

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16152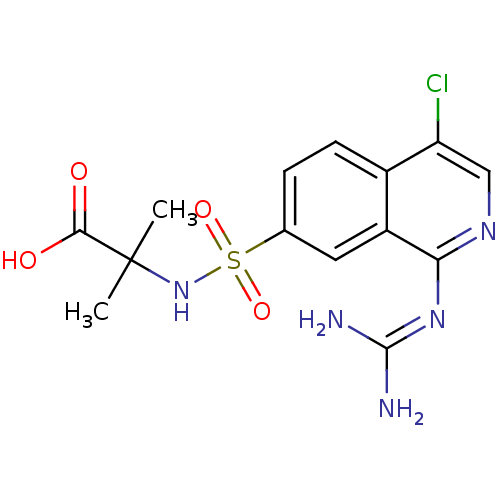 (2-({4-chloro-1-[(diaminomethylidene)amino]isoquino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using S-2444 as substrate | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50231520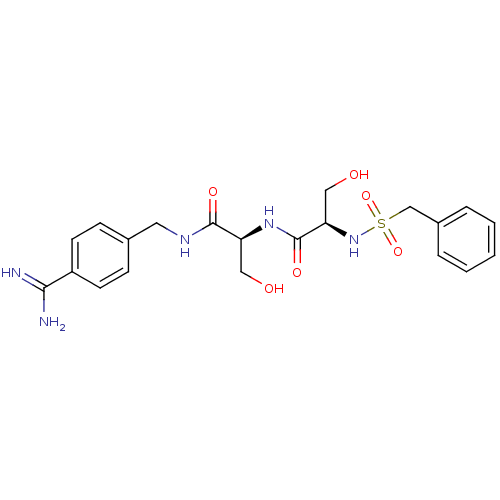 ((R)-N-[(S)-1-(4-carbamimidoyl-benzylcarbamoyl)-2-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50147422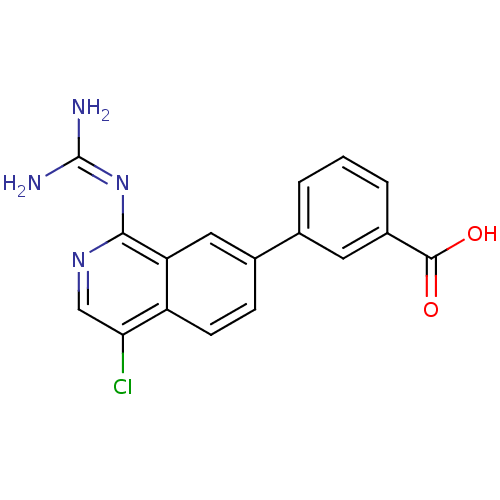 (3-(4-Chloro-1-guanidino-isoquinolin-7-yl)-benzoic ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using S-2444 as substrate | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM23891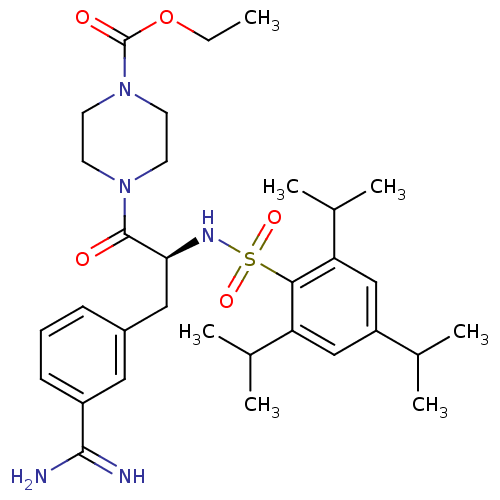 (3-amidinophenylalanine deriv., 35 | CHEMBL107955 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of uPA (unknown origin) | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499900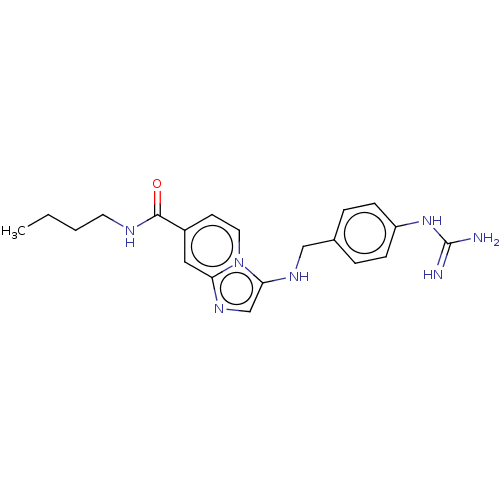 (CHEMBL3740183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499914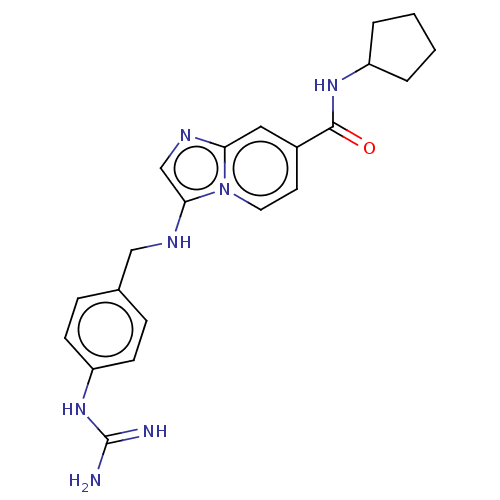 (CHEMBL3740973) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499920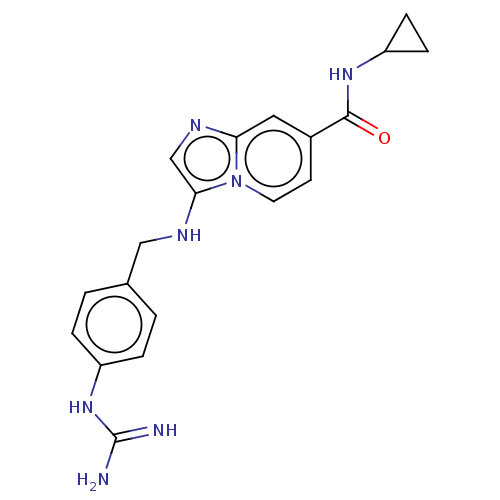 (CHEMBL3741622) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499913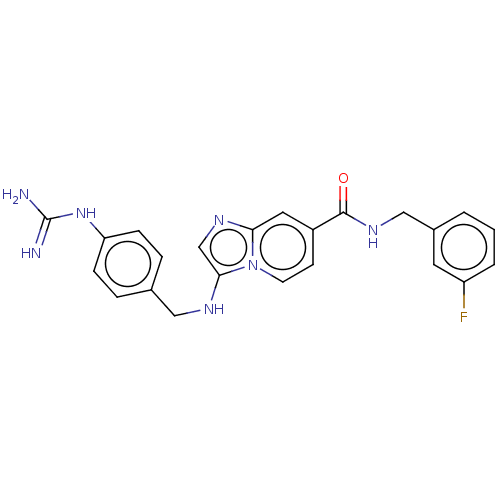 (CHEMBL3741173) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 254 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease ATG4B (Homo sapiens (Human)) | BDBM80861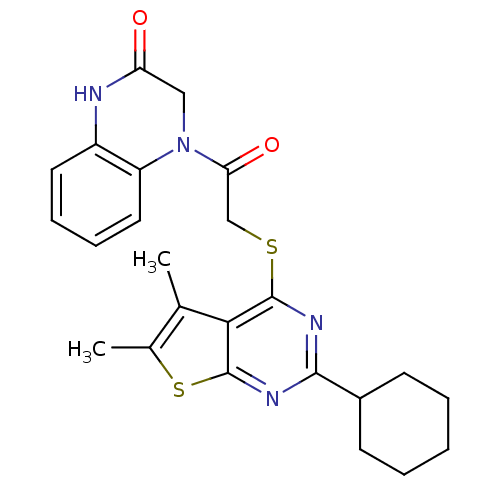 (4-[2-(2-Cyclohexyl-5,6-dimethyl-thieno[2,3-d]pyrim...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of wild type recombinant Atg4B (unknown origin) expressed in Escherichia coli BL21 DE3 using N-terminal His6-tagged LC3B-PLA2 as substrate... | Eur J Med Chem 123: 631-638 (2016) Article DOI: 10.1016/j.ejmech.2016.07.073 BindingDB Entry DOI: 10.7270/Q2FT8P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50363655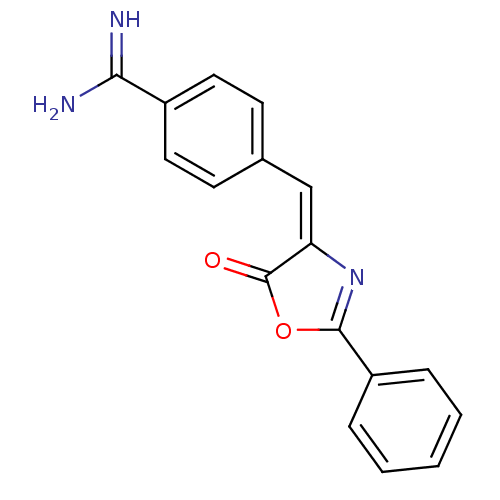 (CHEMBL1945236) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human plasma plasmin using pyroGlu-Pro-Arg-pNA-HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499919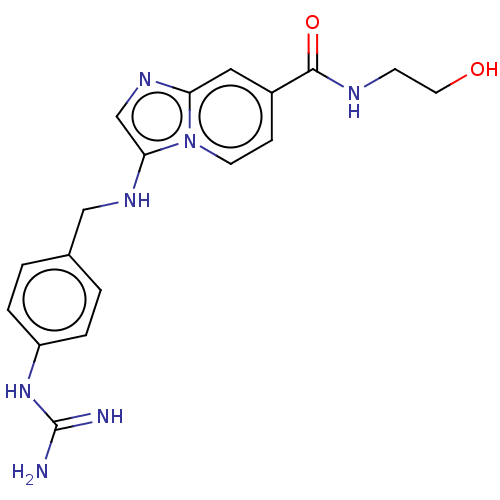 (CHEMBL3740904) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 366 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499916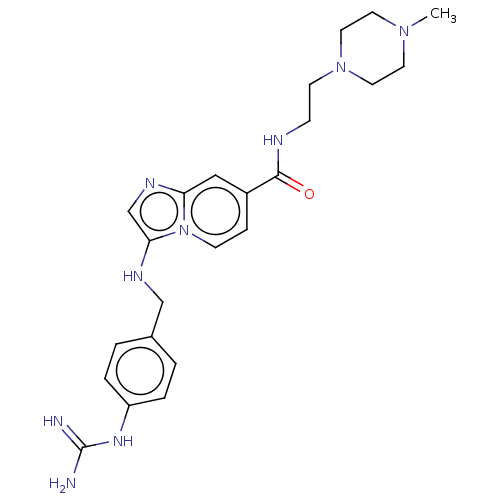 (CHEMBL3741100) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 404 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50104435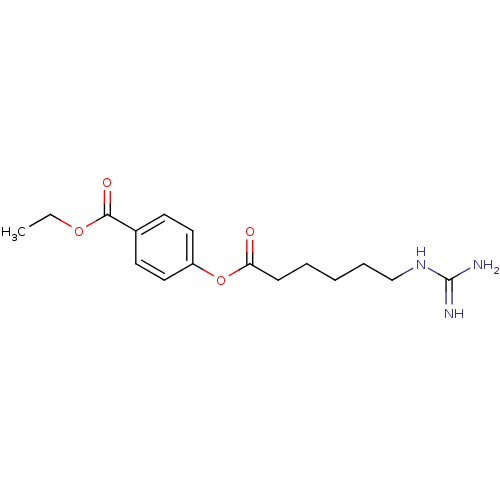 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 431 