 Found 30 hits with Last Name = 'gardner' and Initial = 'ls'
Found 30 hits with Last Name = 'gardner' and Initial = 'ls' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Hexokinase-4
(Rattus norvegicus) | BDBM50161668
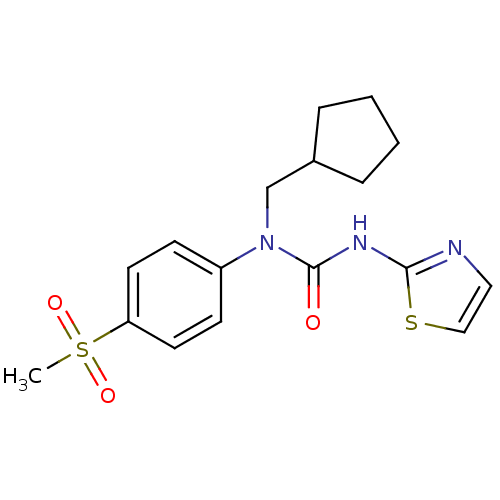
(1-Cyclopentylmethyl-1-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(CC1CCCC1)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H21N3O3S2/c1-25(22,23)15-8-6-14(7-9-15)20(12-13-4-2-3-5-13)17(21)19-16-18-10-11-24-16/h6-11,13H,2-5,12H2,1H3,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161692
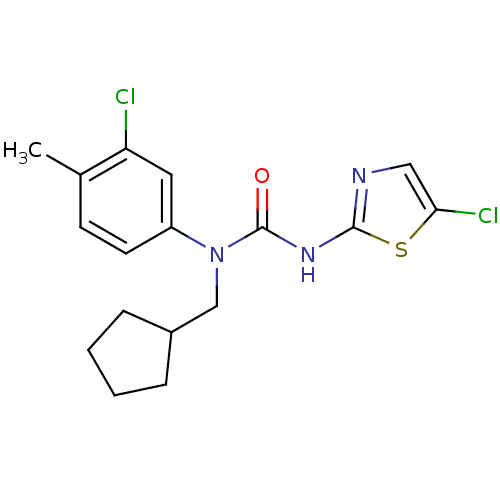
(1-(3-Chloro-4-methyl-phenyl)-3-(5-chloro-thiazol-2...)Show InChI InChI=1S/C17H19Cl2N3OS/c1-11-6-7-13(8-14(11)18)22(10-12-4-2-3-5-12)17(23)21-16-20-9-15(19)24-16/h6-9,12H,2-5,10H2,1H3,(H,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 400 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161670
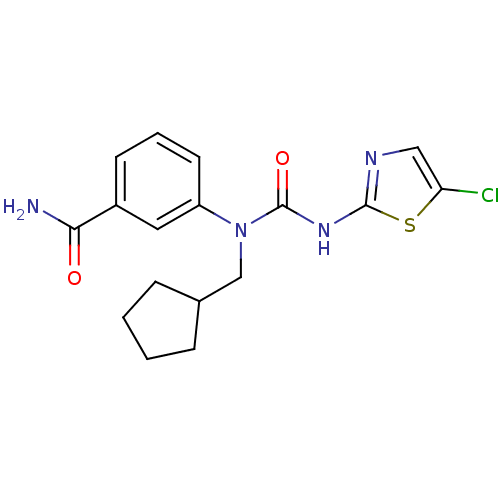
(3-[3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-u...)Show SMILES NC(=O)c1cccc(c1)N(CC1CCCC1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C17H19ClN4O2S/c18-14-9-20-16(25-14)21-17(24)22(10-11-4-1-2-5-11)13-7-3-6-12(8-13)15(19)23/h3,6-9,11H,1-2,4-5,10H2,(H2,19,23)(H,20,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161671
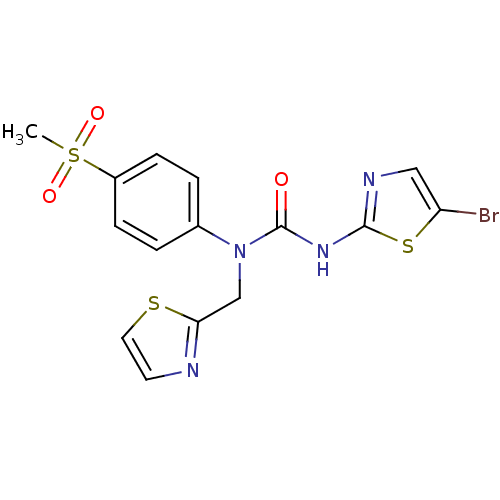
(3-(5-Bromo-thiazol-2-yl)-1-(4-methanesulfonyl-phen...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(Cc1nccs1)C(=O)Nc1ncc(Br)s1 Show InChI InChI=1S/C15H13BrN4O3S3/c1-26(22,23)11-4-2-10(3-5-11)20(9-13-17-6-7-24-13)15(21)19-14-18-8-12(16)25-14/h2-8H,9H2,1H3,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.41E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161672
