 Found 44 hits with Last Name = 'gulyás' and Initial = 'b'
Found 44 hits with Last Name = 'gulyás' and Initial = 'b' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50007522
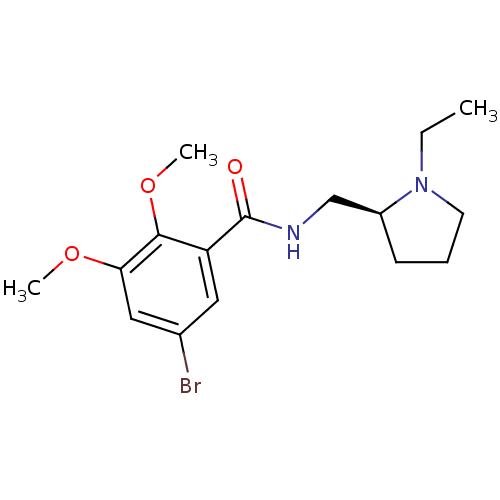
(5-Bromo-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,3-dime...)Show InChI InChI=1S/C16H23BrN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D2 receptor (unknown origin) |
Bioorg Med Chem 16: 6467-73 (2008)
Article DOI: 10.1016/j.bmc.2008.05.039
BindingDB Entry DOI: 10.7270/Q2QN66JM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50007522

(5-Bromo-N-(1-ethyl-pyrrolidin-2-ylmethyl)-2,3-dime...)Show InChI InChI=1S/C16H23BrN2O3/c1-4-19-7-5-6-12(19)10-18-16(20)13-8-11(17)9-14(21-2)15(13)22-3/h8-9,12H,4-7,10H2,1-3H3,(H,18,20)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity to dopamine D3 receptor (unknown origin) |
Bioorg Med Chem 16: 6467-73 (2008)
Article DOI: 10.1016/j.bmc.2008.05.039
BindingDB Entry DOI: 10.7270/Q2QN66JM |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253862
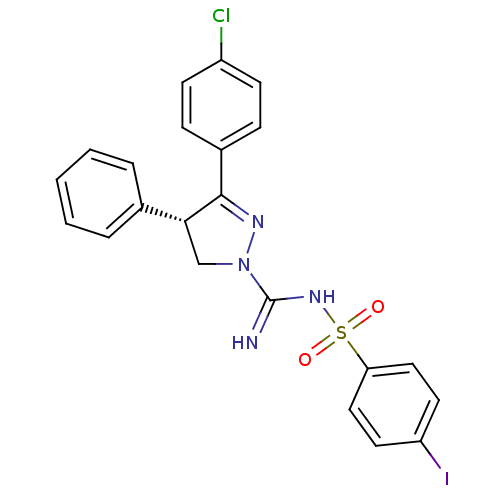
((-)-3-(4-Chlorophenyl)-N'-[(4-iodophenyl)sulfonyl]...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(I)cc1 |r,t:8| Show InChI InChI=1S/C22H18ClIN4O2S/c23-17-8-6-16(7-9-17)21-20(15-4-2-1-3-5-15)14-28(26-21)22(25)27-31(29,30)19-12-10-18(24)11-13-19/h1-13,20H,14H2,(H2,25,27)/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253826
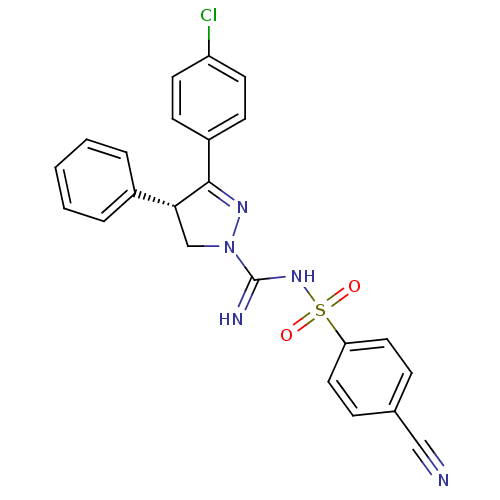
((-)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM35229