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease ATG4B (Homo sapiens (Human)) | BDBM50085339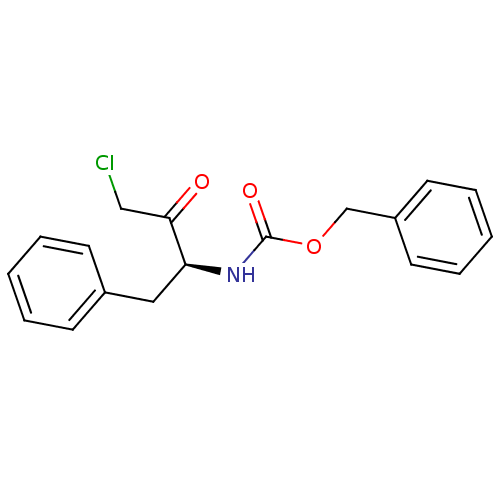 (((S)-1-Benzyl-3-chloro-2-oxo-propyl)-carbamic acid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of Atg4B (unknown origin) using YFP-LC3B-EmGFP as substrate after 40 mins by FRET-based assay | Eur J Med Chem 123: 631-638 (2016) Article DOI: 10.1016/j.ejmech.2016.07.073 BindingDB Entry DOI: 10.7270/Q2FT8P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 687 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human plasma thrombin using pyroGlu-Pro-Arg-pNA-HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease ATG4B (Homo sapiens (Human)) | BDBM60996 (5-[(3-carboxy-4-hydroxy-phenyl)-(3-carboxy-4-keto-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of Atg4B (unknown origin) using YFP-LC3B-EmGFP as substrate after 40 mins by FRET-based assay | Eur J Med Chem 123: 631-638 (2016) Article DOI: 10.1016/j.ejmech.2016.07.073 BindingDB Entry DOI: 10.7270/Q2FT8P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50363655 (CHEMBL1945236) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA-2HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using D-Pro-Phe-Arg-pNA-2HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human plasma