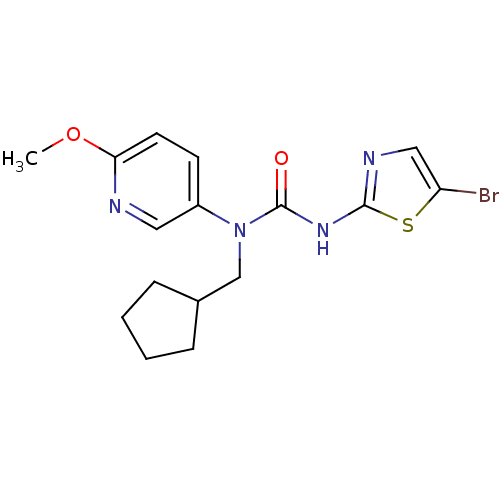
(3-(5-Bromo-thiazol-2-yl)-1-cyclopentylmethyl-1-(6-...)Show InChI InChI=1S/C16H19BrN4O2S/c1-23-14-7-6-12(8-18-14)21(10-11-4-2-3-5-11)16(22)20-15-19-9-13(17)24-15/h6-9,11H,2-5,10H2,1H3,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161673

(1-Cyclopentylmethyl-1-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(CC1CCCC1)C(=O)Nc1nnc(s1)C(F)(F)F Show InChI InChI=1S/C17H19F3N4O3S2/c1-29(26,27)13-8-6-12(7-9-13)24(10-11-4-2-3-5-11)16(25)21-15-23-22-14(28-15)17(18,19)20/h6-9,11H,2-5,10H2,1H3,(H,21,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.42E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161674
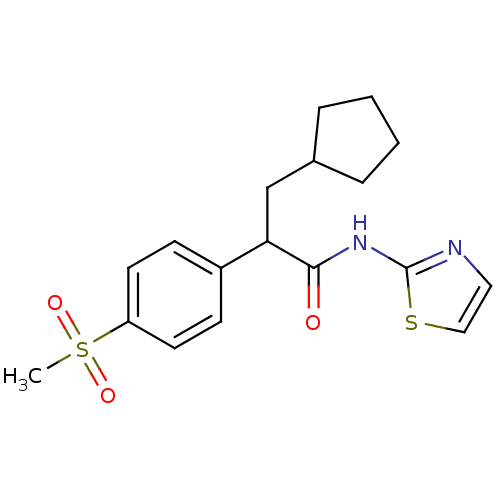
(3-Cyclopentyl-2-(4-methanesulfonyl-phenyl)-N-thiaz...)Show SMILES CS(=O)(=O)c1ccc(cc1)C(CC1CCCC1)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H22N2O3S2/c1-25(22,23)15-8-6-14(7-9-15)16(12-13-4-2-3-5-13)17(21)20-18-19-10-11-24-18/h6-11,13,16H,2-5,12H2,1H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 15 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hexokinase-4
(Rattus norvegicus) | BDBM50161676
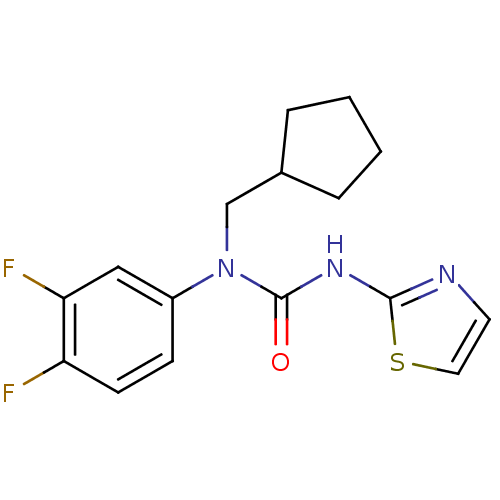
(1-Cyclopentylmethyl-1-(3,4-difluoro-phenyl)-3-thia...)Show InChI InChI=1S/C16H17F2N3OS/c17-13-6-5-12(9-14(13)18)21(10-11-3-1-2-4-11)16(22)20-15-19-7-8-23-15/h5-9,11H,1-4,10H2,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161677