(3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)-N-met...)Show InChI InChI=1S/C18H22N2/c1-19-13-6-14-20-17-9-4-2-7-15(17)11-12-16-8-3-5-10-18(16)20/h2-5,7-10,19H,6,11-14H2,1H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity at NET |
Bioorg Med Chem 15: 616-25 (2006)
Article DOI: 10.1016/j.bmc.2006.10.065
BindingDB Entry DOI: 10.7270/Q22B8XPV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB1 receptor by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50311076
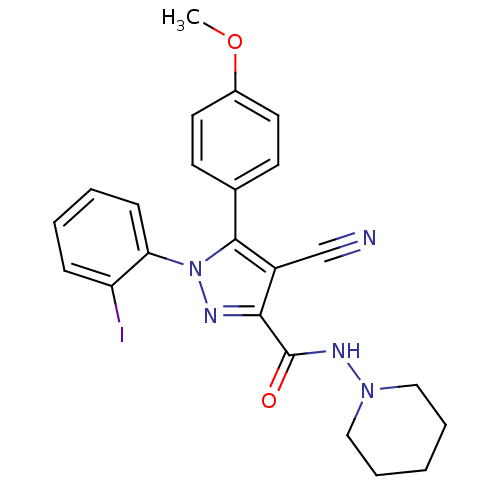
(CHEMBL1079079 | [125I]1-(2-iodophenyl)-4-cyano-5-(...)Show SMILES COc1ccc(cc1)-c1c(C#N)c(nn1-c1ccccc1I)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H22IN5O2/c1-31-17-11-9-16(10-12-17)22-18(15-25)21(23(30)27-28-13-5-2-6-14-28)26-29(22)20-8-4-3-7-19(20)24/h3-4,7-12H,2,5-6,13-14H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB1 receptor by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50199005
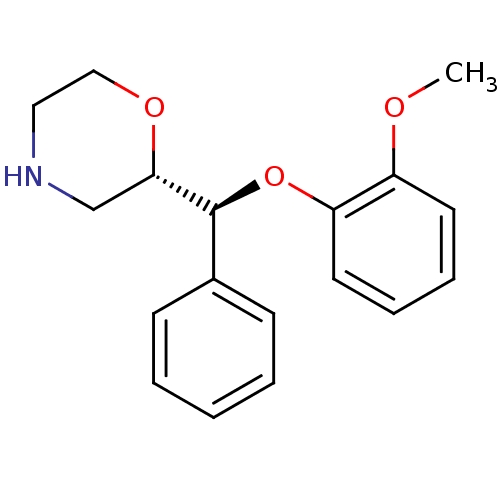
((S)-2-((S)-(2-methoxyphenoxy)(phenyl)methyl)morpho...)Show SMILES COc1ccccc1O[C@H]([C@@H]1CNCCO1)c1ccccc1 |r| Show InChI InChI=1S/C18H21NO3/c1-20-15-9-5-6-10-16(15)22-18(14-7-3-2-4-8-14)17-13-19-11-12-21-17/h2-10,17-19H,11-13H2,1H3/t17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity at NET |
Bioorg Med Chem 15: 616-25 (2006)
Article DOI: 10.1016/j.bmc.2006.10.065
BindingDB Entry DOI: 10.7270/Q22B8XPV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50199003
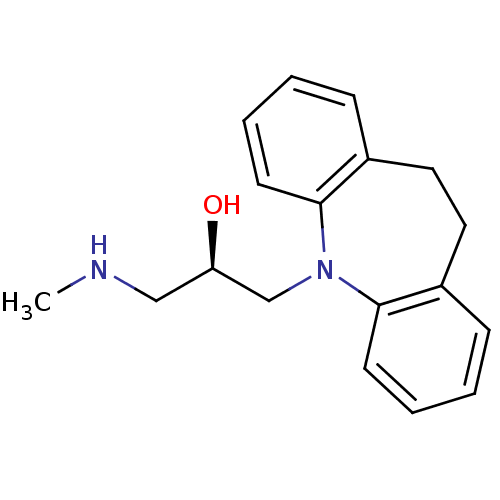
((R)-1-(10,11-dihydro-dibenzo[b,f]azepin-5-yl)-3-me...)Show InChI InChI=1S/C18H22N2O/c1-19-12-16(21)13-20-17-8-4-2-6-14(17)10-11-15-7-3-5-9-18(15)20/h2-9,16,19,21H,10-13H2,1H3/t16-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity at NET |
Bioorg Med Chem 15: 616-25 (2006)
Article DOI: 10.1016/j.bmc.2006.10.065
BindingDB Entry DOI: 10.7270/Q22B8XPV |
More data for this
Ligand-Target Pair | |
Sodium-dependent noradrenaline transporter
(Homo sapiens (Human)) | BDBM50199004
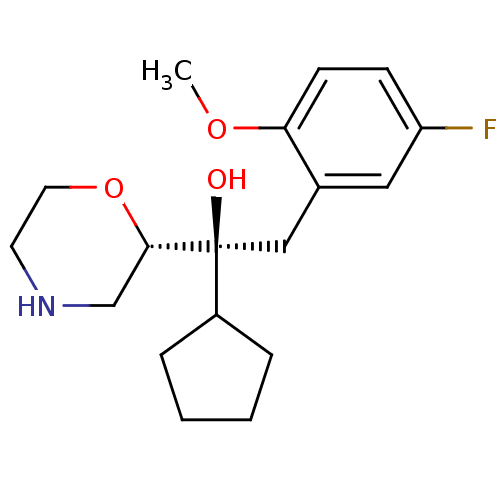
((S,S)-1-cyclopentyl-2-(5-fluoro-2-methoxy-phenyl)-...)Show SMILES COc1ccc(F)cc1C[C@](O)(C1CCCC1)[C@@H]1CNCCO1 Show InChI InChI=1S/C18H26FNO3/c1-22-16-7-6-15(19)10-13(16)11-18(21,14-4-2-3-5-14)17-12-20-8-9-23-17/h6-7,10,14,17,20-21H,2-5,8-9,11-12H2,1H3/t17-,18-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institutet
Curated by ChEMBL
| Assay Description
Binding affinity at NET |
Bioorg Med Chem 15: 616-25 (2006)
Article DOI: 10.1016/j.bmc.2006.10.065
BindingDB Entry DOI: 10.7270/Q22B8XPV |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253861