plasmin using pyroGlu-Pro-Arg-pNA-HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease ATG4B (Homo sapiens (Human)) | BDBM33413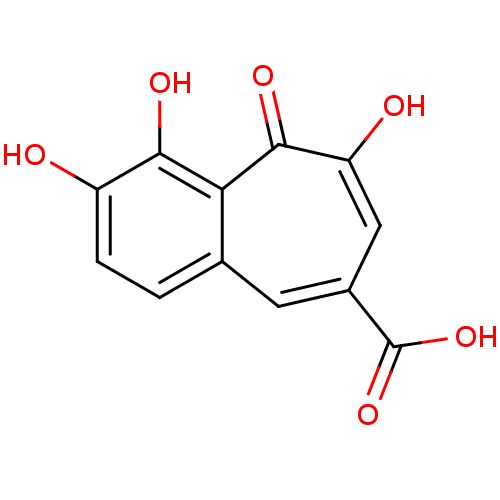 (benzo[7]annulene-8-carboxylic acid, 5) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of wild type recombinant Atg4B (unknown origin) expressed in Escherichia coli BL21 DE3 using N-terminal His6-tagged LC3B-PLA2 as substrate... | Eur J Med Chem 123: 631-638 (2016) Article DOI: 10.1016/j.ejmech.2016.07.073 BindingDB Entry DOI: 10.7270/Q2FT8P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 4.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of purified human factor 10a using Suc-Ile-Glu(gammaPip)-GlyArg-pNa-HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease ATG4B (Homo sapiens (Human)) | BDBM4078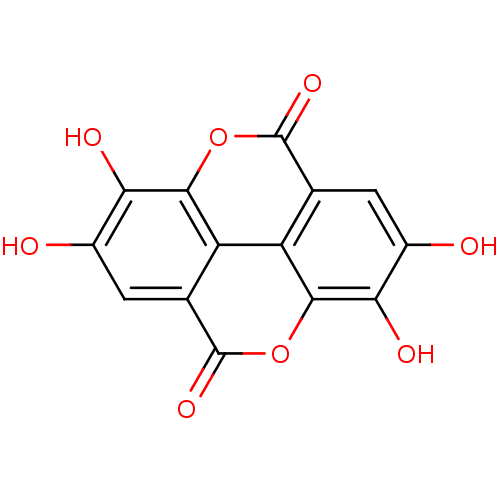 (6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of wild type recombinant Atg4B (unknown origin) expressed in Escherichia coli BL21 DE3 using N-terminal His6-tagged LC3B-PLA2 as substrate... | Eur J Med Chem 123: 631-638 (2016) Article DOI: 10.1016/j.ejmech.2016.07.073 BindingDB Entry DOI: 10.7270/Q2FT8P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50363655 (CHEMBL1945236) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.47E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of