(4-[3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-u...)Show SMILES NS(=O)(=O)c1ccc(cc1)N(CC1CCCC1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C16H19ClN4O3S2/c17-14-9-19-15(25-14)20-16(22)21(10-11-3-1-2-4-11)12-5-7-13(8-6-12)26(18,23)24/h5-9,11H,1-4,10H2,(H2,18,23,24)(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161675
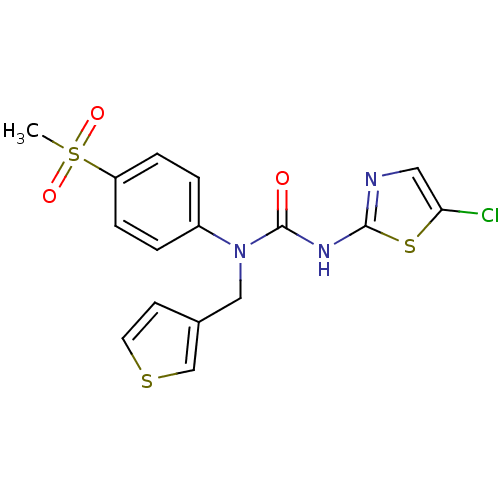
(3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(Cc1ccsc1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C16H14ClN3O3S3/c1-26(22,23)13-4-2-12(3-5-13)20(9-11-6-7-24-10-11)16(21)19-15-18-8-14(17)25-15/h2-8,10H,9H2,1H3,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161680
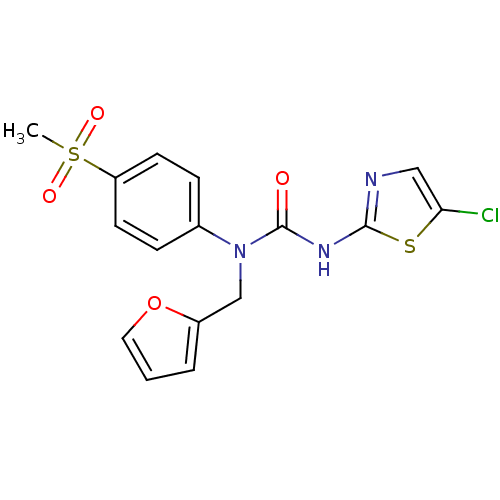
(3-(5-Chloro-thiazol-2-yl)-1-furan-2-ylmethyl-1-(4-...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(Cc1ccco1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C16H14ClN3O4S2/c1-26(22,23)13-6-4-11(5-7-13)20(10-12-3-2-8-24-12)16(21)19-15-18-9-14(17)25-15/h2-9H,10H2,1H3,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161681

(3-(5-Chloro-thiazol-2-yl)-1-(6-cyano-pyridin-3-yl)...)Show InChI InChI=1S/C16H16ClN5OS/c17-14-9-20-15(24-14)21-16(23)22(10-11-3-1-2-4-11)13-6-5-12(7-18)19-8-13/h5-6,8-9,11H,1-4,10H2,(H,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161682

(3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(3...)Show InChI InChI=1S/C17H20ClN3O2S/c1-23-14-8-4-7-13(9-14)21(11-12-5-2-3-6-12)17(22)20-16-19-10-15(18)24-16/h4,7-10,12H,2-3,5-6,11H2,1H3,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161678

