

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM21397 (8-[4-(4-fluorophenyl)-4-keto-butyl]-1-phenyl-1,3,8...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Spiperone from human recombinant dopamine D2S receptor expressed in CHO cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 2 (Homo sapiens (Human)) | BDBM50435127 (CHEMBL2392022) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Peptide YY from neuropeptide Y receptor type 2 in human KAN-TS cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM85035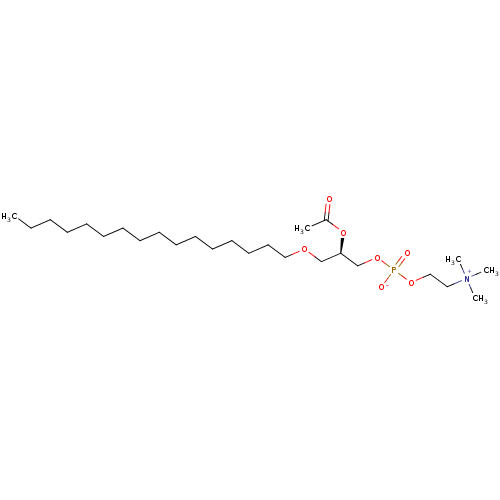 (CAS_65154-06-5 | PAF | bloodplatelet-activatingfac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]PAF from platelet activating factor receptor in human platelets after 3 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50015490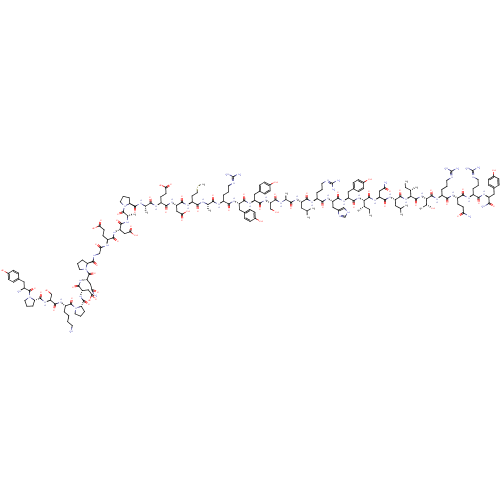 (CHEMBL438945 | H-YPSKPDNPGEDAPAEDMARYYSALRHYINLITR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Peptide YY from neuropeptide Y receptor type 1 in human SK-N-MC cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B1 bradykinin receptor (Homo sapiens (Human)) | BDBM50156454 (CHEMBL264100 | des-Arg10-Kallidin) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H](Des-Arg10)-Kallidin from bradykinin B1 receptor in human IMR90 cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50165019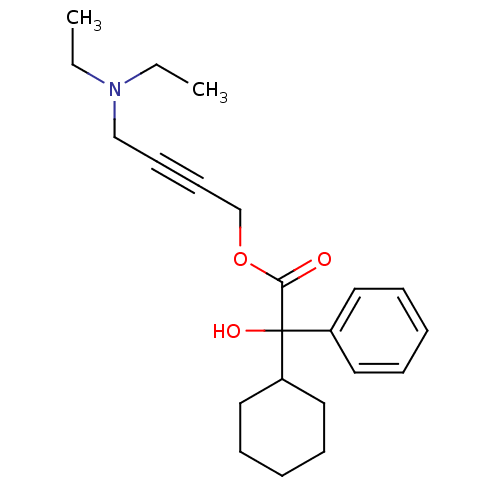 (4-(Diethylamino)-2-butynyl alpha-phenylcyclohexane...