 Found 361 hits with Last Name = 'grädler' and Initial = 'u'
Found 361 hits with Last Name = 'grädler' and Initial = 'u' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50386178
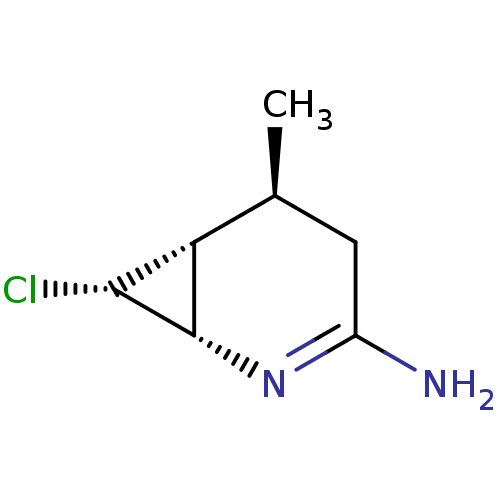
(CHEMBL1800346 | ONO-1714)Show SMILES C[C@H]1CC(N)=N[C@@H]2[C@H](Cl)[C@H]12 |r,c:4| Show InChI InChI=1S/C7H11ClN2/c1-3-2-4(9)10-7-5(3)6(7)8/h3,5-7H,2H2,1H3,(H2,9,10)/t3-,5?,6+,7-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50386178

(CHEMBL1800346 | ONO-1714)Show SMILES C[C@H]1CC(N)=N[C@@H]2[C@H](Cl)[C@H]12 |r,c:4| Show InChI InChI=1S/C7H11ClN2/c1-3-2-4(9)10-7-5(3)6(7)8/h3,5-7H,2H2,1H3,(H2,9,10)/t3-,5?,6+,7-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125769
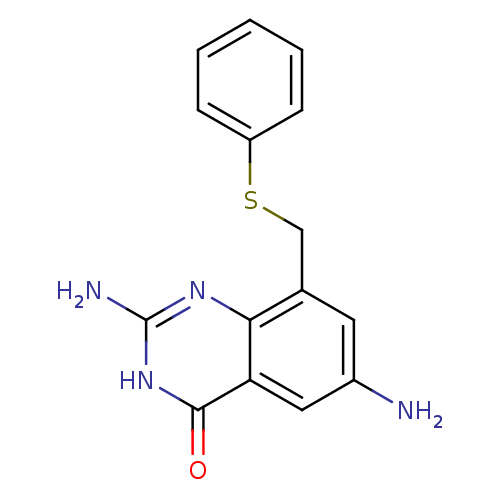
(2,6-Diamino-8-phenylsulfanylmethyl-3H-quinazolin-4...)Show InChI InChI=1S/C15H14N4OS/c16-10-6-9(8-21-11-4-2-1-3-5-11)13-12(7-10)14(20)19-15(17)18-13/h1-7H,8,16H2,(H3,17,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125773
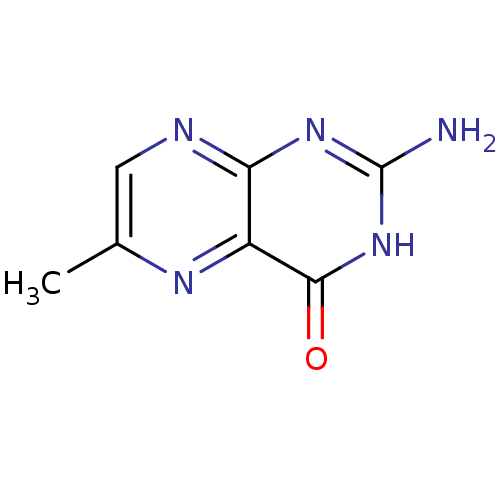
(2-Amino-6-methyl-3H-pteridin-4-one | CHEMBL14913)Show InChI InChI=1S/C7H7N5O/c1-3-2-9-5-4(10-3)6(13)12-7(8)11-5/h2H,1H3,(H3,8,9,11,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125771
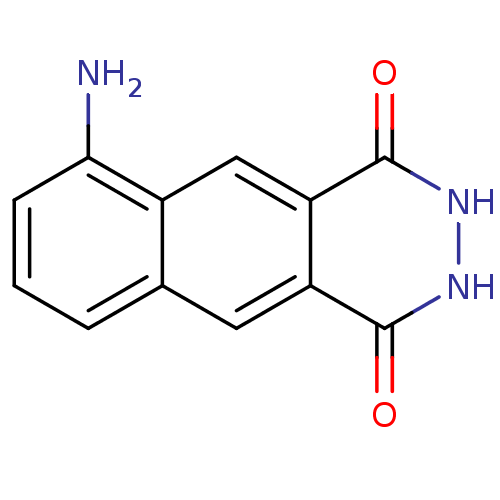
(6-Amino-2,3-dihydro-benzo[g]phthalazine-1,4-dione ...)Show InChI InChI=1S/C12H9N3O2/c13-10-3-1-2-6-4-8-9(5-7(6)10)12(17)15-14-11(8)16/h1-5H,13H2,(H,14,16)(H,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125772
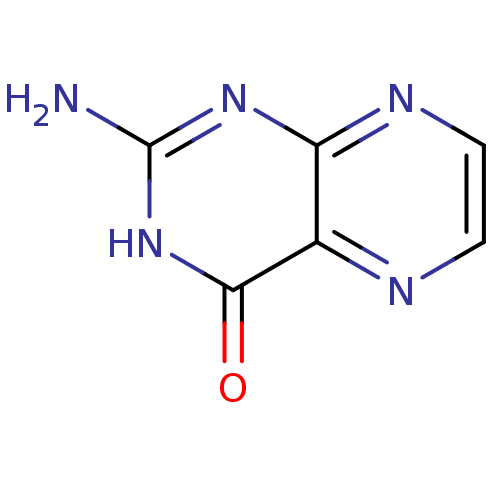
(2-amino-4-hydroxypteridine | 2-aminopteridin-4-ol ...)Show InChI InChI=1S/C6H5N5O/c7-6-10-4-3(5(12)11-6)8-1-2-9-4/h1-2H,(H3,7,9,10,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125760
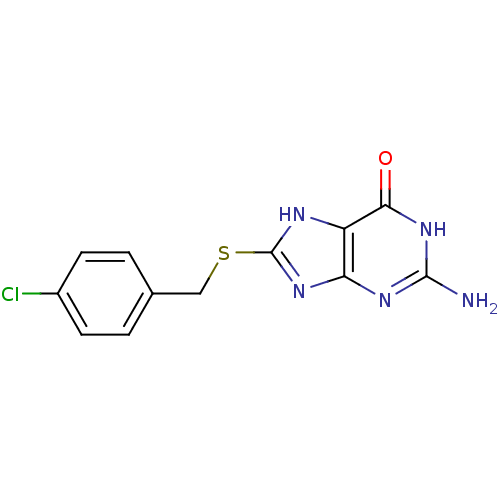
(2-Amino-8-(4-chloro-benzylsulfanyl)-1,9-dihydro-pu...)Show InChI InChI=1S/C12H10ClN5OS/c13-7-3-1-6(2-4-7)5-20-12-15-8-9(17-12)16-11(14)18-10(8)19/h1-4H,5H2,(H4,14,15,16,17,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125766
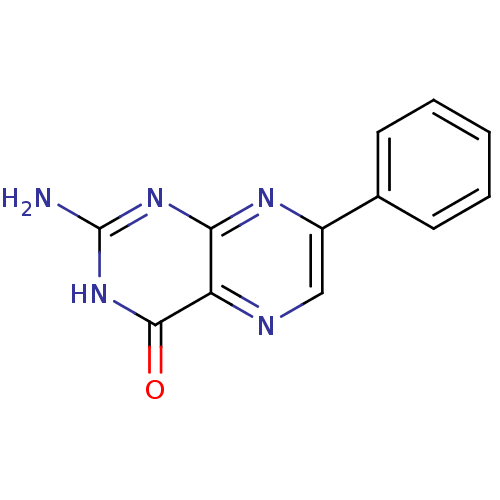
(2-Amino-7-phenyl-3H-pteridin-4-one | CHEMBL277561)Show InChI InChI=1S/C12H9N5O/c13-12-16-10-9(11(18)17-12)14-6-8(15-10)7-4-2-1-3-5-7/h1-6H,(H3,13,15,16,17,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125761
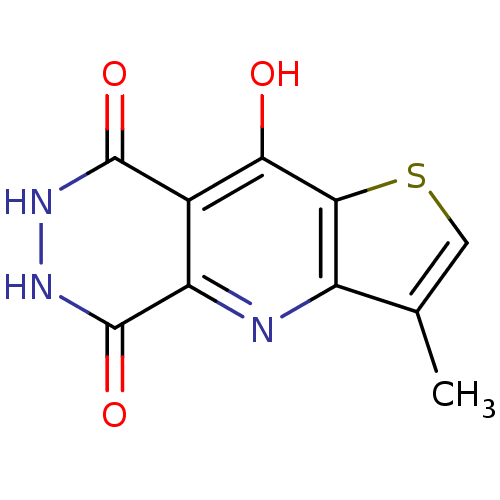