(3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(6...)Show InChI InChI=1S/C16H19ClN4O2S/c1-23-14-7-6-12(8-18-14)21(10-11-4-2-3-5-11)16(22)20-15-19-9-13(17)24-15/h6-9,11H,2-5,10H2,1H3,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161679
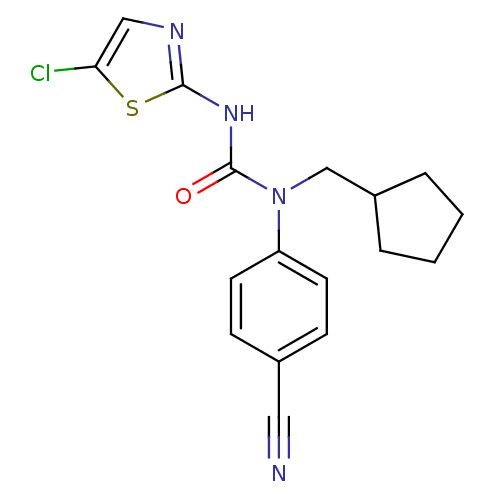
(3-(5-Chloro-thiazol-2-yl)-1-(4-cyano-phenyl)-1-cyc...)Show InChI InChI=1S/C17H17ClN4OS/c18-15-10-20-16(24-15)21-17(23)22(11-13-3-1-2-4-13)14-7-5-12(9-19)6-8-14/h5-8,10,13H,1-4,11H2,(H,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161684
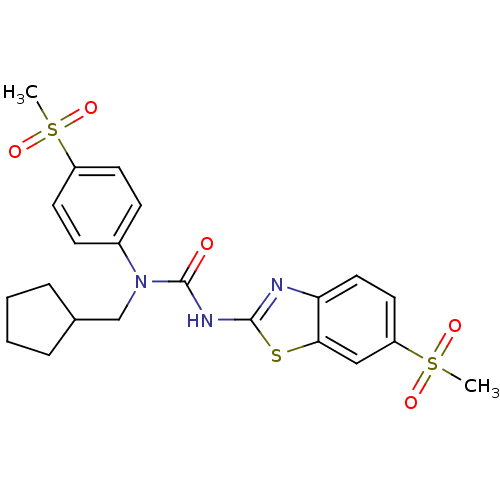
(1-Cyclopentylmethyl-3-(6-methanesulfonyl-benzothia...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(CC1CCCC1)C(=O)Nc1nc2ccc(cc2s1)S(C)(=O)=O Show InChI InChI=1S/C22H25N3O5S3/c1-32(27,28)17-9-7-16(8-10-17)25(14-15-5-3-4-6-15)22(26)24-21-23-19-12-11-18(33(2,29)30)13-20(19)31-21/h7-13,15H,3-6,14H2,1-2H3,(H,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.29E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161685
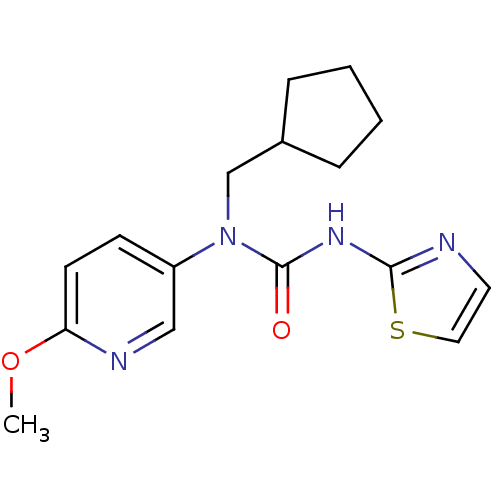
(1-Cyclopentylmethyl-1-(6-methoxy-pyridin-3-yl)-3-t...)Show InChI InChI=1S/C16H20N4O2S/c1-22-14-7-6-13(10-18-14)20(11-12-4-2-3-5-12)16(21)19-15-17-8-9-23-15/h6-10,12H,2-5,11H2,1H3,(H,17,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.92E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161683
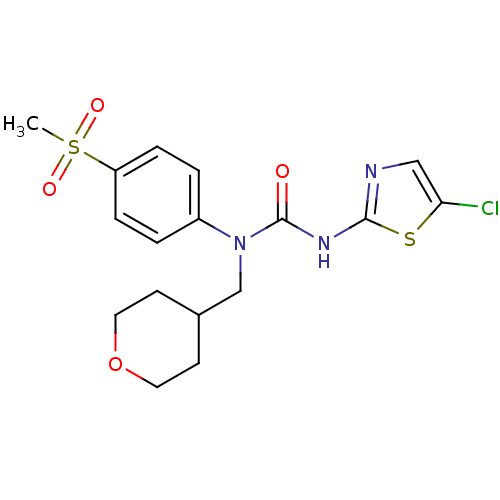
(3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(CC1CCOCC1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C17H20ClN3O4S2/c1-27(23,24)14-4-2-13(3-5-14)21(11-12-6-8-25-9-7-12)17(22)20-16-19-10-15(18)26-16/h2-5,10,12H,6-9,11H2,1H3,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161674

