

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2Y purinoceptor 1 (Homo sapiens (Human)) | BDBM50274638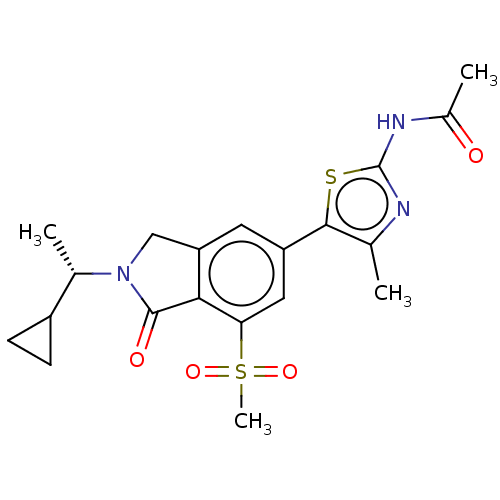 (CHEMBL4126156 | US10858355, Example 4) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | <1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Binding affinity to human P2Y1 receptor | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274648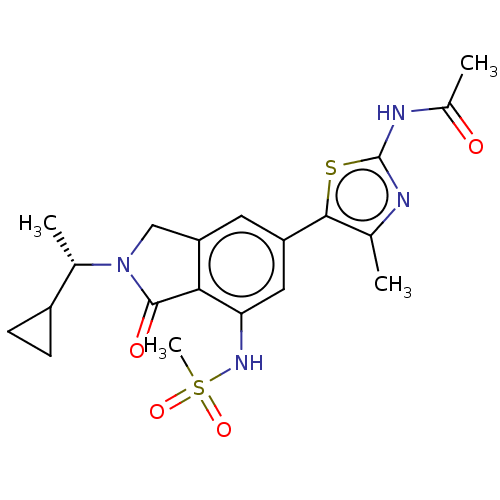 (CHEMBL4127396 | US10858355, Example 22) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274640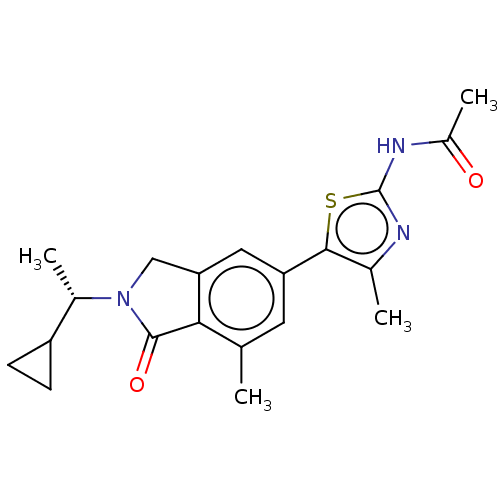 (CHEMBL4126445) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274660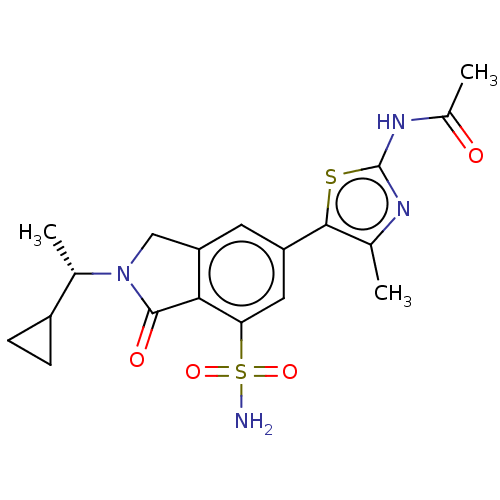 (CHEMBL4128537 | US10858355, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274675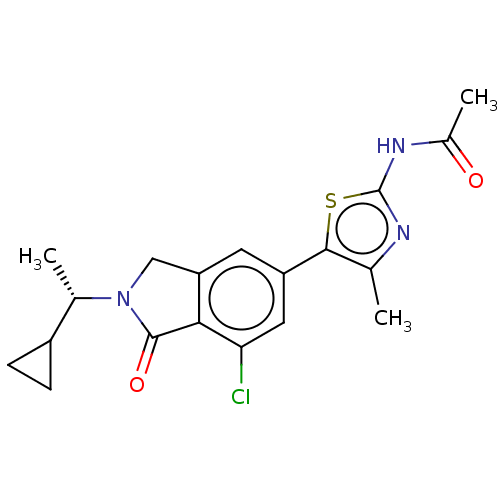 (CHEMBL4126601) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274649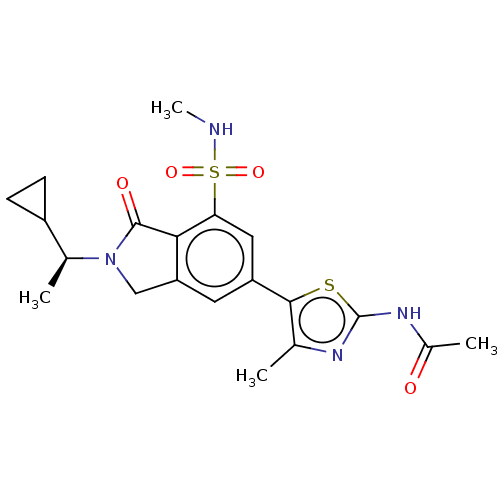 (CHEMBL4129600 | US10858355, Example 30) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274641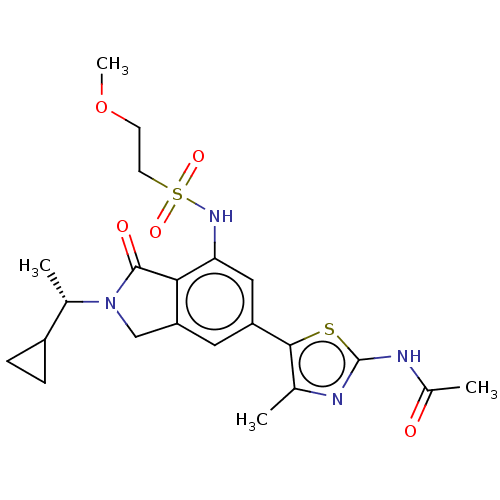 (CHEMBL4126010 | US10858355, Example 27) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274639 (CHEMBL4128230 | US10858355, Example 18) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274657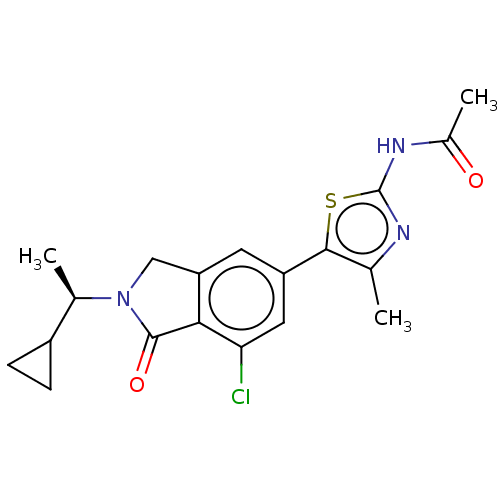 (CHEMBL4127833) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274642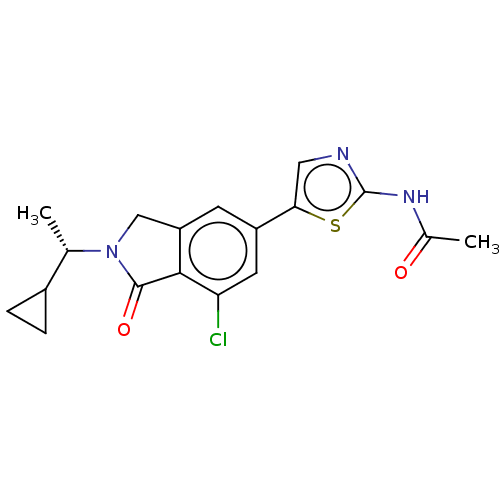 (CHEMBL4129877) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274655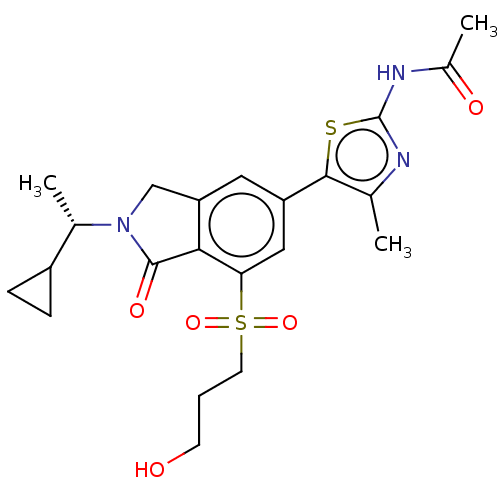 (CHEMBL4129457) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50049478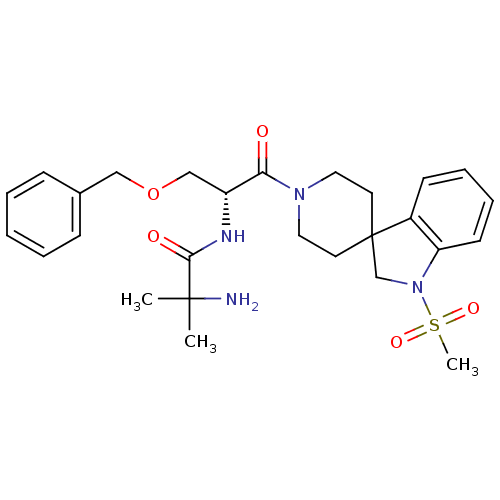 (1-{[(2R)-3-(benzyloxy)-1-{1-methanesulfonyl-1,2-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of human [125I]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes after 1 hr by radioligand binding assay | J Med Chem 61: 5974-5987 (2018) Article DOI: 10.1021/acs.jmedchem.8b00322 BindingDB Entry DOI: 10.7270/Q2WD433X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM25330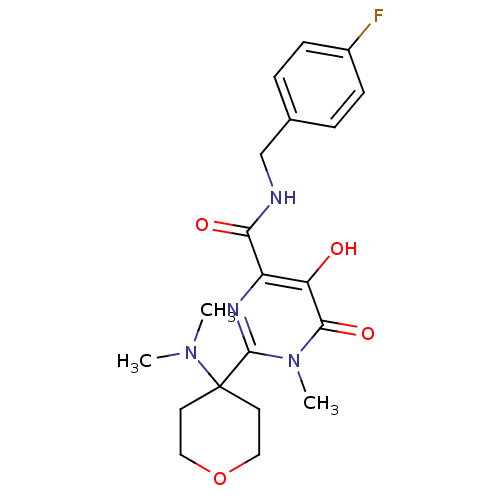 (2-[4-(dimethylamino)oxan-4-yl]-N-[(4-fluorophenyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Merck Research Laboratories | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 5843-55 (2008) Article DOI: 10.1021/jm800245z BindingDB Entry DOI: 10.7270/Q2QJ7FMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50049478 (1-{[(2R)-3-(benzyloxy)-1-{1-methanesulfonyl-1,2-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of human [125I]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes after 1 hr by radioligand binding assay | J Med Chem 61: 5974-5987 (2018) Article DOI: 10.1021/acs.jmedchem.8b00322 BindingDB Entry DOI: 10.7270/Q2WD433X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Mus musculus (Mouse)) | BDBM50274648 (CHEMBL4127396 | US10858355, Example 22) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22682 (Bicyclic pyrimidinone, 15b | N-[(4-fluorophenyl)me...