((+)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253863
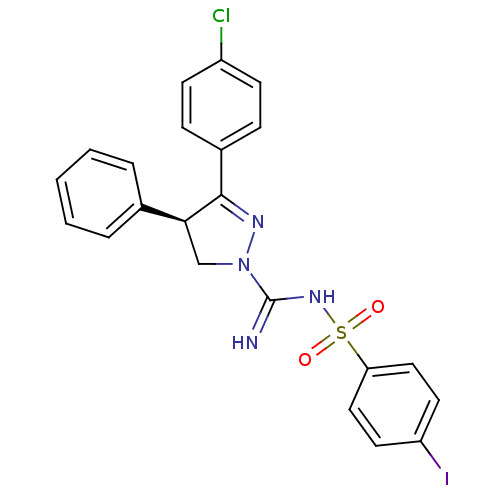
((+)-3-(4-Chlorophenyl)-N'-[(4-iodophenyl)sulfonyl]...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(I)cc1 |r,t:8| Show InChI InChI=1S/C22H18ClIN4O2S/c23-17-8-6-16(7-9-17)21-20(15-4-2-1-3-5-15)14-28(26-21)22(25)27-31(29,30)19-12-10-18(24)11-13-19/h1-13,20H,14H2,(H2,25,27)/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50311076

(CHEMBL1079079 | [125I]1-(2-iodophenyl)-4-cyano-5-(...)Show SMILES COc1ccc(cc1)-c1c(C#N)c(nn1-c1ccccc1I)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H22IN5O2/c1-31-17-11-9-16(10-12-17)22-18(15-25)21(23(30)27-28-13-5-2-6-14-28)26-29(22)20-8-4-3-7-19(20)24/h3-4,7-12H,2,5-6,13-14H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]8OH-DPAT from 5HT1A receptor at 10 uM by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50311076

(CHEMBL1079079 | [125I]1-(2-iodophenyl)-4-cyano-5-(...)Show SMILES COc1ccc(cc1)-c1c(C#N)c(nn1-c1ccccc1I)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H22IN5O2/c1-31-17-11-9-16(10-12-17)22-18(15-25)21(23(30)27-28-13-5-2-6-14-28)26-29(22)20-8-4-3-7-19(20)24/h3-4,7-12H,2,5-6,13-14H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 548 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB2 receptor by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 697 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 927 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP-55940 from CB2 receptor by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50311076