(3-Cyclopentyl-2-(4-methanesulfonyl-phenyl)-N-thiaz...)Show SMILES CS(=O)(=O)c1ccc(cc1)C(CC1CCCC1)C(=O)Nc1nccs1 Show InChI InChI=1S/C18H22N2O3S2/c1-25(22,23)15-8-6-14(7-9-15)16(12-13-4-2-3-5-13)17(21)20-18-19-10-11-24-18/h6-11,13,16H,2-5,12H2,1H3,(H,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Hexokinase-4
(Rattus norvegicus) | BDBM50161686

(3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(4...)Show SMILES FC(F)(F)c1ccc(cc1)N(CC1CCCC1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C17H17ClF3N3OS/c18-14-9-22-15(26-14)23-16(25)24(10-11-3-1-2-4-11)13-7-5-12(6-8-13)17(19,20)21/h5-9,11H,1-4,10H2,(H,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161687
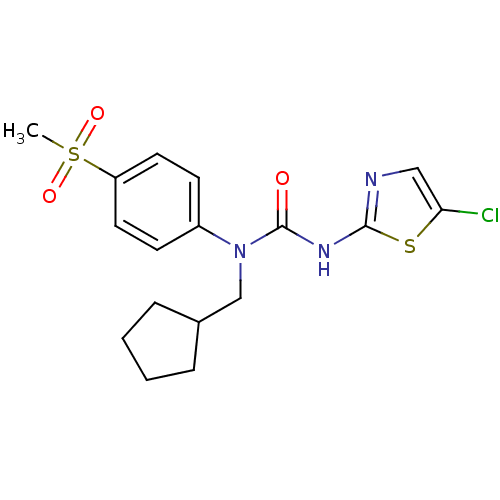
(3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(4...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(CC1CCCC1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C17H20ClN3O3S2/c1-26(23,24)14-8-6-13(7-9-14)21(11-12-4-2-3-5-12)17(22)20-16-19-10-15(18)25-16/h6-10,12H,2-5,11H2,1H3,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161688

(3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(Cc1cccs1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C16H14ClN3O3S3/c1-26(22,23)13-6-4-11(5-7-13)20(10-12-3-2-8-24-12)16(21)19-15-18-9-14(17)25-15/h2-9H,10H2,1H3,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161689
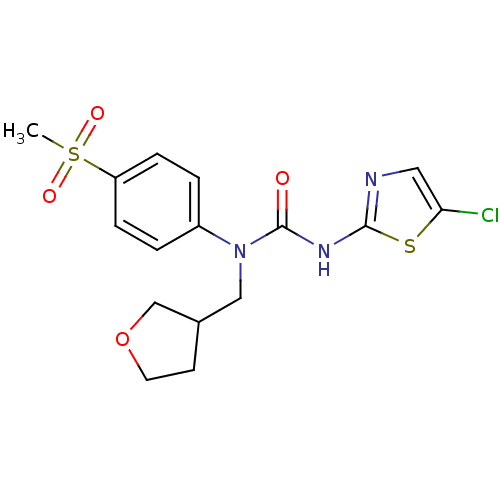
