

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431807 (CHEMBL2347041) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 458 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50147009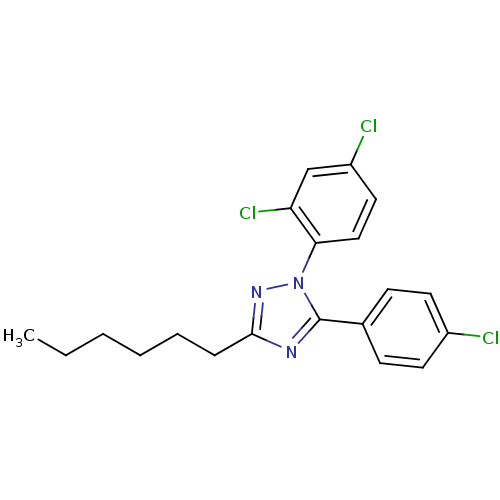 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-3-hexy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 829 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431808 (CHEMBL2347040) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50147009 (5-(4-Chloro-phenyl)-1-(2,4-dichloro-phenyl)-3-hexy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | 3.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50431803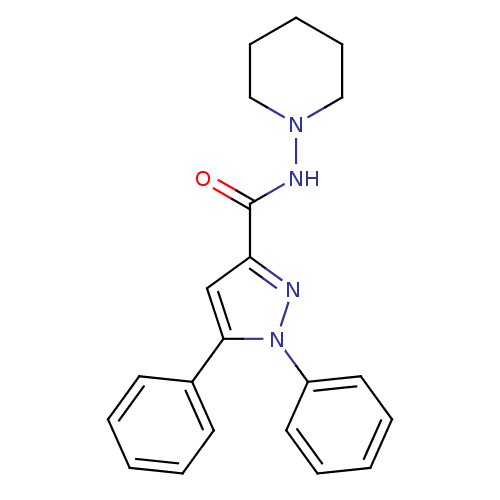 (CHEMBL2347045) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.59E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50431808 (CHEMBL2347040) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 6.