(CHEMBL1079079 | [125I]1-(2-iodophenyl)-4-cyano-5-(...)Show SMILES COc1ccc(cc1)-c1c(C#N)c(nn1-c1ccccc1I)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H22IN5O2/c1-31-17-11-9-16(10-12-17)22-18(15-25)21(23(30)27-28-13-5-2-6-14-28)26-29(22)20-8-4-3-7-19(20)24/h3-4,7-12H,2,5-6,13-14H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]U69593 from kappa opioid receptor at 10 uM by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50311076

(CHEMBL1079079 | [125I]1-(2-iodophenyl)-4-cyano-5-(...)Show SMILES COc1ccc(cc1)-c1c(C#N)c(nn1-c1ccccc1I)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H22IN5O2/c1-31-17-11-9-16(10-12-17)22-18(15-25)21(23(30)27-28-13-5-2-6-14-28)26-29(22)20-8-4-3-7-19(20)24/h3-4,7-12H,2,5-6,13-14H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from mu opioid at 10 uM by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Translocator protein
(Homo sapiens (Human)) | BDBM50311076

(CHEMBL1079079 | [125I]1-(2-iodophenyl)-4-cyano-5-(...)Show SMILES COc1ccc(cc1)-c1c(C#N)c(nn1-c1ccccc1I)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H22IN5O2/c1-31-17-11-9-16(10-12-17)22-18(15-25)21(23(30)27-28-13-5-2-6-14-28)26-29(22)20-8-4-3-7-19(20)24/h3-4,7-12H,2,5-6,13-14H2,1H3,(H,27,30) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]PK11195 from TSPO at 10 uM by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50253862

((-)-3-(4-Chlorophenyl)-N'-[(4-iodophenyl)sulfonyl]...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(I)cc1 |r,t:8| Show InChI InChI=1S/C22H18ClIN4O2S/c23-17-8-6-16(7-9-17)21-20(15-4-2-1-3-5-15)14-28(26-21)22(25)27-31(29,30)19-12-10-18(24)11-13-19/h1-13,20H,14H2,(H2,25,27)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50253861

((+)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50253826

((-)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50253863

((+)-3-(4-Chlorophenyl)-N'-[(4-iodophenyl)sulfonyl]...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(I)cc1 |r,t:8| Show InChI InChI=1S/C22H18ClIN4O2S/c23-17-8-6-16(7-9-17)21-20(15-4-2-1-3-5-15)14-28(26-21)22(25)27-31(29,30)19-12-10-18(24)11-13-19/h1-13,20H,14H2,(H2,25,27)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Homo sapiens (Human)) | BDBM50253826

((-)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >7.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]ketanserin from human cloned 5HT2A receptor |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Histamine H2 receptor
(Homo sapiens (Human)) | BDBM50253826

((-)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]tiotidine from human cloned histamine H2 receptor |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50253826

((-)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [3H]alpha methyl histamine from human cloned histamine H3 receptor |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(Homo sapiens (Human)) | BDBM50311076

(CHEMBL1079079 | [125I]1-(2-iodophenyl)-4-cyano-5-(...)Show SMILES COc1ccc(cc1)-c1c(C#N)c(nn1-c1ccccc1I)C(=O)NN1CCCCC1 Show InChI InChI=1S/C23H22IN5O2/c1-31-17-11-9-16(10-12-17)22-18(15-25)21(23(30)27-28-13-5-2-6-14-28)26-29(22)20-8-4-3-7-19(20)24/h3-4,7-12H,2,5-6,13-14H2,1H3,(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodoclonidine from alpha-2C adrenergic receptor at 10 uM by scintillation counting |
Bioorg Med Chem Lett 19: 6209-12 (2009)
Article DOI: 10.1016/j.bmcl.2009.08.092
BindingDB Entry DOI: 10.7270/Q23J3D3H |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253862

((-)-3-(4-Chlorophenyl)-N'-[(4-iodophenyl)sulfonyl]...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(I)cc1 |r,t:8| Show InChI InChI=1S/C22H18ClIN4O2S/c23-17-8-6-16(7-9-17)21-20(15-4-2-1-3-5-15)14-28(26-21)22(25)27-31(29,30)19-12-10-18(24)11-13-19/h1-13,20H,14H2,(H2,25,27)/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253826