purified human factor 10a using Suc-Ile-Glu(gammaPip)-GlyArg-pNa-HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499906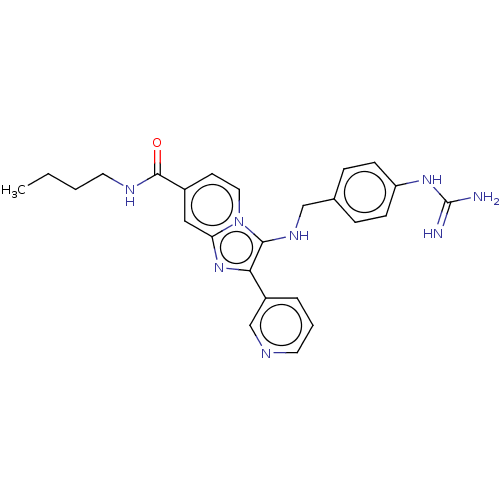 (CHEMBL3742152) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50363655 (CHEMBL1945236) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499912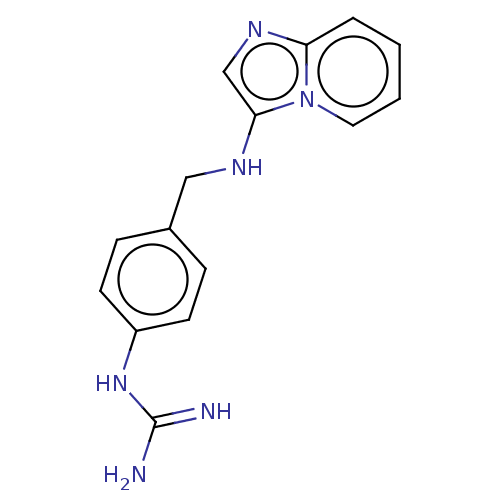 (CHEMBL3740931) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499933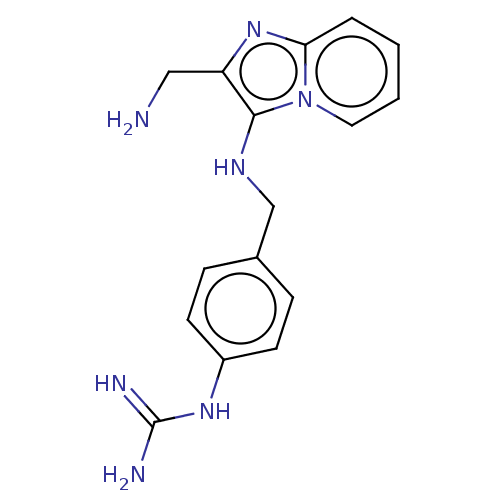 (CHEMBL3740914) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM16173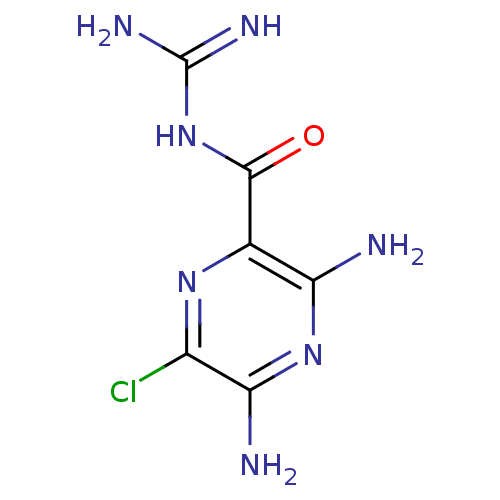 (3,5-diamino-6-chloro-N-(diaminomethylene)pyrazinam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499915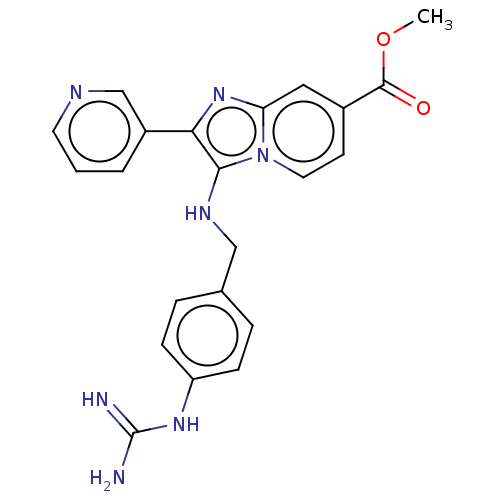 (CHEMBL3740642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50104435 (4-(6-Guanidino-hexanoyloxy)-benzoic acid ethyl est...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of recombinant human tPA using H-D-Ile-Pro-L-Arg-pNA-2HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499922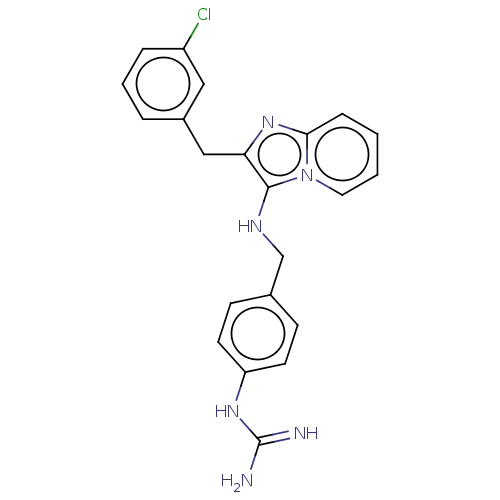 (CHEMBL3740676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499911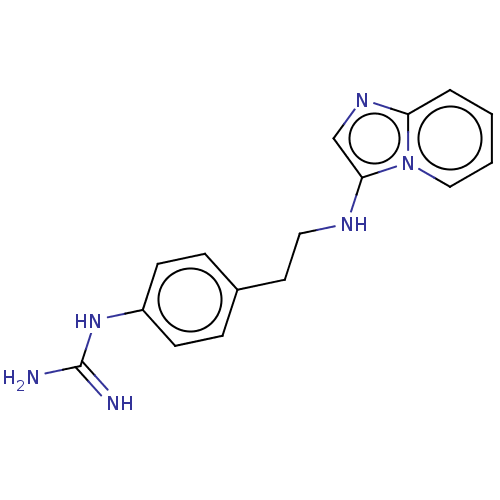 (CHEMBL3739833) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499905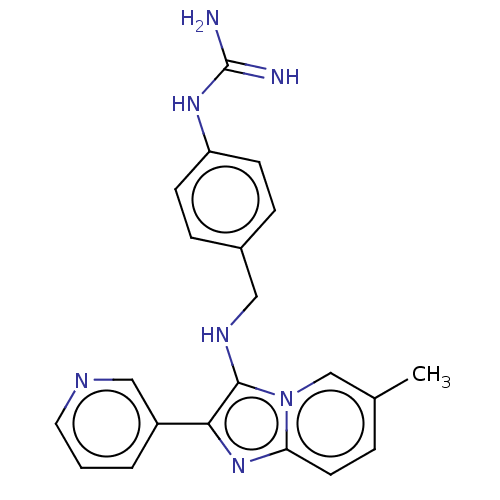 (CHEMBL3741004) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499917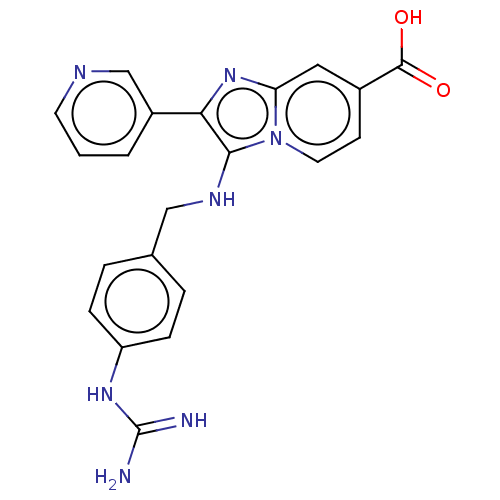 (CHEMBL3739519) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499925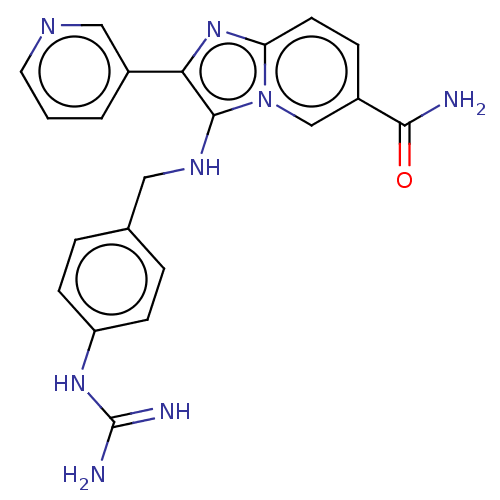 (CHEMBL3741887) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.73E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499921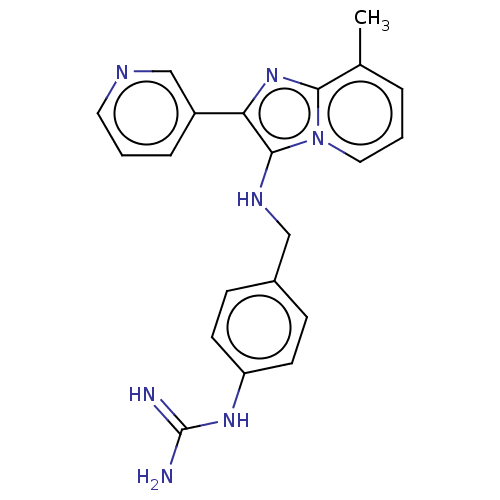 (CHEMBL3740294) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.74E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499926 (CHEMBL3740145) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499929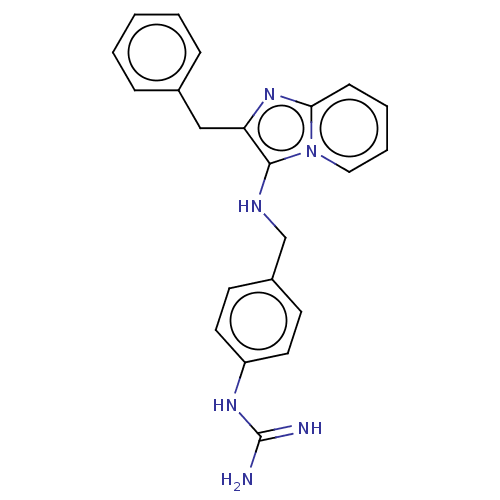 (CHEMBL3741697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease ATG4B (Homo sapiens (Human)) | BDBM60996 (5-[(3-carboxy-4-hydroxy-phenyl)-(3-carboxy-4-keto-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of human recombinant GST-tagged Atg4B expressed in Escherichia coli using LC3B-GST as substrate after 6 mins by SDS-PAGE assay | Eur J Med Chem 123: 631-638 (2016) Article DOI: 10.1016/j.ejmech.2016.07.073 BindingDB Entry DOI: 10.7270/Q2FT8P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499908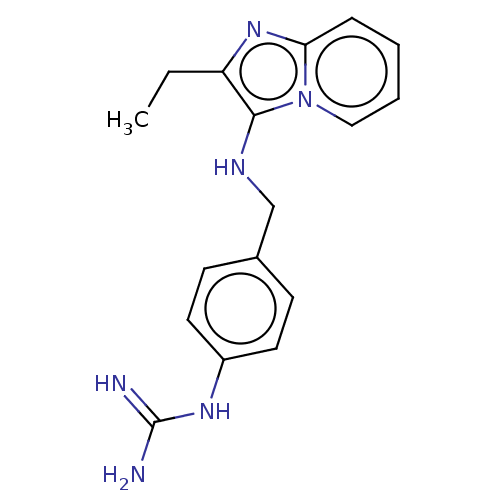 (CHEMBL3742000) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease ATG4B (Homo sapiens (Human)) | BDBM34545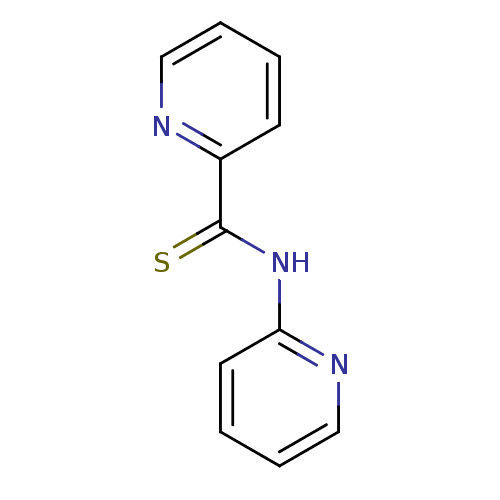 (MLS000104342 | N-(2-pyridinyl)-2-pyridinecarbothio...