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 37 |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM25329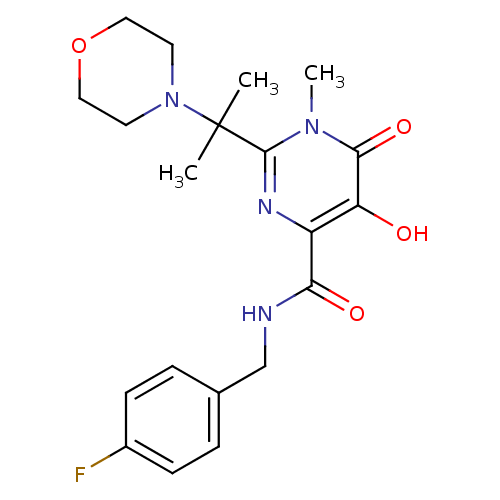 (N-[(4-fluorophenyl)methyl]-5-hydroxy-1-methyl-2-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Merck Research Laboratories | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 5843-55 (2008) Article DOI: 10.1021/jm800245z BindingDB Entry DOI: 10.7270/Q2QJ7FMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM25343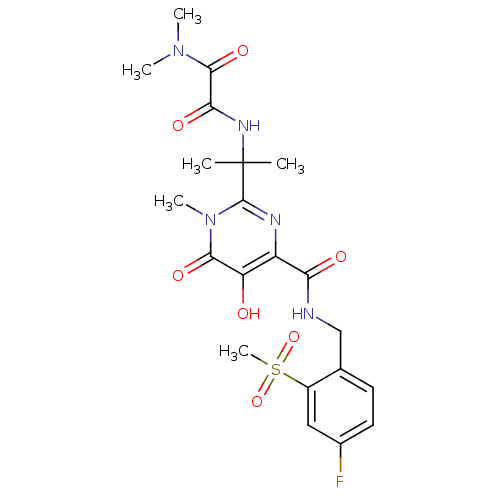 (N-[2-(4-{[(4-fluoro-2-methanesulfonylphenyl)methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Merck Research Laboratories | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 5843-55 (2008) Article DOI: 10.1021/jm800245z BindingDB Entry DOI: 10.7270/Q2QJ7FMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM25337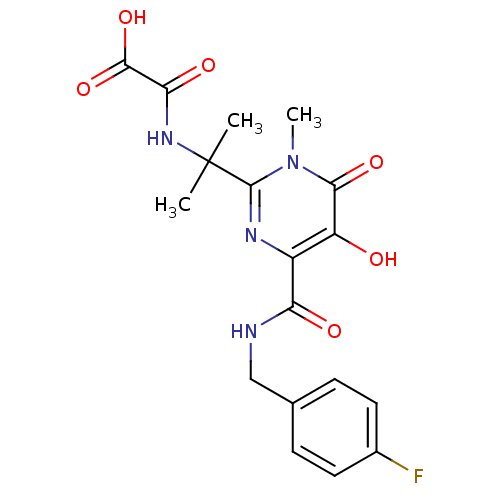 (N-methyl-pyrimidinone derivative, 13 | {[2-(4-{[(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Merck Research Laboratories | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 5843-55 (2008) Article DOI: 10.1021/jm800245z BindingDB Entry DOI: 10.7270/Q2QJ7FMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22673 (9-[benzyl(methyl)amino]-N-[(4-fluorophenyl)methyl]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 37 |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22641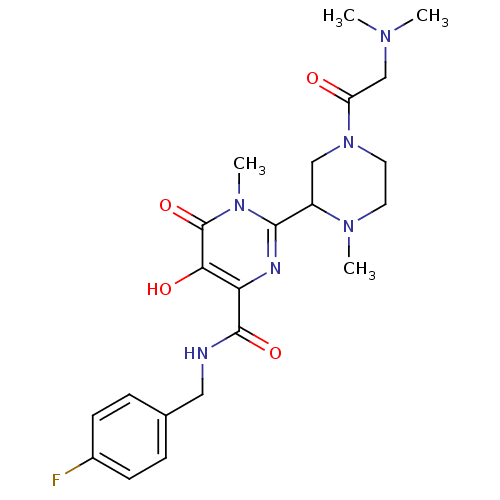 (2-{4-[2-(dimethylamino)acetyl]-1-methylpiperazin-2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 50: 4953-75 (2007) Article DOI: 10.1021/jm0704705 BindingDB Entry DOI: 10.7270/Q2BZ64BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22670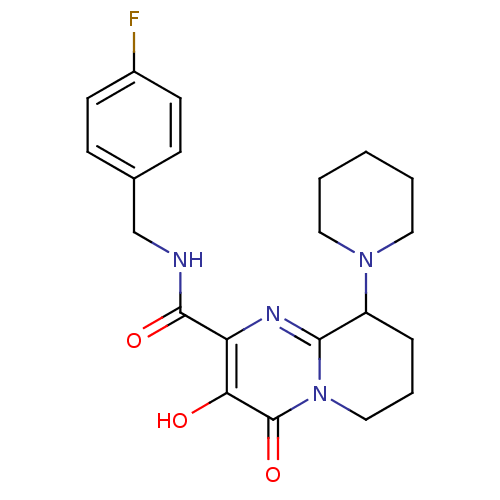 (Bicyclic pyrimidinone, 9b | N-[(4-fluorophenyl)met...