((-)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM15581

(CHEMBL8706 | CLG | CLORGILINE | Clorgyline | N-[3-...)Show InChI InChI=1S/C13H15Cl2NO/c1-3-7-16(2)8-4-9-17-13-6-5-11(14)10-12(13)15/h1,5-6,10H,4,7-9H2,2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 30.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAO-A expressed in insect cells assessed as inhibition of kynuramine oxidation after 30 mins by fluorescence assay |
J Med Chem 54: 7023-9 (2011)
Article DOI: 10.1021/jm200710b
BindingDB Entry DOI: 10.7270/Q2765FQJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM15579

(CHEMBL972 | DEPRENYL | L-Deprenyl | N-methyl-N-[(2...)Show InChI InChI=1S/C13H17N/c1-4-10-14(3)12(2)11-13-8-6-5-7-9-13/h1,5-9,12H,10-11H2,2-3H3/t12-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 85.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAO-B expressed in insect cells assessed as inhibition of kynuramine oxidation after 30 mins by fluorescence assay |
J Med Chem 54: 7023-9 (2011)
Article DOI: 10.1021/jm200710b
BindingDB Entry DOI: 10.7270/Q2765FQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253861

((+)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50253863

((+)-3-(4-Chlorophenyl)-N'-[(4-iodophenyl)sulfonyl]...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(I)cc1 |r,t:8| Show InChI InChI=1S/C22H18ClIN4O2S/c23-17-8-6-16(7-9-17)21-20(15-4-2-1-3-5-15)14-28(26-21)22(25)27-31(29,30)19-12-10-18(24)11-13-19/h1-13,20H,14H2,(H2,25,27)/t20-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB1 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50355808
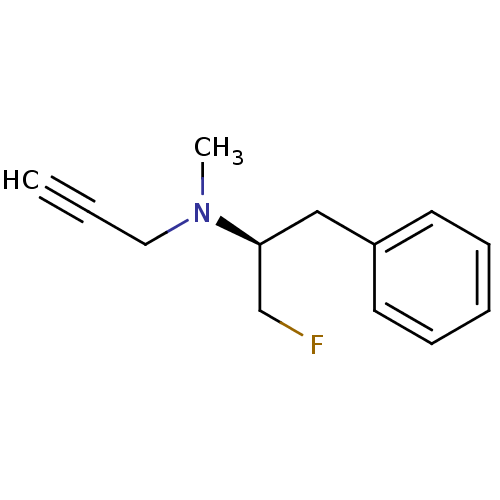
(CHEMBL1911928)Show InChI InChI=1S/C13H16FN/c1-3-9-15(2)13(11-14)10-12-7-5-4-6-8-12/h1,4-8,13H,9-11H2,2H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 171 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAO-B expressed in insect cells assessed as inhibition of kynuramine oxidation after 30 mins by fluorescence assay |
J Med Chem 54: 7023-9 (2011)
Article DOI: 10.1021/jm200710b
BindingDB Entry DOI: 10.7270/Q2765FQJ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50355806
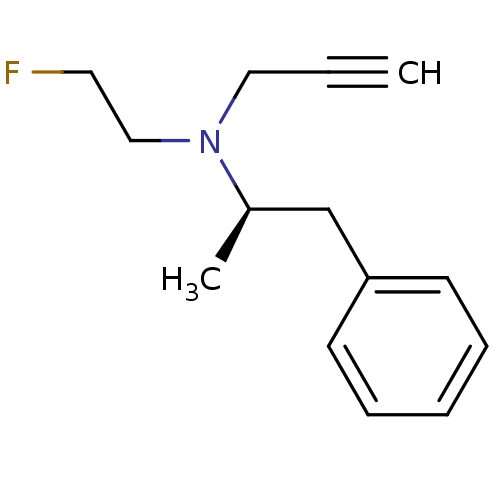
(CHEMBL1911926)Show InChI InChI=1S/C14H18FN/c1-3-10-16(11-9-15)13(2)12-14-7-5-4-6-8-14/h1,4-8,13H,9-12H2,2H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAO-B expressed in insect cells assessed as inhibition of kynuramine oxidation after 30 mins by fluorescence assay |
J Med Chem 54: 7023-9 (2011)
Article DOI: 10.1021/jm200710b
BindingDB Entry DOI: 10.7270/Q2765FQJ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM50355808

