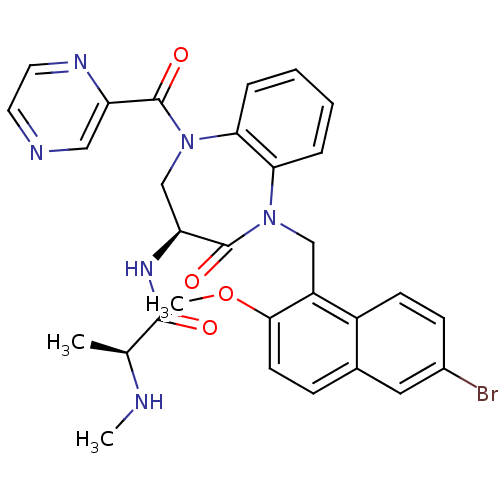 (CHEMBL2436211 | US10053431, 76c) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441821 (CHEMBL2436223 | US9422331, 27) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441826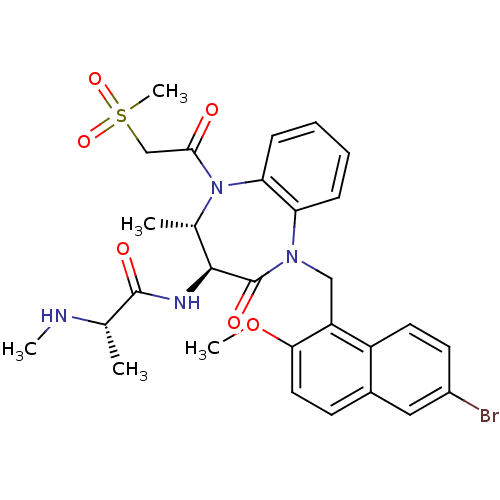 (CHEMBL2436216 | US9422331, 10) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50233281 (CHEMBL4084995) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES1 in human whole blood assessed as reduction in LPS-induced PGE2 production preincubated for 30 mins followed by LPS addition meas... | Bioorg Med Chem Lett 27: 1478-1483 (2017) Article DOI: 10.1016/j.bmcl.2016.11.011 BindingDB Entry DOI: 10.7270/Q28P62RZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Canis familiaris) | BDBM50194138 (CHEMBL3928608) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of dog mPGES-1 | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50194140 (CHEMBL3947494) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mPGES-1 in human A549 cells assessed as reduction in recombinant human interleukin-1 beta-induced PGE2 production preincubated for 30 m... | Bioorg Med Chem Lett 26: 4824-4828 (2016) Article DOI: 10.1016/j.bmcl.2016.08.023 BindingDB Entry DOI: 10.7270/Q2125VMX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 260 total ) | Next | Last >> |