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50431800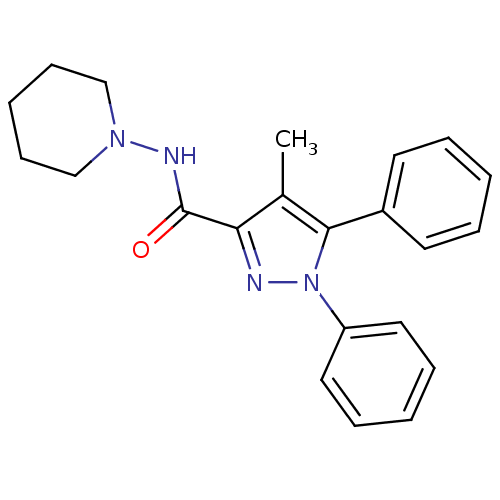 (CHEMBL2347048) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431804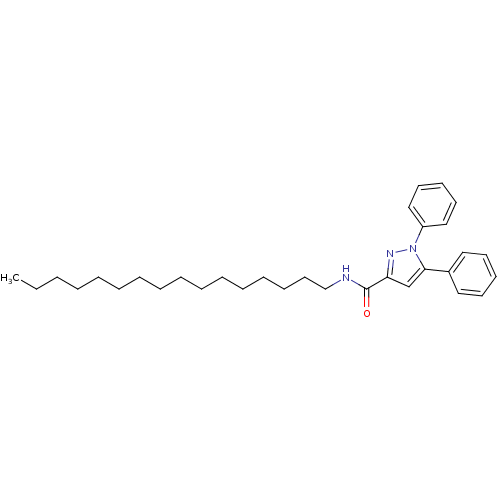 (CHEMBL2347044) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50431798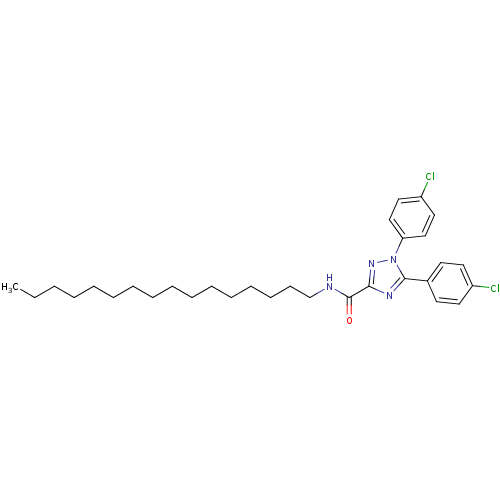 (CHEMBL2347050) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431801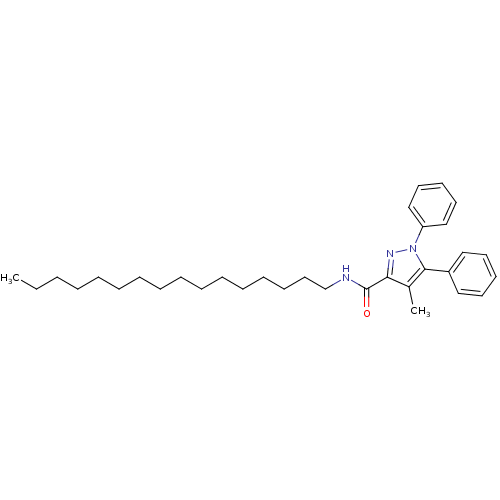 (CHEMBL2347047) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50431801 (CHEMBL2347047) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431798 (CHEMBL2347050) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431800 (CHEMBL2347048) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.82E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431803 (CHEMBL2347045) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50431807 (CHEMBL2347041) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50431806 (CHEMBL2347042) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50431805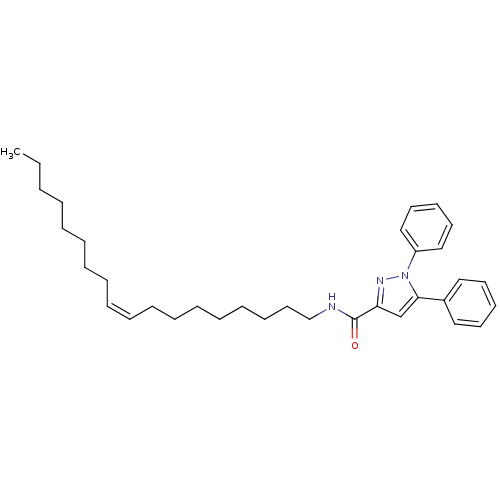 (CHEMBL2347043) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50431804 (CHEMBL2347044) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50431802 (CHEMBL2347046) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50431799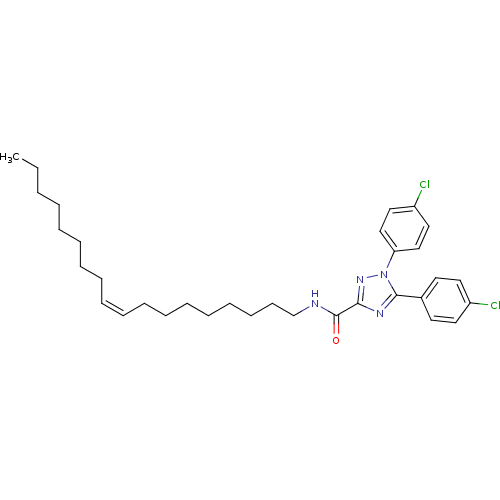 (CHEMBL2347049) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431806 (CHEMBL2347042) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431805 (CHEMBL2347043) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431802 (CHEMBL2347046) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50431799 (CHEMBL2347049) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica Curated by ChEMBL | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor after 90 mins by liquid scintillation spectrophotometer analysis | Bioorg Med Chem 21: 1708-16 (2013) Article DOI: 10.1016/j.bmc.2013.01.055 BindingDB Entry DOI: 10.7270/Q2MG7QW1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447027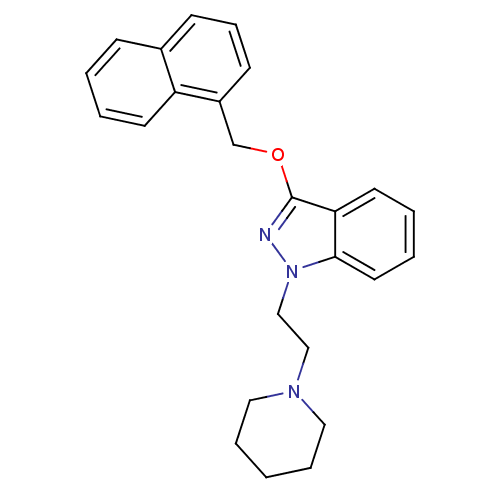 (CHEMBL3116284) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 