(CHEMBL1911928)Show InChI InChI=1S/C13H16FN/c1-3-9-15(2)13(11-14)10-12-7-5-4-6-8-12/h1,4-8,13H,9-11H2,2H3/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAO-A expressed in insect cells assessed as inhibition of kynuramine oxidation after 30 mins by fluorescence assay |
J Med Chem 54: 7023-9 (2011)
Article DOI: 10.1021/jm200710b
BindingDB Entry DOI: 10.7270/Q2765FQJ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM50355807
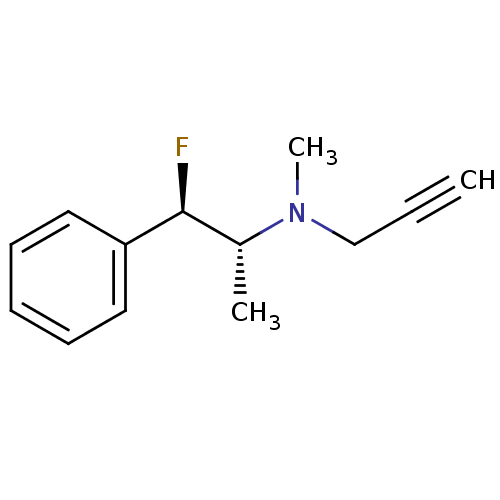
(CHEMBL1911927)Show InChI InChI=1S/C13H16FN/c1-4-10-15(3)11(2)13(14)12-8-6-5-7-9-12/h1,5-9,11,13H,10H2,2-3H3/t11-,13+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Karolinska Institutet
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant MAO-B expressed in insect cells assessed as inhibition of kynuramine oxidation after 30 mins by fluorescence assay |
J Med Chem 54: 7023-9 (2011)
Article DOI: 10.1021/jm200710b
BindingDB Entry DOI: 10.7270/Q2765FQJ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21278

(5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...)Show SMILES Cc1c(nn(c1-c1ccc(Cl)cc1)-c1ccc(Cl)cc1Cl)C(=O)NN1CCCCC1 Show InChI InChI=1S/C22H21Cl3N4O/c1-14-20(22(30)27-28-11-3-2-4-12-28)26-29(19-10-9-17(24)13-18(19)25)21(14)15-5-7-16(23)8-6-15/h5-10,13H,2-4,11-12H2,1H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50253826

((-)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50253861

((+)-3-(4-Chlorophenyl)-N'-[(4-cyanophenyl)sulfonyl...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(cc1)C#N |r,t:8| Show InChI InChI=1S/C23H18ClN5O2S/c24-19-10-8-18(9-11-19)22-21(17-4-2-1-3-5-17)15-29(27-22)23(26)28-32(30,31)20-12-6-16(14-25)7-13-20/h1-13,21H,15H2,(H2,26,28)/t21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50253863

((+)-3-(4-Chlorophenyl)-N'-[(4-iodophenyl)sulfonyl]...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(I)cc1 |r,t:8| Show InChI InChI=1S/C22H18ClIN4O2S/c23-17-8-6-16(7-9-17)21-20(15-4-2-1-3-5-15)14-28(26-21)22(25)27-31(29,30)19-12-10-18(24)11-13-19/h1-13,20H,14H2,(H2,25,27)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50253862

((-)-3-(4-Chlorophenyl)-N'-[(4-iodophenyl)sulfonyl]...)Show SMILES Clc1ccc(cc1)C1=NN(C[C@@H]1c1ccccc1)C(=N)NS(=O)(=O)c1ccc(I)cc1 |r,t:8| Show InChI InChI=1S/C22H18ClIN4O2S/c23-17-8-6-16(7-9-17)21-20(15-4-2-1-3-5-15)14-28(26-21)22(25)27-31(29,30)19-12-10-18(24)11-13-19/h1-13,20H,14H2,(H2,25,27)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Mental Health
Curated by ChEMBL
| Assay Description
Binding affinity to human CB2 receptor by [35S]GTPgammaS binding assay |
J Med Chem 51: 5608-16 (2008)
Article DOI: 10.1021/jm800329z
BindingDB Entry DOI: 10.7270/Q2GT5N00 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data