(3-Methyl-6,7-dihydro-4H-1-thia-4,6,7-triaza-cyclop...)Show InChI InChI=1S/C10H7N3O3S/c1-3-2-17-8-5(3)11-6-4(7(8)14)9(15)12-13-10(6)16/h2H,1H3,(H,11,14)(H,12,15)(H,13,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125768
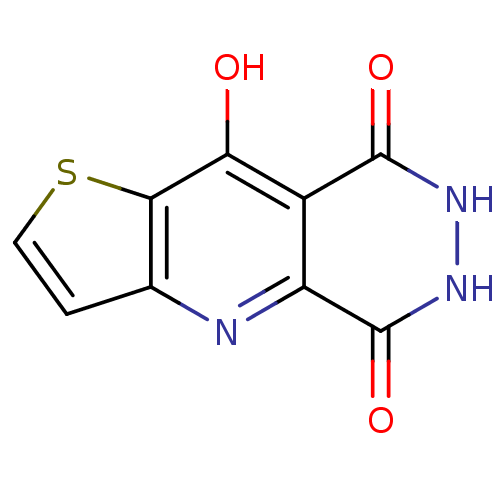
(6,7-Dihydro-4H-1-thia-4,6,7-triaza-cyclopenta[b]na...)Show InChI InChI=1S/C9H5N3O3S/c13-6-4-5(9(15)12-11-8(4)14)10-3-1-2-16-7(3)6/h1-2H,(H,10,13)(H,11,14)(H,12,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125765
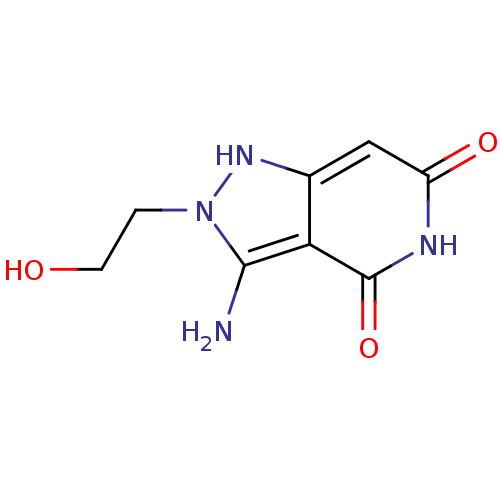
(3-Amino-6-hydroxy-2-(2-hydroxy-ethyl)-2,5-dihydro-...)Show InChI InChI=1S/C8H10N4O3/c9-7-6-4(11-12(7)1-2-13)3-5(14)10-8(6)15/h3,11,13H,1-2,9H2,(H,10,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125767
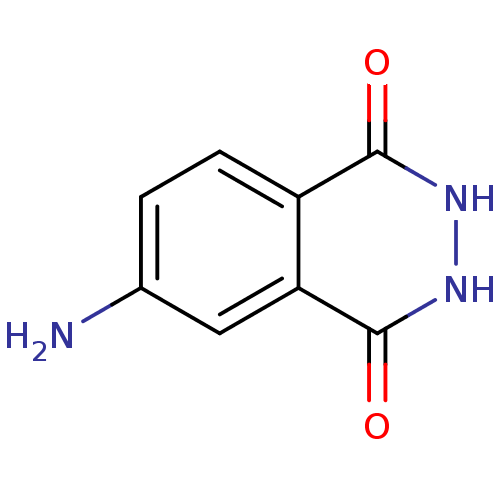
(4-AMINOPHTHALHYDRAZIDE | 6-Amino-2,3-dihydro-phtha...)Show InChI InChI=1S/C8H7N3O2/c9-4-1-2-5-6(3-4)8(13)11-10-7(5)12/h1-3H,9H2,(H,10,12)(H,11,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125759
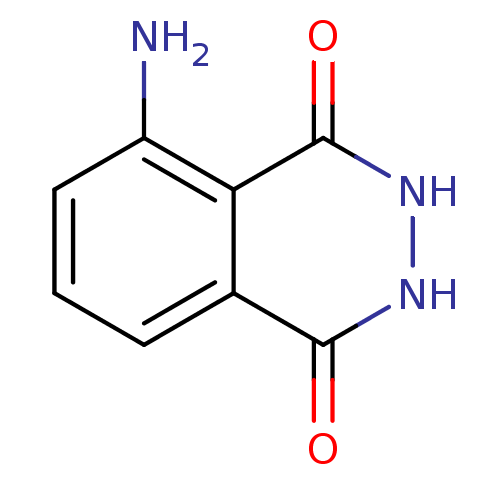
(5-Amino-2,3-dihydro-phthalazine-1,4-dione | CHEMBL...)Show InChI InChI=1S/C8H7N3O2/c9-5-3-1-2-4-6(5)8(13)11-10-7(4)12/h1-3H,9H2,(H,10,12)(H,11,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125775
(2-Amino-8-piperidin-1-yl-1,9-dihydro-purin-6-one |...)Show InChI InChI=1S/C10H14N6O/c11-9-13-7-6(8(17)15-9)12-10(14-7)16-4-2-1-3-5-16/h1-5H2,(H4,11,12,13,14,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125774
(5-Fluoro-1H-indole-2-carboxylic acid hydrazide | C...)Show InChI InChI=1S/C9H8FN3O/c10-6-1-2-7-5(3-6)4-8(12-7)9(14)13-11/h1-4,12H,11H2,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125763
(2-BUTYL-5,6-DIHYDRO-1H-IMIDAZO[4,5-D]PYRIDAZINE-4,...)Show InChI InChI=1S/C9H12N4O2/c1-2-3-4-5-10-6-7(11-5)9(15)13-12-8(6)14/h2-4H2,1H3,(H,10,11)(H,12,14)(H,13,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
| DrugBank
PDB
Article
PubMed
| 8.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125770
(6-Methyl-2H-pyrazolo[3,4-b]quinolin-3-ylamine | CH...)Show InChI InChI=1S/C11H10N4/c1-6-2-3-9-7(4-6)5-8-10(12)14-15-11(8)13-9/h2-5H,1H3,(H3,12,13,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.56E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125762
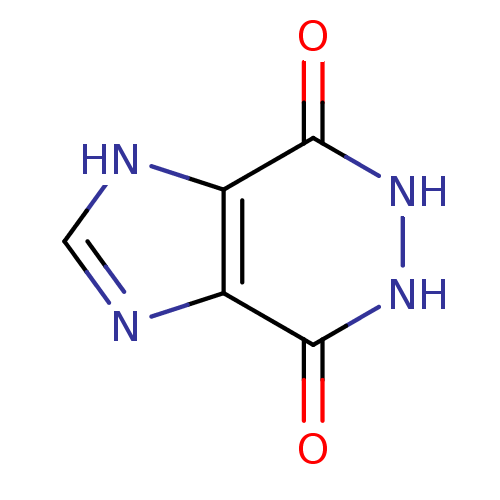