(3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(CC1CCOC1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C16H18ClN3O4S2/c1-26(22,23)13-4-2-12(3-5-13)20(9-11-6-7-24-10-11)16(21)19-15-18-8-14(17)25-15/h2-5,8,11H,6-7,9-10H2,1H3,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.19E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161690
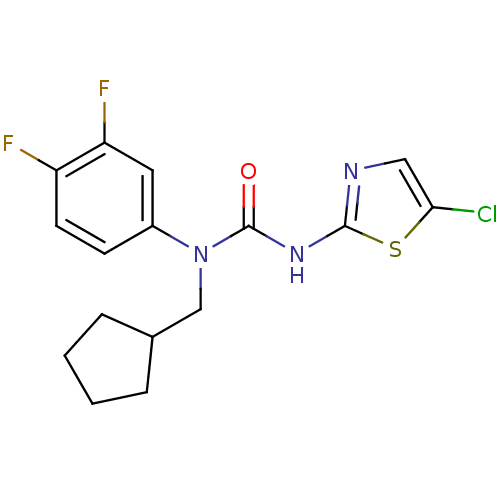
(3-(5-Chloro-thiazol-2-yl)-1-cyclopentylmethyl-1-(3...)Show InChI InChI=1S/C16H16ClF2N3OS/c17-14-8-20-15(24-14)21-16(23)22(9-10-3-1-2-4-10)11-5-6-12(18)13(19)7-11/h5-8,10H,1-4,9H2,(H,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161691

(CHEMBL180172 | N-{3-[3-(5-Chloro-thiazol-2-yl)-1-c...)Show SMILES CC(=O)Nc1cccc(c1)N(CC1CCCC1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C18H21ClN4O2S/c1-12(24)21-14-7-4-8-15(9-14)23(11-13-5-2-3-6-13)18(25)22-17-20-10-16(19)26-17/h4,7-10,13H,2-3,5-6,11H2,1H3,(H,21,24)(H,20,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 300 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161694
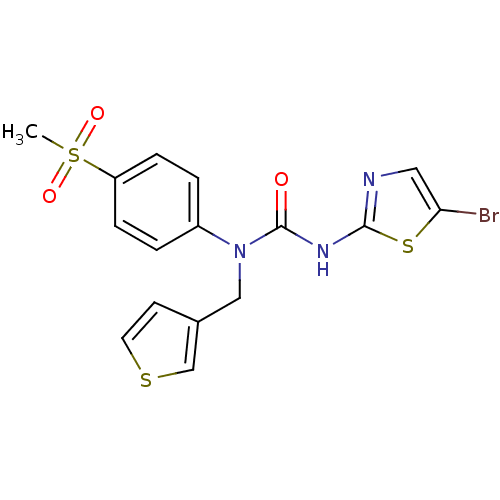
(3-(5-Bromo-thiazol-2-yl)-1-(4-methanesulfonyl-phen...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(Cc1ccsc1)C(=O)Nc1ncc(Br)s1 Show InChI InChI=1S/C16H14BrN3O3S3/c1-26(22,23)13-4-2-12(3-5-13)20(9-11-6-7-24-10-11)16(21)19-15-18-8-14(17)25-15/h2-8,10H,9H2,1H3,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161693
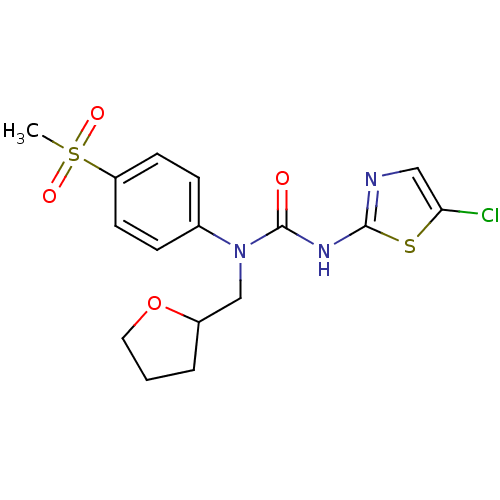