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 37 |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480787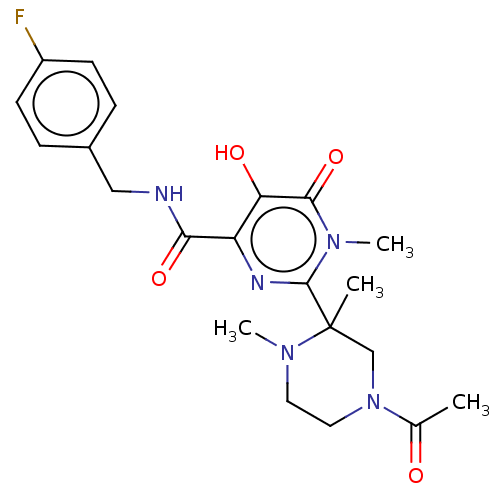 (CHEMBL549592) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 integrase strand transfer activity | Bioorg Med Chem Lett 19: 4617-21 (2009) Article DOI: 10.1016/j.bmcl.2009.06.091 BindingDB Entry DOI: 10.7270/Q24F1TJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50280783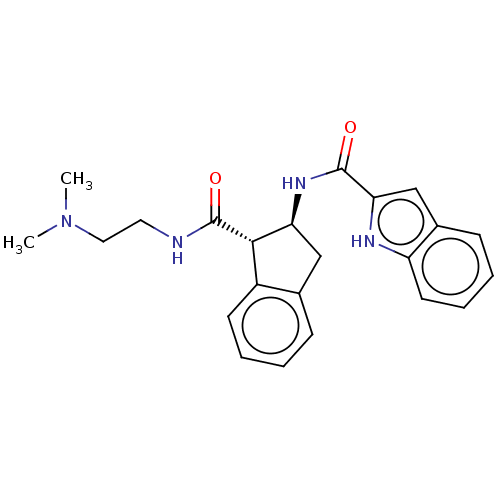 (CHEMBL4164328) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description In vitro inhibition of dihydrofolate reductase enzymes in Neisseria gonorrhoeae | J Med Chem 61: 5974-5987 (2018) Article DOI: 10.1021/acs.jmedchem.8b00322 BindingDB Entry DOI: 10.7270/Q2WD433X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50280783 (CHEMBL4164328) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of human [125I]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes after 1 hr by radioligand binding assay | J Med Chem 61: 5974-5987 (2018) Article DOI: 10.1021/acs.jmedchem.8b00322 BindingDB Entry DOI: 10.7270/Q2WD433X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50544339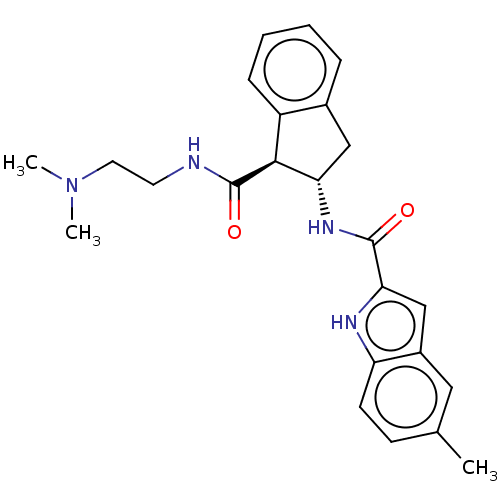 (CHEMBL4639379) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [His[125I]]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | J Med Chem 63: 9705-9730 (2020) Article DOI: 10.1021/acs.jmedchem.0c00828 BindingDB Entry DOI: 10.7270/Q2445R3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50544339 (CHEMBL4639379) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [His[125I]]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | J Med Chem 63: 9705-9730 (2020) Article DOI: 10.1021/acs.jmedchem.0c00828 BindingDB Entry DOI: 10.7270/Q2445R3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM25352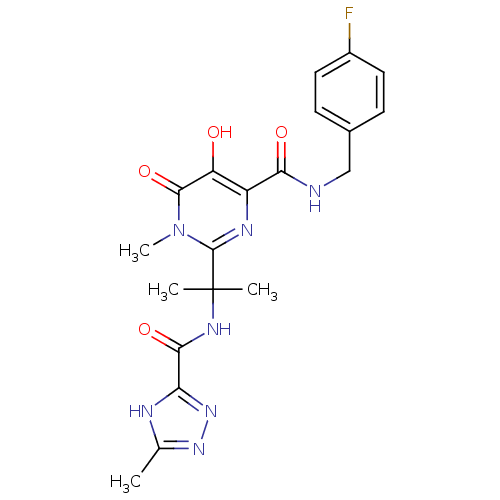 (N-[(4-fluorophenyl)methyl]-5-hydroxy-1-methyl-2-{2...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Merck Research Laboratories | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 5843-55 (2008) Article DOI: 10.1021/jm800245z BindingDB Entry DOI: 10.7270/Q2QJ7FMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50544341 (CHEMBL4647797) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [His[125I]]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | J Med Chem 63: 9705-9730 (2020) Article DOI: 10.1021/acs.jmedchem.0c00828 BindingDB Entry DOI: 10.7270/Q2445R3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Mus musculus (Mouse)) | BDBM50274649 (CHEMBL4129600 | US10858355, Example 30) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22676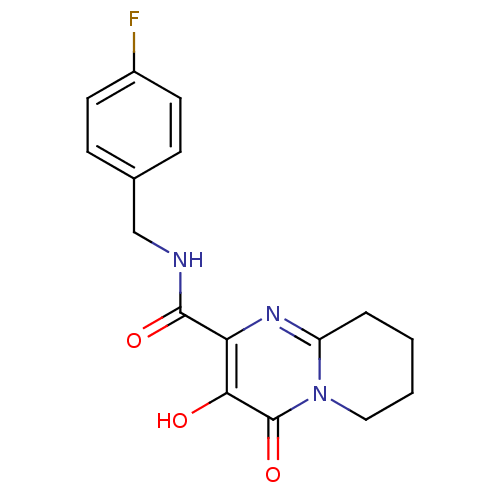 (Bicyclic pyrimidinone, 12b | N-[(4-fluorophenyl)me...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.8 | 37 |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22696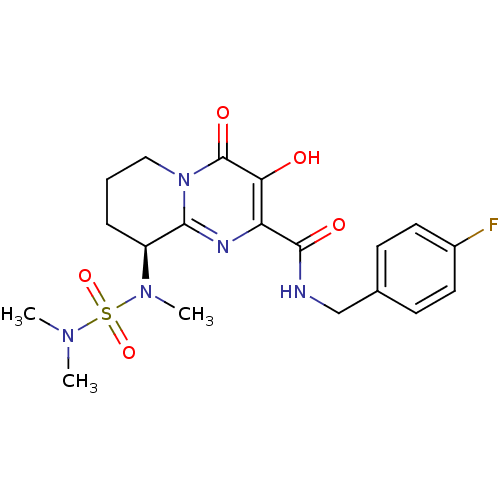 ((9S)-9-[(dimethylsulfamoyl)(methyl)amino]-N-[(4-fl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22694 (9-[(dimethylsulfamoyl)(methyl)amino]-N-[(4-fluorop...