(5,6-Dihydro-1H-imidazo[4,5-d]pyridazine-4,7-dione ...)Show InChI InChI=1S/C5H4N4O2/c10-4-2-3(7-1-6-2)5(11)9-8-4/h1H,(H,6,7)(H,8,10)(H,9,11) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Queuine tRNA-ribosyltransferase
(Zymomonas mobilis) | BDBM50125764
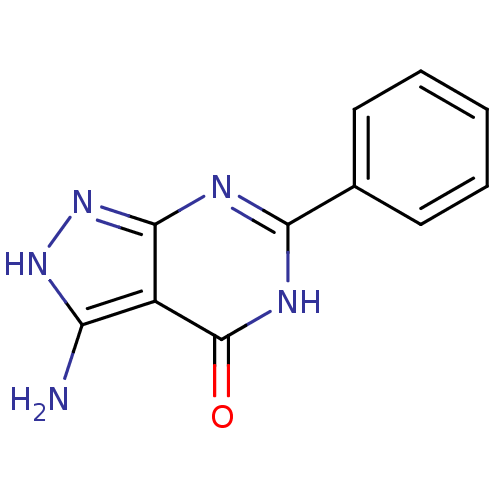
(3-Amino-6-phenyl-2,7-dihydro-pyrazolo[3,4-d]pyrimi...)Show InChI InChI=1S/C11H9N5O/c12-8-7-10(16-15-8)13-9(14-11(7)17)6-4-2-1-3-5-6/h1-5H,(H4,12,13,14,15,16,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 2.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Philipps-Universität Marburg
Curated by ChEMBL
| Assay Description
Inhibitory activity against eubacterial tRNA guanine transglycosylase (TGT) from Zymomonas mobilis |
J Med Chem 46: 1133-43 (2003)
Article DOI: 10.1021/jm0209937
BindingDB Entry DOI: 10.7270/Q2B27TNH |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50318884

(CHEMBL1084546 | CHEMBL2430359 | N-methyl-N-(3-((2-...)Show SMILES CN(c1ncccc1CNc1nc(Nc2ccc3NC(=O)Cc3c2)ncc1C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H20F3N7O3S/c1-31(35(2,33)34)19-12(4-3-7-25-19)10-26-18-15(21(22,23)24)11-27-20(30-18)28-14-5-6-16-13(8-14)9-17(32)29-16/h3-8,11H,9-10H2,1-2H3,(H,29,32)(H2,26,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Competitive binding affinity to FAK kinase domain (410 to 689) (unknown origin) assessed as phosphorylation of p(Glu/Tyr) in presence of ATP |
Bioorg Med Chem Lett 23: 5401-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.050
BindingDB Entry DOI: 10.7270/Q2891782 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425681
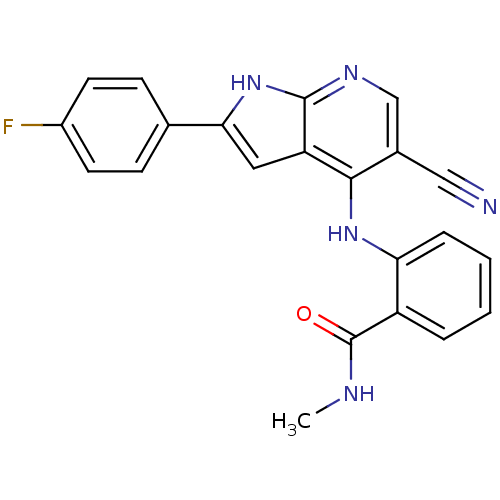
(CHEMBL2315564)Show SMILES CNC(=O)c1ccccc1Nc1c(cnc2[nH]c(cc12)-c1ccc(F)cc1)C#N Show InChI InChI=1S/C22H16FN5O/c1-25-22(29)16-4-2-3-5-18(16)27-20-14(11-24)12-26-21-17(20)10-19(28-21)13-6-8-15(23)9-7-13/h2-10,12H,1H3,(H,25,29)(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425672
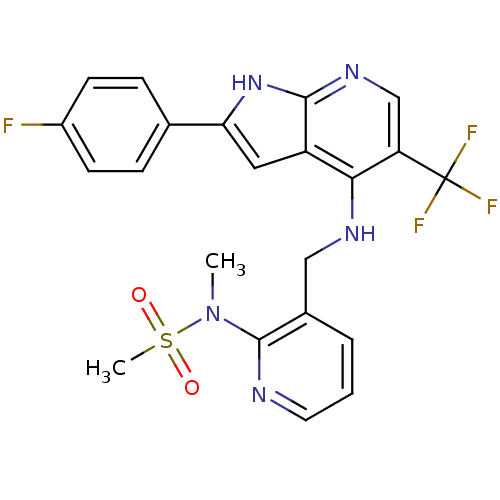
(CHEMBL2315584)Show SMILES CN(c1ncccc1CNc1c(cnc2[nH]c(cc12)-c1ccc(F)cc1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C22H19F4N5O2S/c1-31(34(2,32)33)21-14(4-3-9-27-21)11-28-19-16-10-18(13-5-7-15(23)8-6-13)30-20(16)29-12-17(19)22(24,25)26/h3-10,12H,11H2,1-2H3,(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50124535
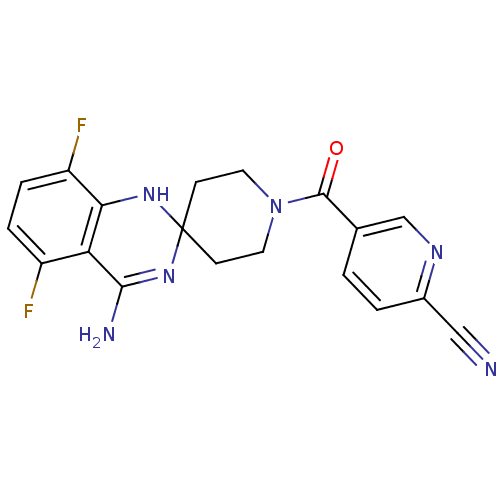