(3-(5-Chloro-thiazol-2-yl)-1-(4-methanesulfonyl-phe...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(CC1CCCO1)C(=O)Nc1ncc(Cl)s1 Show InChI InChI=1S/C16H18ClN3O4S2/c1-26(22,23)13-6-4-11(5-7-13)20(10-12-3-2-8-24-12)16(21)19-15-18-9-14(17)25-15/h4-7,9,12H,2-3,8,10H2,1H3,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161668

(1-Cyclopentylmethyl-1-(4-methanesulfonyl-phenyl)-3...)Show SMILES CS(=O)(=O)c1ccc(cc1)N(CC1CCCC1)C(=O)Nc1nccs1 Show InChI InChI=1S/C17H21N3O3S2/c1-25(22,23)15-8-6-14(7-9-15)20(12-13-4-2-3-5-13)17(21)19-16-18-10-11-24-16/h6-11,13H,2-5,12H2,1H3,(H,18,19,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 15 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161695
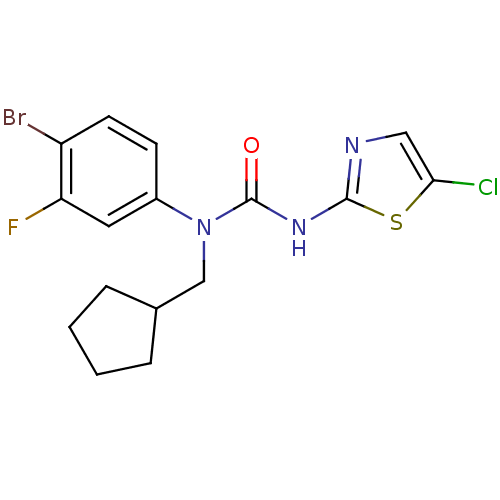
(1-(4-Bromo-3-fluoro-phenyl)-3-(5-chloro-thiazol-2-...)Show InChI InChI=1S/C16H16BrClFN3OS/c17-12-6-5-11(7-13(12)19)22(9-10-3-1-2-4-10)16(23)21-15-20-8-14(18)24-15/h5-8,10H,1-4,9H2,(H,20,21,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 700 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |
Hexokinase-4
(Rattus norvegicus) | BDBM50161669
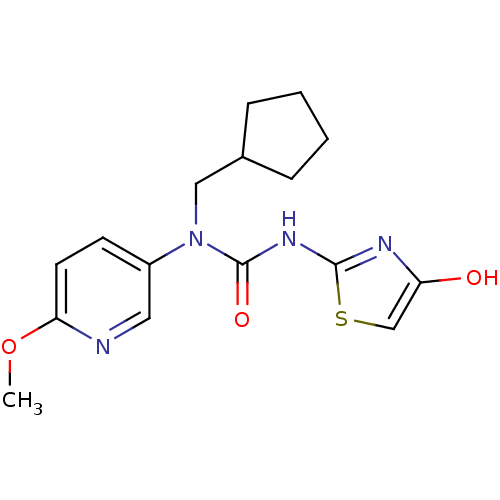
(1-Cyclopentylmethyl-1-(6-methoxy-pyridin-3-yl)-3-(...)Show InChI InChI=1S/C16H20N4O3S/c1-23-14-7-6-12(8-17-14)20(9-11-4-2-3-5-11)16(22)19-15-18-13(21)10-24-15/h6-8,10-11,21H,2-5,9H2,1H3,(H,18,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.91E+4 | n/a | n/a | n/a | n/a |
OSI Pharmaceuticals
Curated by ChEMBL
| Assay Description
Effective concentration for glucokinase activation with 5 mM glucose |
Bioorg Med Chem Lett 15: 1501-4 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.083
BindingDB Entry DOI: 10.7270/Q2HT2NVC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data