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of Atg4B (unknown origin) using LC3B-GST as substrate preincubated for 24 hrs followed by substrate addition measured for 3 mins by SDS-PA... | Eur J Med Chem 123: 631-638 (2016) Article DOI: 10.1016/j.ejmech.2016.07.073 BindingDB Entry DOI: 10.7270/Q2FT8P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50499919 (CHEMBL3740904) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of purified human factor 10a using Suc-Ile-Glu(gammaPip)-GlyArg-pNa-HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499931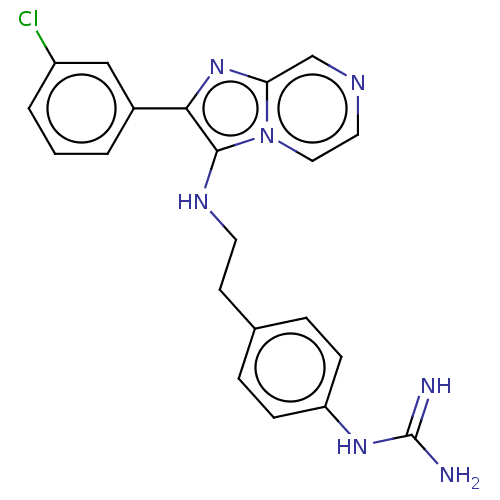 (CHEMBL3740795) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50363655 (CHEMBL1945236) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human plasma thrombin using pyroGlu-Pro-Arg-pNA-HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease ATG4B (Homo sapiens (Human)) | BDBM50060874 (1,3,4,6,8,13-hexahydroxy-10,11-dimethylphenanthro[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp Curated by ChEMBL | Assay Description Inhibition of Atg4B (unknown origin) using YFP-LC3B-EmGFP as substrate after 40 mins