(1-(6-CYANO-3-PYRIDYLCARBONYL)-5',8'-DIFLUOROSPIRO[...)Show SMILES NC1=NC2(CCN(CC2)C(=O)c2ccc(nc2)C#N)Nc2c(F)ccc(F)c12 |t:1| Show InChI InChI=1S/C19H16F2N6O/c20-13-3-4-14(21)16-15(13)17(23)26-19(25-16)5-7-27(8-6-19)18(28)11-1-2-12(9-22)24-10-11/h1-4,10,25H,5-8H2,(H2,23,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 37.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418856
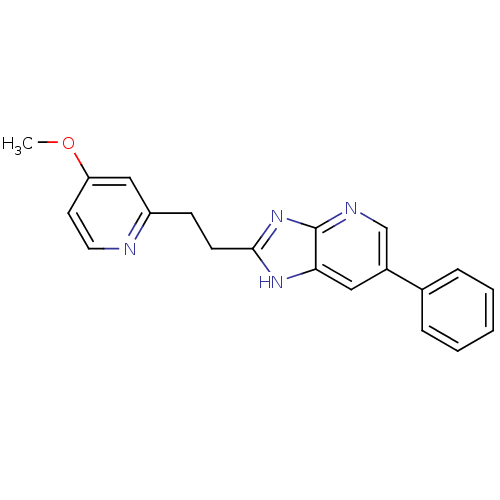
(CHEMBL1800534)Show InChI InChI=1S/C20H18N4O/c1-25-17-9-10-21-16(12-17)7-8-19-23-18-11-15(13-22-20(18)24-19)14-5-3-2-4-6-14/h2-6,9-13H,7-8H2,1H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, endothelial
(Homo sapiens (Human)) | BDBM50091805

(2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...)Show InChI InChI=1S/C7H10N2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3,(H2,8,9) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human eNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425686
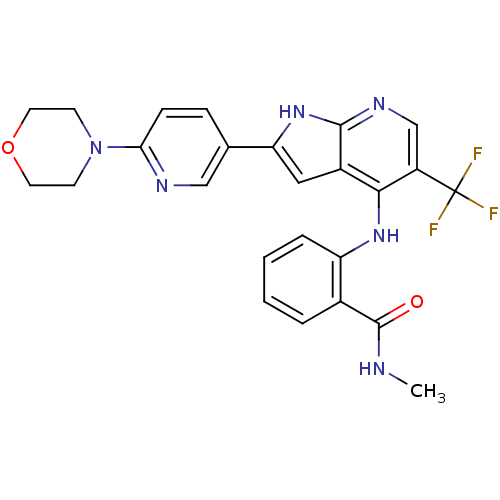
(CHEMBL2315566)Show SMILES CNC(=O)c1ccccc1Nc1c(cnc2[nH]c(cc12)-c1ccc(nc1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C25H23F3N6O2/c1-29-24(35)16-4-2-3-5-19(16)32-22-17-12-20(33-23(17)31-14-18(22)25(26,27)28)15-6-7-21(30-13-15)34-8-10-36-11-9-34/h2-7,12-14H,8-11H2,1H3,(H,29,35)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425687
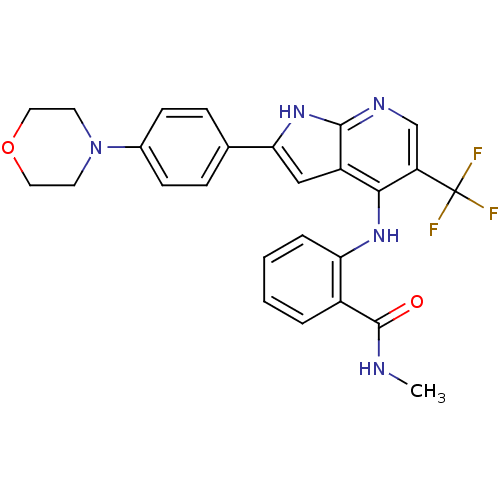
(CHEMBL2315565)Show SMILES CNC(=O)c1ccccc1Nc1c(cnc2[nH]c(cc12)-c1ccc(cc1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C26H24F3N5O2/c1-30-25(35)18-4-2-3-5-21(18)32-23-19-14-22(33-24(19)31-15-20(23)26(27,28)29)16-6-8-17(9-7-16)34-10-12-36-13-11-34/h2-9,14-15H,10-13H2,1H3,(H,30,35)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425679
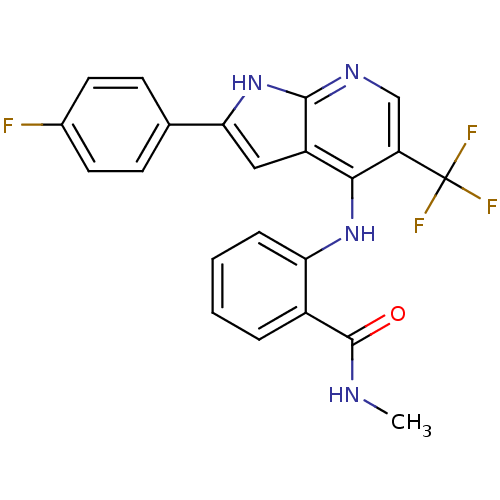
(CHEMBL2315562)Show SMILES CNC(=O)c1ccccc1Nc1c(cnc2[nH]c(cc12)-c1ccc(F)cc1)C(F)(F)F Show InChI InChI=1S/C22H16F4N4O/c1-27-21(31)14-4-2-3-5-17(14)29-19-15-10-18(12-6-8-13(23)9-7-12)30-20(15)28-11-16(19)22(24,25)26/h2-11H,1H3,(H,27,31)(H2,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418855

(CHEMBL1800533)Show InChI InChI=1S/C18H22N4O/c1-3-4-5-13-10-16-18(20-12-13)22-17(21-16)7-6-14-11-15(23-2)8-9-19-14/h8-12H,3-7H2,1-2H3,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55.0 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425688