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Affinity for rat Muscarinic acetylcholine receptor M3 | Bioorg Med Chem Lett 15: 2093-6 (2005) Article DOI: 10.1016/j.bmcl.2005.02.036 BindingDB Entry DOI: 10.7270/Q2DJ5F4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| B2 bradykinin receptor (Homo sapiens (Human)) | BDBM50049949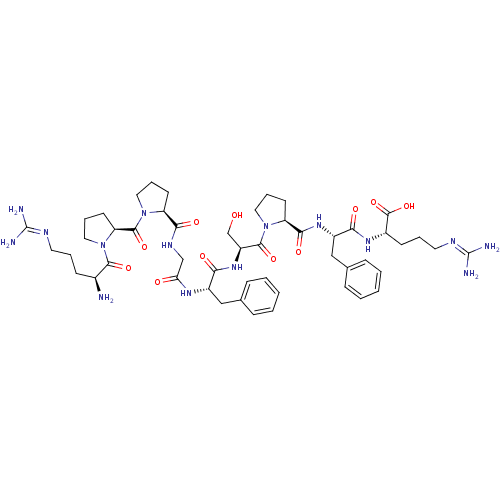 ((BK) H-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH | (b...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Bradykinin from human recombinant bradykinin B2 receptor expressed in CHEM1 cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H1 receptor (Homo sapiens (Human)) | BDBM22567 (3H]pyrilamine | CHEMBL511 | Dorantamin | Mepyramin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Pyrilamine from human recombinant histamine H1 receptor expressed in CHOK1 cells after 3 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50218053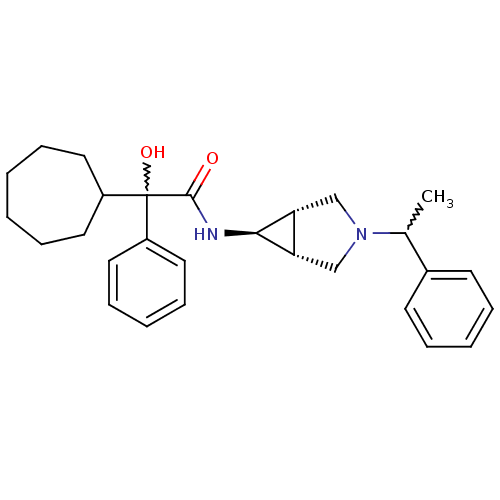 ((R)-2-cycloheptyl-2-hydroxy-2-phenyl-N-((1S,5R,6s)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine from muscarinic M3 receptor in rat submandibular gland | Bioorg Med Chem Lett 17: 5256-60 (2007) Article DOI: 10.1016/j.bmcl.2007.06.081 BindingDB Entry DOI: 10.7270/Q27D2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18207 ((1R,2S,10S,11S,13R,14R,15S,17S)-1-fluoro-14,17-dih...