by FRET-based assay | Eur J Med Chem 123: 631-638 (2016) Article DOI: 10.1016/j.ejmech.2016.07.073 BindingDB Entry DOI: 10.7270/Q2FT8P1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499930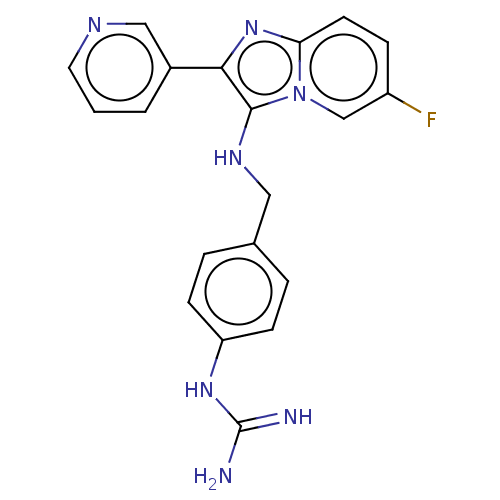 (CHEMBL3740195) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499903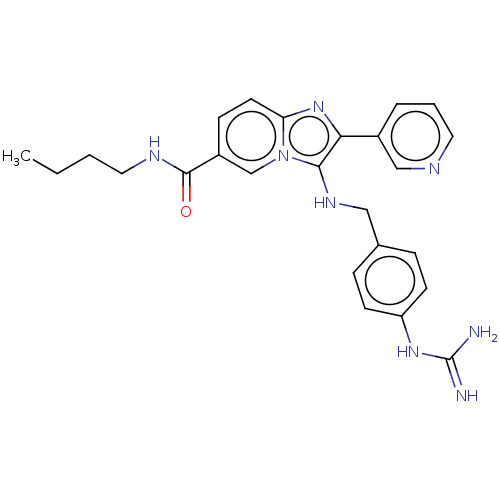 (CHEMBL3741341) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499918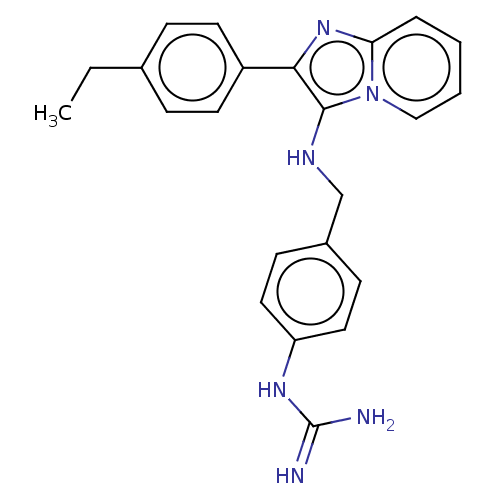 (CHEMBL3739939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50363655 (CHEMBL1945236) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of recombinant human tPA using H-D-Ile-Pro-L-Arg-pNA-2HCl as substrate measured for 5 mins by spectrophotometric assay | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50499928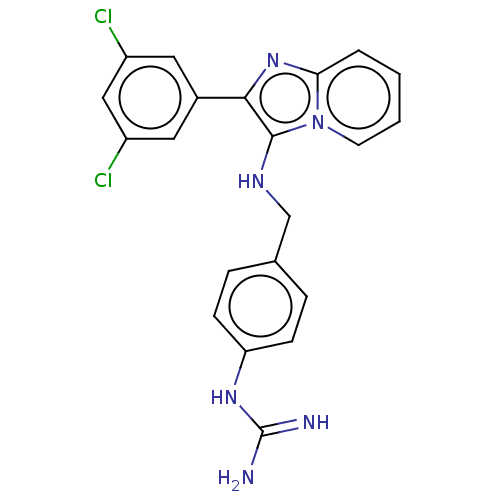 (CHEMBL3741486) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Antwerp (UA) Curated by ChEMBL | Assay Description Inhibition of human uPA using pyro-Glu-Gly-Arg-pNA as substrate assessed as para-nitroaniline release from substrate measured for 5 mins by spectroph... | J Med Chem 58: 9238-57 (2015) Article DOI: 10.1021/acs.jmedchem.5b01171 BindingDB Entry DOI: 10.7270/Q241713M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 68 total ) | Next | Last >> |