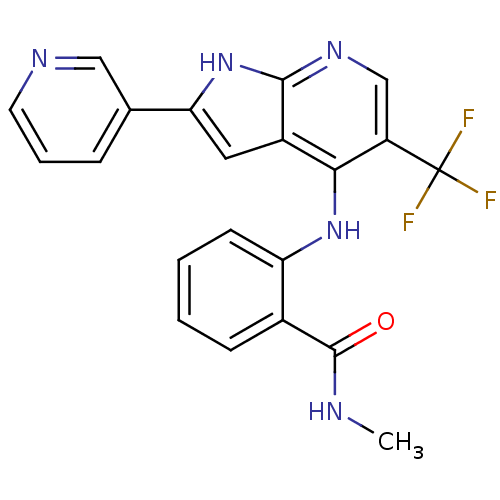
(CHEMBL2315563)Show SMILES CNC(=O)c1ccccc1Nc1c(cnc2[nH]c(cc12)-c1cccnc1)C(F)(F)F Show InChI InChI=1S/C21H16F3N5O/c1-25-20(30)13-6-2-3-7-16(13)28-18-14-9-17(12-5-4-8-26-10-12)29-19(14)27-11-15(18)21(22,23)24/h2-11H,1H3,(H,25,30)(H2,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50386043
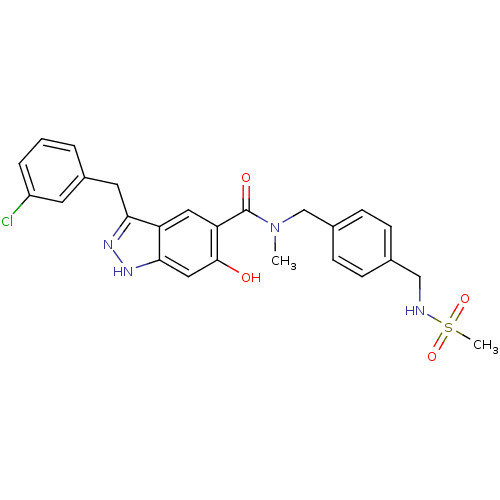
(CHEMBL2042883)Show SMILES CN(Cc1ccc(CNS(C)(=O)=O)cc1)C(=O)c1cc2c(Cc3cccc(Cl)c3)n[nH]c2cc1O Show InChI InChI=1S/C25H25ClN4O4S/c1-30(15-17-8-6-16(7-9-17)14-27-35(2,33)34)25(32)21-12-20-22(28-29-23(20)13-24(21)31)11-18-4-3-5-19(26)10-18/h3-10,12-13,27,31H,11,14-15H2,1-2H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]17AAG from human recombinant Hsp90 after 30 mins by beta scintillation counting |
Bioorg Med Chem Lett 22: 4396-403 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.121
BindingDB Entry DOI: 10.7270/Q2T154QC |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418844

(CHEMBL1800347)Show InChI InChI=1S/C18H19N5O2/c1-25-16-4-7-20-17(10-16)22-14-5-8-23(9-6-14)18(24)13-2-3-15(11-19)21-12-13/h2-4,7,10,12,14H,5-6,8-9H2,1H3,(H,20,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418849

(CHEMBL1738840)Show InChI InChI=1S/C14H14N4O/c1-19-11-6-8-15-10(9-11)4-5-13-17-12-3-2-7-16-14(12)18-13/h2-3,6-9H,4-5H2,1H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 81.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425687

(CHEMBL2315565)Show SMILES CNC(=O)c1ccccc1Nc1c(cnc2[nH]c(cc12)-c1ccc(cc1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C26H24F3N5O2/c1-30-25(35)18-4-2-3-5-21(18)32-23-19-14-22(33-24(19)31-15-20(23)26(27,28)29)16-6-8-17(9-7-16)34-10-12-36-13-11-34/h2-9,14-15H,10-13H2,1H3,(H,30,35)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK in human HT-29 cells assessed as phosphorylation at tyrosine 397 after 45 mins |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425686

(CHEMBL2315566)Show SMILES CNC(=O)c1ccccc1Nc1c(cnc2[nH]c(cc12)-c1ccc(nc1)N1CCOCC1)C(F)(F)F Show InChI InChI=1S/C25H23F3N6O2/c1-29-24(35)16-4-2-3-5-19(16)32-22-17-12-20(33-23(17)31-14-18(22)25(26,27)28)15-6-7-21(30-13-15)34-8-10-36-11-9-34/h2-7,12-14H,8-11H2,1H3,(H,29,35)(H2,31,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK in human HT-29 cells assessed as phosphorylation at tyrosine 397 after 45 mins |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50091805