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50544340 (CHEMBL4637980) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [His[125I]]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | J Med Chem 63: 9705-9730 (2020) Article DOI: 10.1021/acs.jmedchem.0c00828 BindingDB Entry DOI: 10.7270/Q2445R3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22671 (Bicyclic pyrimidinone, 10b | N-[(4-fluorophenyl)me...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.8 | 37 |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50544341 (CHEMBL4647797) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [His[125I]]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | J Med Chem 63: 9705-9730 (2020) Article DOI: 10.1021/acs.jmedchem.0c00828 BindingDB Entry DOI: 10.7270/Q2445R3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Growth hormone secretagogue receptor type 1 (Homo sapiens (Human)) | BDBM50544340 (CHEMBL4637980) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [His[125I]]-ghrelin from GHS-R1a (unknown origin) expressed in HEK293 cell membranes measured after 1 hr by microbeta counting method | J Med Chem 63: 9705-9730 (2020) Article DOI: 10.1021/acs.jmedchem.0c00828 BindingDB Entry DOI: 10.7270/Q2445R3K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22687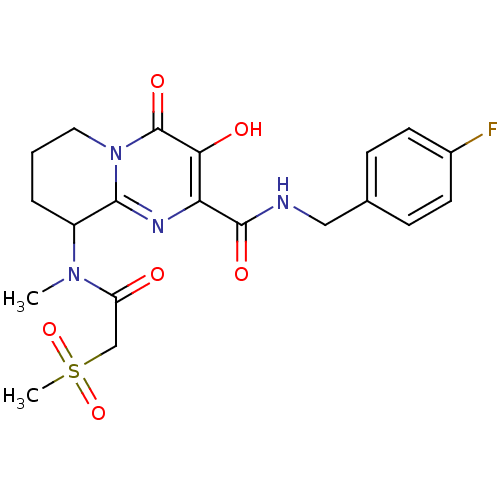 (Bicyclic pyrimidinone, 18b | N-[(4-fluorophenyl)me...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.8 | 37 |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM22638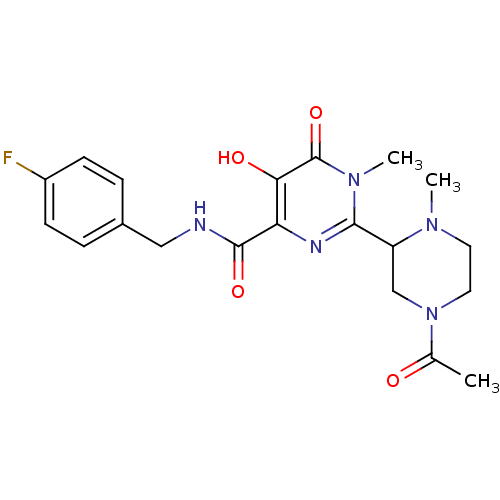 (2-(4-acetyl-1-methylpiperazin-2-yl)-N-[(4-fluoroph...