910 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447026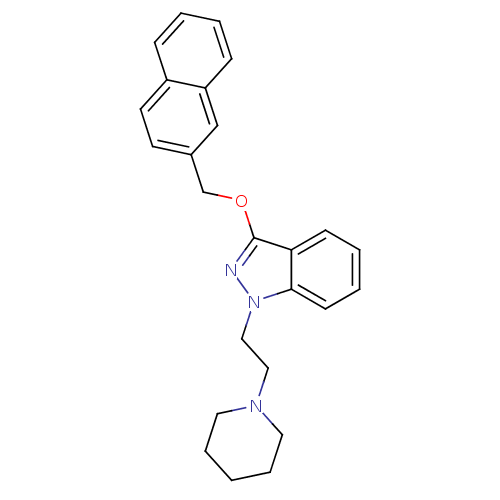 (CHEMBL3116286) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447015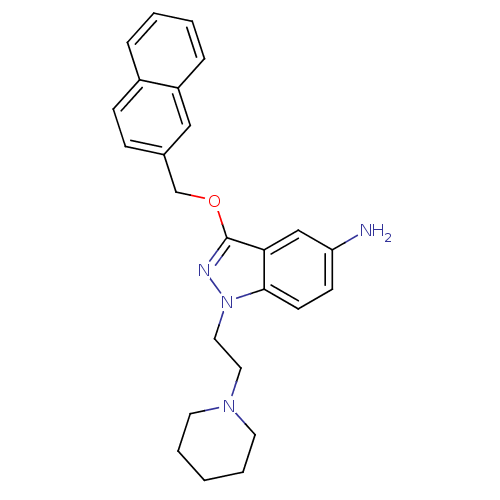 (CHEMBL3116300) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447020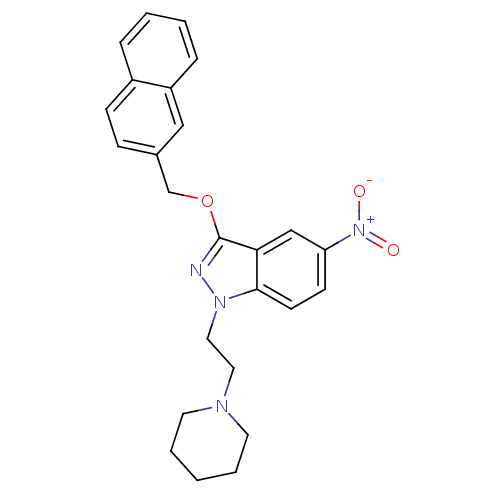 (CHEMBL3116294) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447030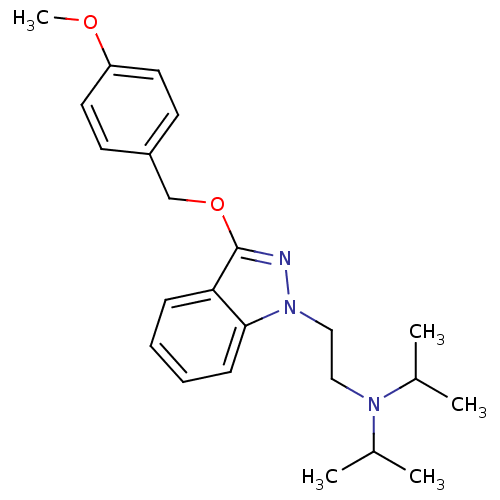 (CHEMBL3116280) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447028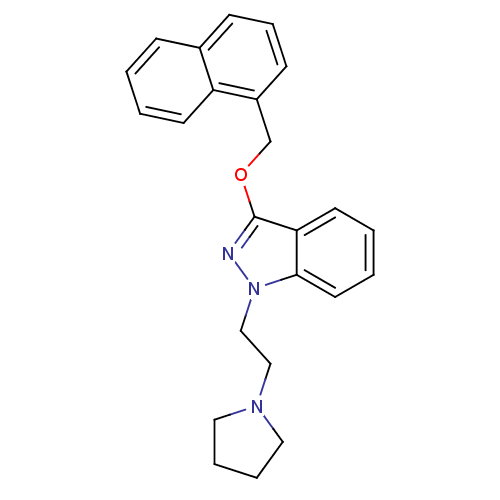 (CHEMBL3116283) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447021 (CHEMBL3116293) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447022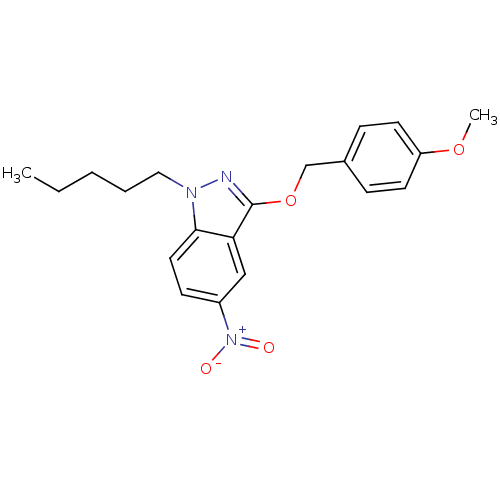 (CHEMBL3116289) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447023 (CHEMBL1973869) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447024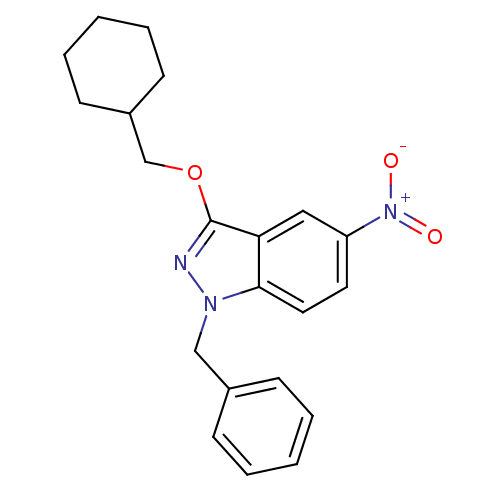 (CHEMBL3116288) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447018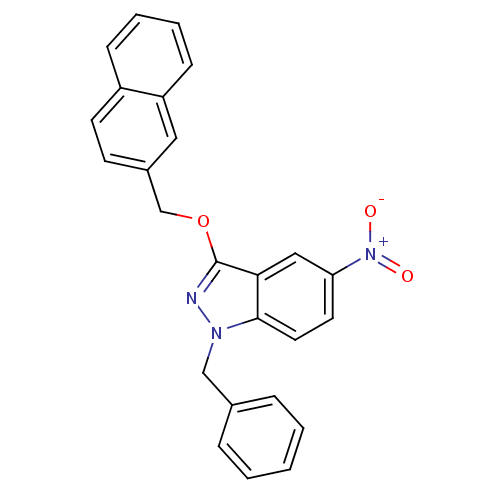 (CHEMBL3116296) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447016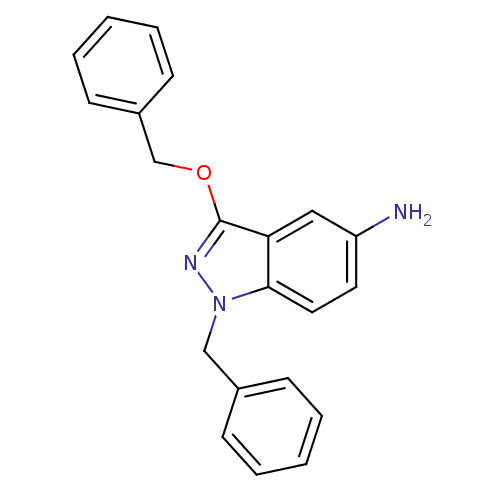 (CHEMBL3116298) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447029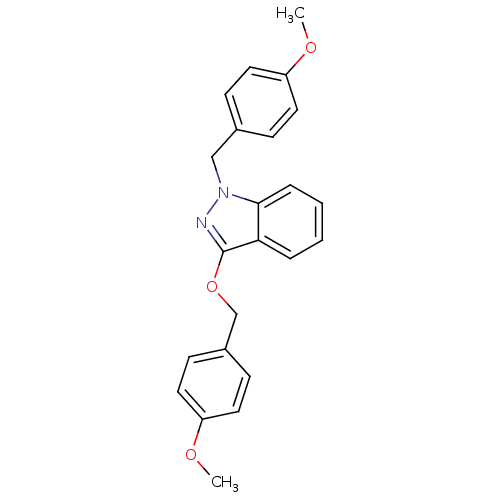 (CHEMBL3116281) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447031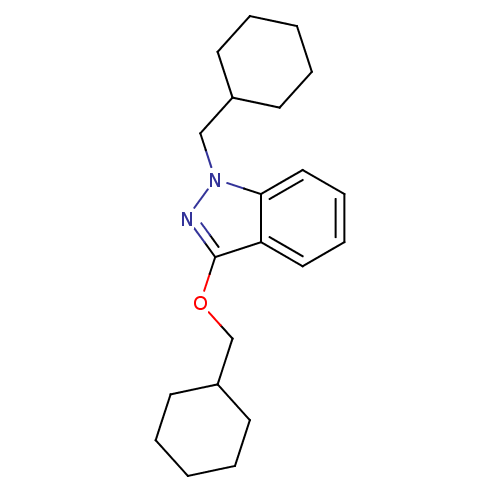 (CHEMBL3116278) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447017 (CHEMBL3116297) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447019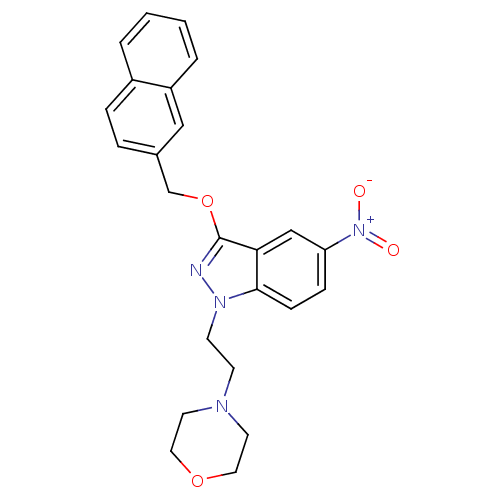 (CHEMBL3116295) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50447025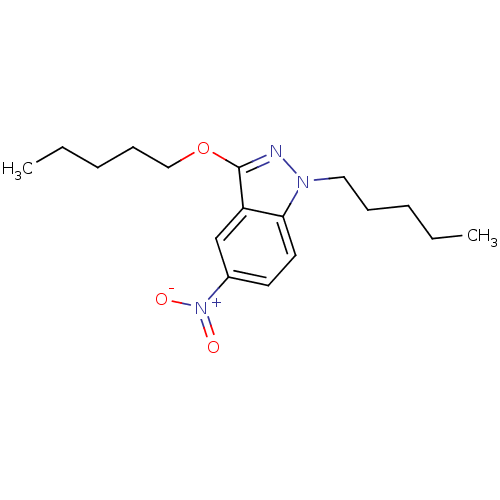 (CHEMBL3116287) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibition of horse serum BuChE using butyrylthiocholine as substrate by Ellman's method | Eur J Med Chem 73: 56-72 (2014) Article DOI: 10.1016/j.ejmech.2013.11.026 BindingDB Entry DOI: 10.7270/Q2474CC8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||