(2-amino-4,6-dimethylpyridine | 4,6-Dimethyl-pyridi...)Show InChI InChI=1S/C7H10N2/c1-5-3-6(2)9-7(8)4-5/h3-4H,1-2H3,(H2,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418845
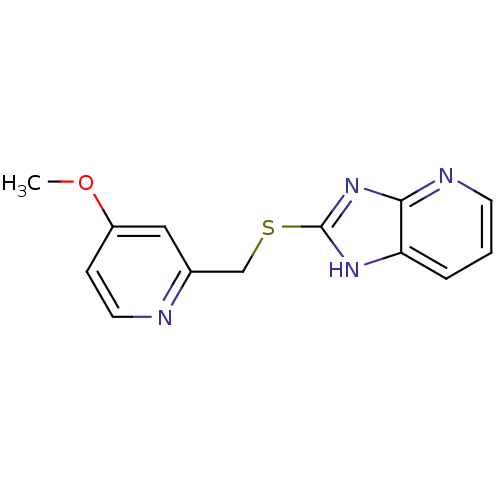
(CHEMBL1800348)Show InChI InChI=1S/C13H12N4OS/c1-18-10-4-6-14-9(7-10)8-19-13-16-11-3-2-5-15-12(11)17-13/h2-7H,8H2,1H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425685
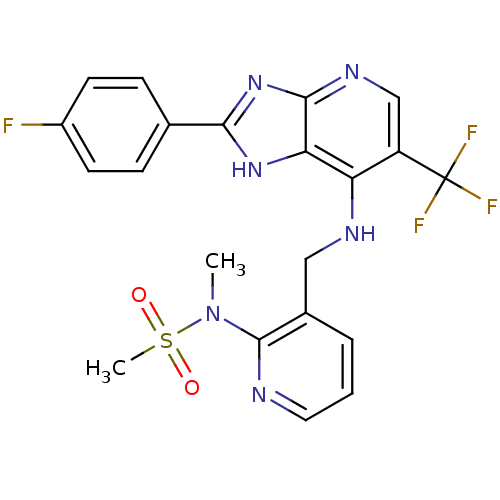
(CHEMBL2315572)Show SMILES CN(c1ncccc1CNc1c(cnc2nc([nH]c12)-c1ccc(F)cc1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C21H18F4N6O2S/c1-31(34(2,32)33)20-13(4-3-9-26-20)10-27-16-15(21(23,24)25)11-28-19-17(16)29-18(30-19)12-5-7-14(22)8-6-12/h3-9,11H,10H2,1-2H3,(H2,27,28,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418851
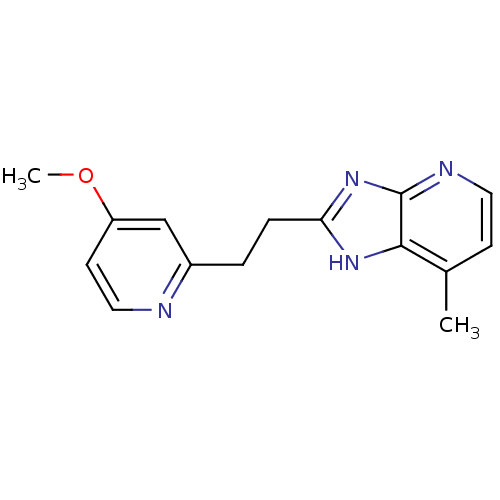
(CHEMBL1800528)Show InChI InChI=1S/C15H16N4O/c1-10-5-7-17-15-14(10)18-13(19-15)4-3-11-9-12(20-2)6-8-16-11/h5-9H,3-4H2,1-2H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 145 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50386053
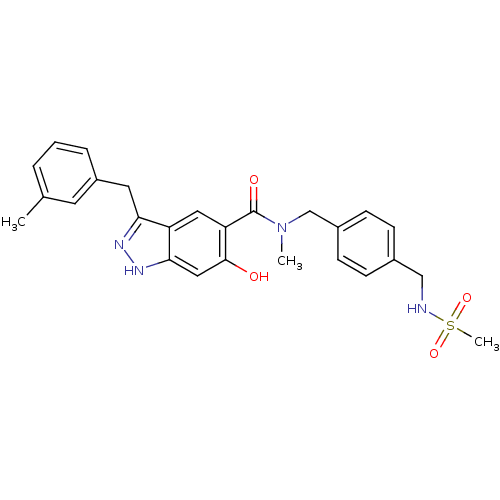
(CHEMBL2042769)Show SMILES CN(Cc1ccc(CNS(C)(=O)=O)cc1)C(=O)c1cc2c(Cc3cccc(C)c3)n[nH]c2cc1O Show InChI InChI=1S/C26H28N4O4S/c1-17-5-4-6-20(11-17)12-23-21-13-22(25(31)14-24(21)29-28-23)26(32)30(2)16-19-9-7-18(8-10-19)15-27-35(3,33)34/h4-11,13-14,27,31H,12,15-16H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]17AAG from human recombinant Hsp90 after 30 mins by beta scintillation counting |
Bioorg Med Chem Lett 22: 4396-403 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.121
BindingDB Entry DOI: 10.7270/Q2T154QC |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418847
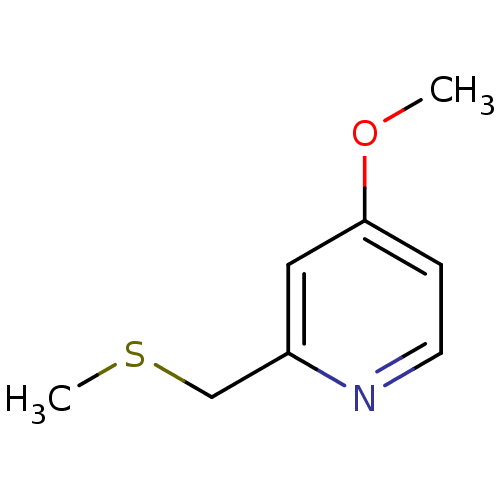
(CHEMBL1800523)Show InChI InChI=1S/C8H11NOS/c1-10-8-3-4-9-7(5-8)6-11-2/h3-5H,6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 155 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425684
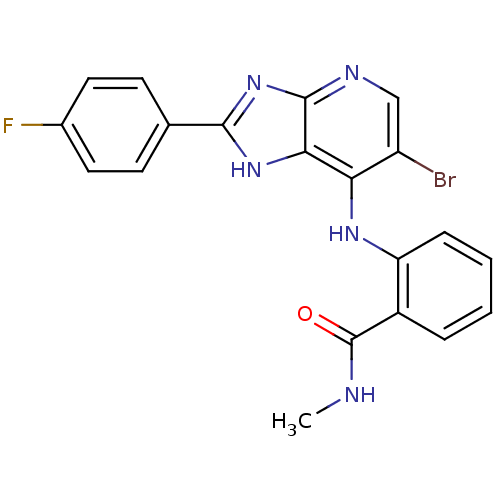
(CHEMBL2315571)Show SMILES CNC(=O)c1ccccc1Nc1c(Br)cnc2nc([nH]c12)-c1ccc(F)cc1 Show InChI InChI=1S/C20H15BrFN5O/c1-23-20(28)13-4-2-3-5-15(13)25-16-14(21)10-24-19-17(16)26-18(27-19)11-6-8-12(22)9-7-11/h2-10H,1H3,(H,23,28)(H2,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50386047