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome Curated by ChEMBL | Assay Description Inhibition of recombinant HIV-1 integrase strand transfer activity | Bioorg Med Chem Lett 19: 4617-21 (2009) Article DOI: 10.1016/j.bmcl.2009.06.091 BindingDB Entry DOI: 10.7270/Q24F1TJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22679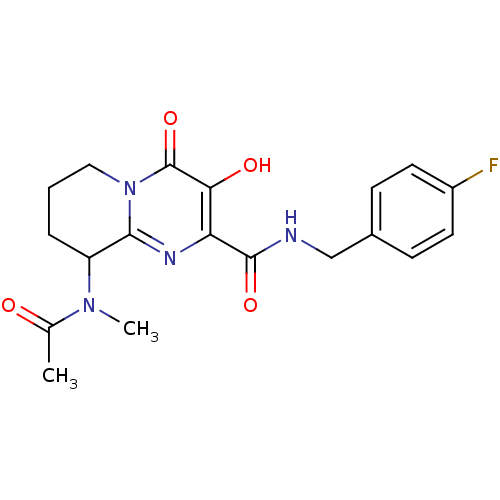 (Bicyclic pyrimidinone, 13b | N-[(4-fluorophenyl)me...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.8 | 37 |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22699 (Bicyclic pyrimidinone, 23c | N-[(4-fluorophenyl)me...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM25350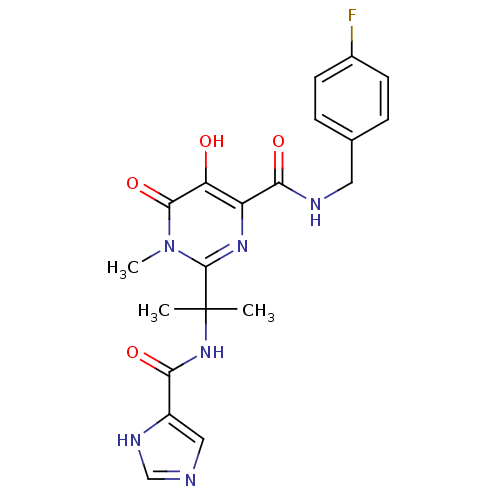 (N-[(4-fluorophenyl)methyl]-5-hydroxy-2-[2-(1H-imid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.8 | 37 |
Merck Research Laboratories | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 5843-55 (2008) Article DOI: 10.1021/jm800245z BindingDB Entry DOI: 10.7270/Q2QJ7FMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Homo sapiens (Human)) | BDBM50274656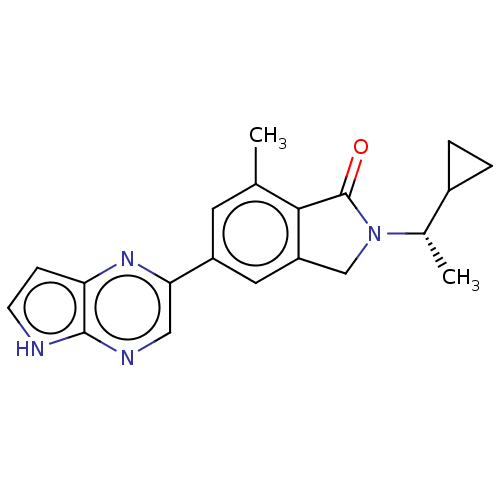 (CHEMBL4128652) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human 6His-tagged PI3Kgamma (144 to 1102 residues) using DiC8-PIP2 as substrate preincubated for 10 mins followed by substr... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Mus musculus (Mouse)) | BDBM50274640 (CHEMBL4126445) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Mus musculus (Mouse)) | BDBM50274641 (CHEMBL4126010 | US10858355, Example 27) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform (Mus musculus (Mouse)) | BDBM50274675 (CHEMBL4126601) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaron-Beijing Co., Ltd Curated by ChEMBL | Assay Description