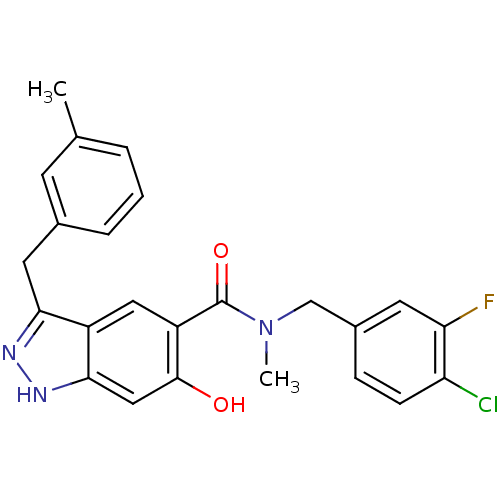
(CHEMBL2042764)Show SMILES CN(Cc1ccc(Cl)c(F)c1)C(=O)c1cc2c(Cc3cccc(C)c3)n[nH]c2cc1O Show InChI InChI=1S/C24H21ClFN3O2/c1-14-4-3-5-15(8-14)10-21-17-11-18(23(30)12-22(17)28-27-21)24(31)29(2)13-16-6-7-19(25)20(26)9-16/h3-9,11-12,30H,10,13H2,1-2H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]17AAG from human recombinant Hsp90 after 30 mins by beta scintillation counting |
Bioorg Med Chem Lett 22: 4396-403 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.121
BindingDB Entry DOI: 10.7270/Q2T154QC |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425673
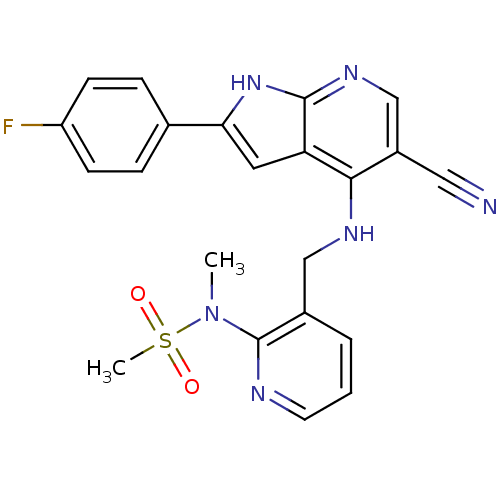
(CHEMBL2315580)Show SMILES CN(c1ncccc1CNc1c(cnc2[nH]c(cc12)-c1ccc(F)cc1)C#N)S(C)(=O)=O Show InChI InChI=1S/C22H19FN6O2S/c1-29(32(2,30)31)22-15(4-3-9-25-22)12-26-20-16(11-24)13-27-21-18(20)10-19(28-21)14-5-7-17(23)8-6-14/h3-10,13H,12H2,1-2H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Nitric oxide synthase, inducible
(Homo sapiens (Human)) | BDBM50418860

(CHEMBL1800526)Show InChI InChI=1S/C14H13BrN4O/c1-20-11-4-5-16-10(7-11)2-3-13-18-12-6-9(15)8-17-14(12)19-13/h4-8H,2-3H2,1H3,(H,17,18,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Nycomed GmbH
Curated by ChEMBL
| Assay Description
Inhibition of human iNOS assessed as inhibition of [3H]L-arginine to [3H]L-citrulline conversion by scintillation counting |
Bioorg Med Chem Lett 21: 4228-32 (2011)
Article DOI: 10.1016/j.bmcl.2011.05.073
BindingDB Entry DOI: 10.7270/Q2H996GJ |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425675
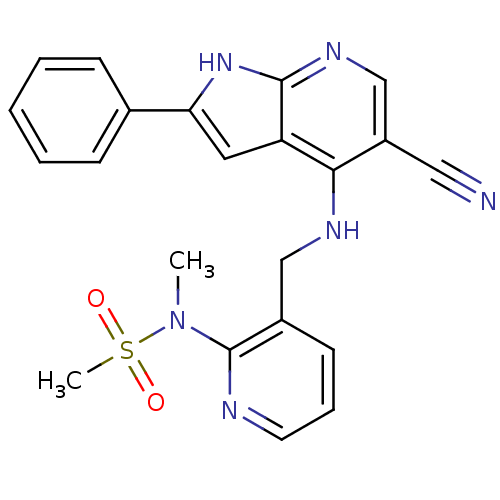
(CHEMBL2315579)Show SMILES CN(c1ncccc1CNc1c(cnc2[nH]c(cc12)-c1ccccc1)C#N)S(C)(=O)=O Show InChI InChI=1S/C22H20N6O2S/c1-28(31(2,29)30)22-16(9-6-10-24-22)13-25-20-17(12-23)14-26-21-18(20)11-19(27-21)15-7-4-3-5-8-15/h3-11,14H,13H2,1-2H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 195 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50386051
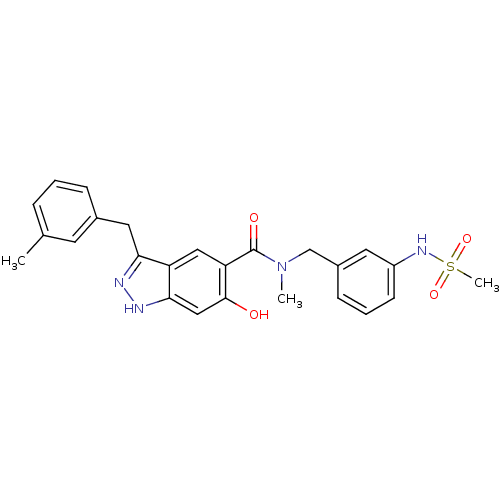
(CHEMBL2042767)Show SMILES CN(Cc1cccc(NS(C)(=O)=O)c1)C(=O)c1cc2c(Cc3cccc(C)c3)n[nH]c2cc1O Show InChI InChI=1S/C25H26N4O4S/c1-16-6-4-7-17(10-16)12-22-20-13-21(24(30)14-23(20)27-26-22)25(31)29(2)15-18-8-5-9-19(11-18)28-34(3,32)33/h4-11,13-14,28,30H,12,15H2,1-3H3,(H,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]17AAG from human recombinant Hsp90 after 30 mins by beta scintillation counting |