Inhibition of PI3Kgamma in mouse RAW264 cells assessed as reduction in Akt phosphorylation at Ser473 residue preincubated for 15 mins followed by C5a... | J Med Chem 61: 5435-5441 (2018) Article DOI: 10.1021/acs.jmedchem.8b00447 BindingDB Entry DOI: 10.7270/Q27M0BFZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22644 (2-[4-(benzenesulfonyl)-1-methylpiperazin-2-yl]-N-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 50: 4953-75 (2007) Article DOI: 10.1021/jm0704705 BindingDB Entry DOI: 10.7270/Q2BZ64BS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22666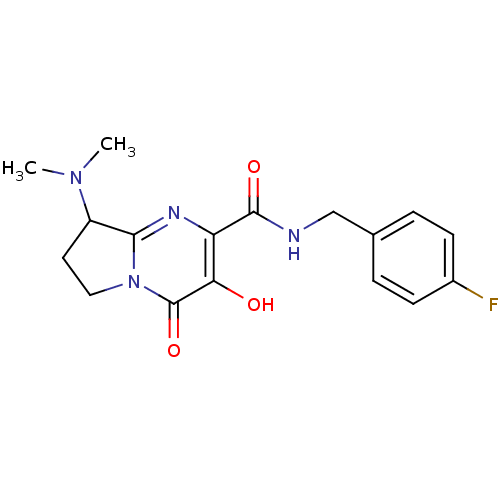 (8-(dimethylamino)-N-[(4-fluorophenyl)methyl]-3-hyd...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.8 | 37 |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22685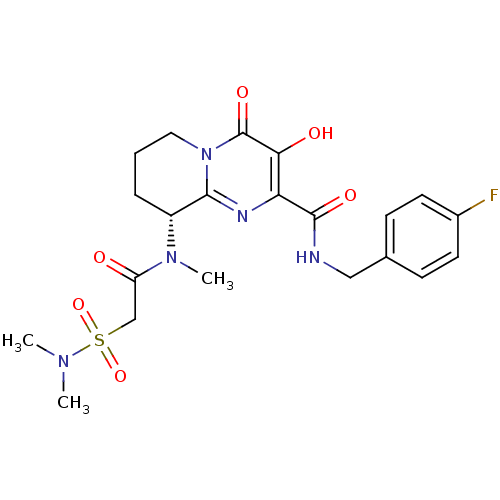 ((9R)-9-[2-(dimethylsulfamoyl)-N-methylacetamido]-N...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.8 | 37 |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein (Human immunodeficiency virus type 1 group M subtyp...) | BDBM22686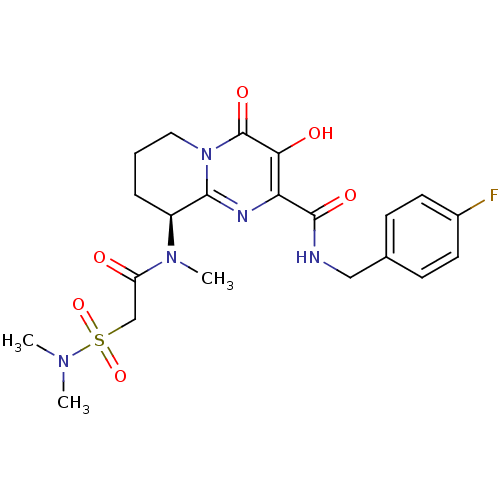 ((9S)-9-[2-(dimethylsulfamoyl)-N-methylacetamido]-N...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.8 | 37 |
IRBM-MRL | Assay Description The microtiter plate assay for stand transfer was performed with an immobilized donor substrate and a target substrate biotinylated at the 3-prime en... | J Med Chem 51: 861-74 (2008) Article DOI: 10.1021/jm701164t BindingDB Entry DOI: 10.7270/Q2765CNT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 661 total ) | Next | Last >> |