Bioorg Med Chem Lett 22: 4396-403 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.121
BindingDB Entry DOI: 10.7270/Q2T154QC |
More data for this
Ligand-Target Pair | |
Focal adhesion kinase 1
(Homo sapiens (Human)) | BDBM50425667
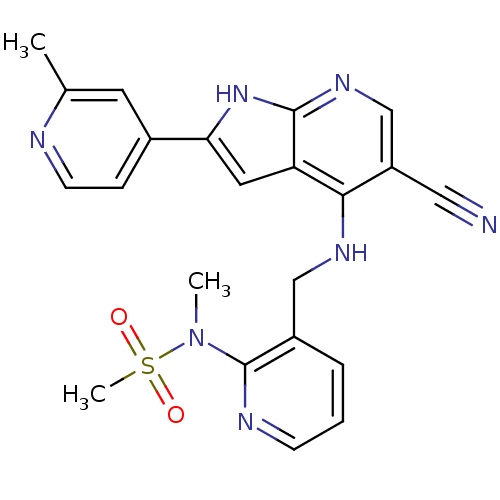
(CHEMBL2315583)Show SMILES CN(c1ncccc1CNc1c(cnc2[nH]c(cc12)-c1ccnc(C)c1)C#N)S(C)(=O)=O Show InChI InChI=1S/C22H21N7O2S/c1-14-9-15(6-8-24-14)19-10-18-20(17(11-23)13-27-21(18)28-19)26-12-16-5-4-7-25-22(16)29(2)32(3,30)31/h4-10,13H,12H2,1-3H3,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Inhibition of FAK (unknown origin) using biotinylated His-TEV-hsFAK(31-686)(K454R) substrate after 2 hrs by scintillation counting analysis |
J Med Chem 56: 1160-70 (2013)
Article DOI: 10.1021/jm3016014
BindingDB Entry DOI: 10.7270/Q2GT5PGP |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50386005
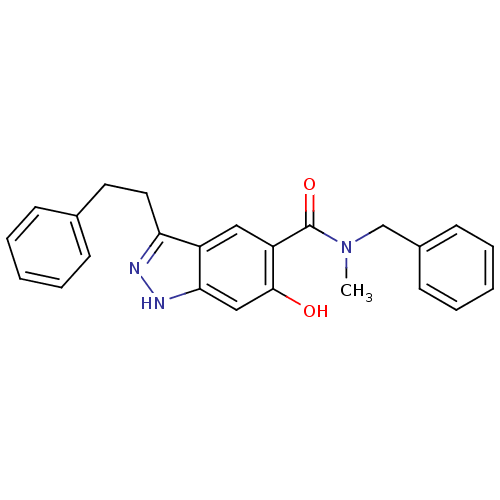
(CHEMBL2042895)Show SMILES CN(Cc1ccccc1)C(=O)c1cc2c(CCc3ccccc3)n[nH]c2cc1O Show InChI InChI=1S/C24H23N3O2/c1-27(16-18-10-6-3-7-11-18)24(29)20-14-19-21(25-26-22(19)15-23(20)28)13-12-17-8-4-2-5-9-17/h2-11,14-15,28H,12-13,16H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]17AAG from human recombinant Hsp90 after 30 mins by beta scintillation counting |
Bioorg Med Chem Lett 22: 4396-403 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.121
BindingDB Entry DOI: 10.7270/Q2T154QC |
More data for this
Ligand-Target Pair | |
Heat shock protein HSP 90-beta
(Homo sapiens (Human)) | BDBM50386031
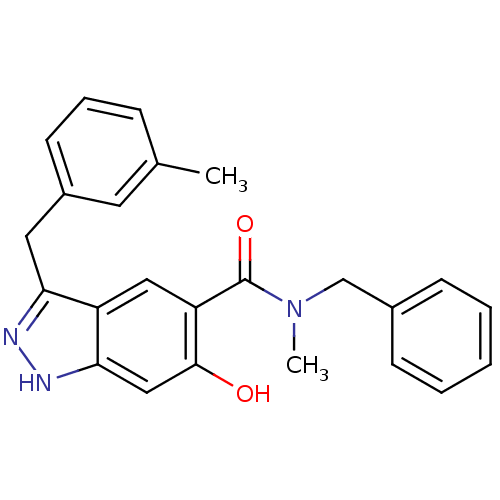
(CHEMBL2042870)Show SMILES CN(Cc1ccccc1)C(=O)c1cc2c(Cc3cccc(C)c3)n[nH]c2cc1O Show InChI InChI=1S/C24H23N3O2/c1-16-7-6-10-18(11-16)12-21-19-13-20(23(28)14-22(19)26-25-21)24(29)27(2)15-17-8-4-3-5-9-17/h3-11,13-14,28H,12,15H2,1-2H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Serono Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]17AAG from human recombinant Hsp90 after 30 mins by beta scintillation counting |
Bioorg Med Chem Lett 22: 4396-403 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.121
BindingDB Entry DOI: 10.7270/Q2T154QC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data