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Dexamethasone from glucocorticoid receptor in human HeLaS3 cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50109647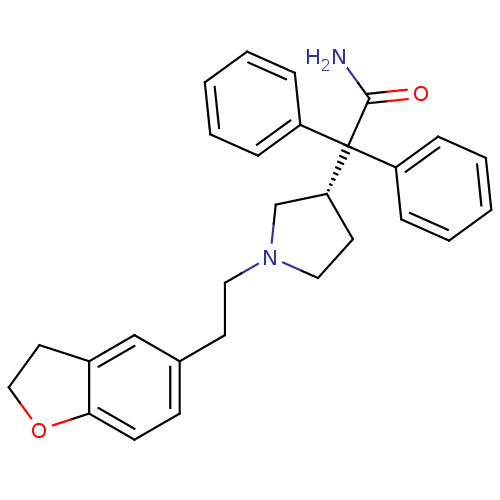 (2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine from muscarinic M3 receptor in rat submandibular gland | Bioorg Med Chem Lett 17: 5256-60 (2007) Article DOI: 10.1016/j.bmcl.2007.06.081 BindingDB Entry DOI: 10.7270/Q27D2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM82561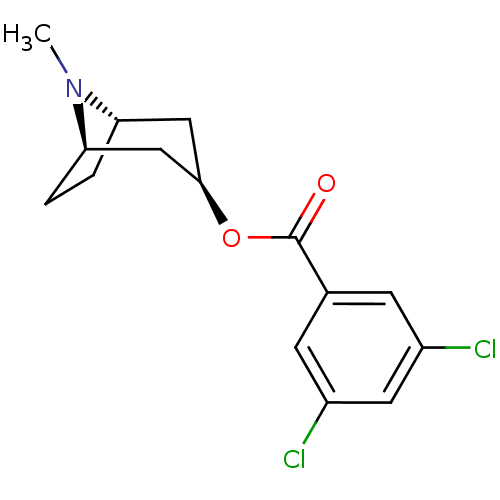 (CAS_40796-97-2 | TROPANYL 3,5-DICHLOROBENZOATE | T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]GR65630 from human recombinant 5HT3 receptor expressed in HEK293 cells after 60 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50435126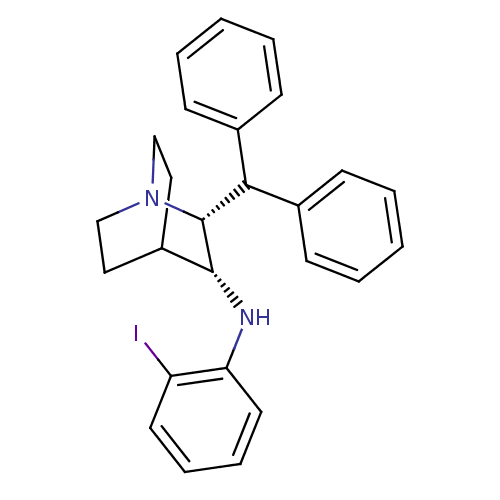 (CHEMBL2392023) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Substance P from human recombinant substance P receptor expressed in CHO cells after 90 mins | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid type B receptor subunit 1 (Homo sapiens (Human)) | BDBM50435128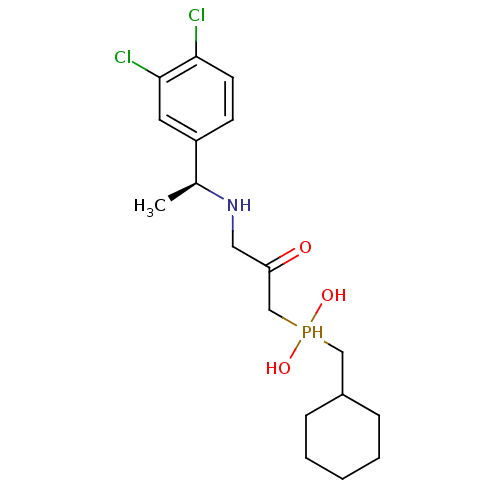 (CHEMBL2391908) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]CGP54626 from human recombinant GABAB1A receptor expressed in CHO cells after 3 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319849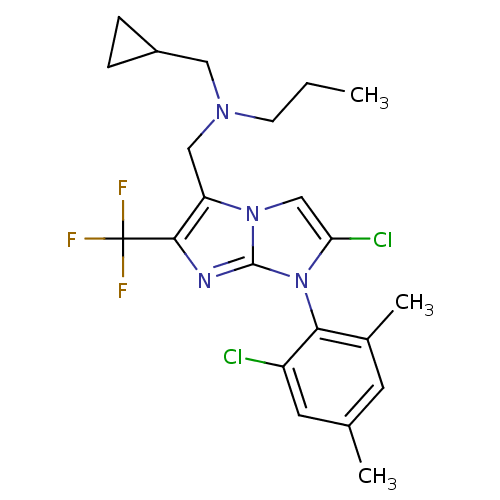 (CHEMBL1083921 | N-((2-chloro-1-(2-chloro-4,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Rattus norvegicus (Rat)) | BDBM8885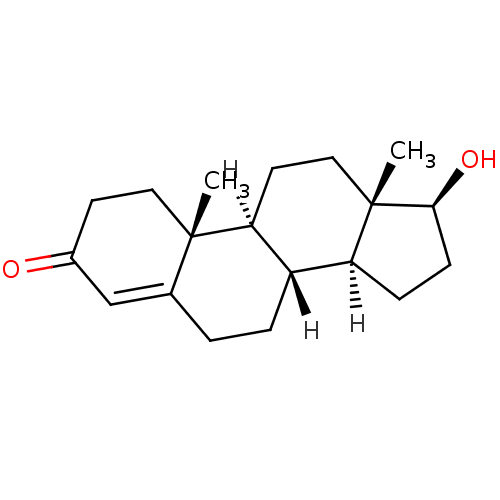 ((1S,2R,10R,11S,14S,15S)-14-hydroxy-2,15-dimethylte...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Mibolerone from rat recombinant androgen receptor expressed in Escherichia coli after 4 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319896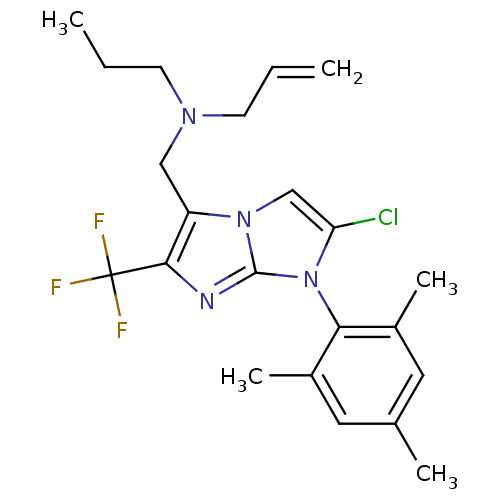 (CHEMBL1084999 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319906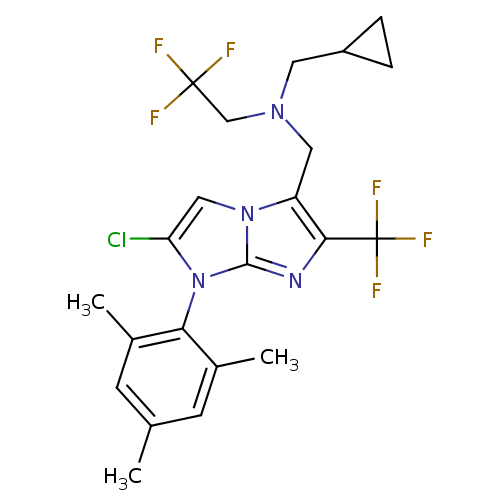 (CHEMBL1083933 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319893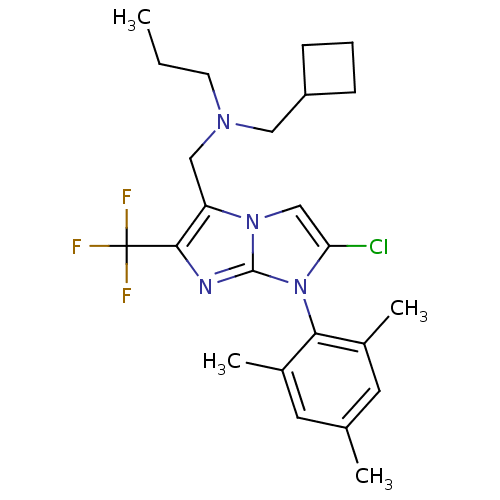 (CHEMBL1083952 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50218045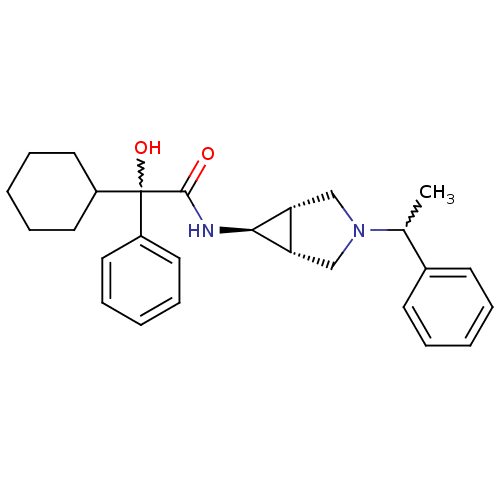 ((R)-2-cyclohexyl-2-hydroxy-2-phenyl-N-((1S,5R,6s)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine from muscarinic M3 receptor in rat submandibular gland | Bioorg Med Chem Lett 17: 5256-60 (2007) Article DOI: 10.1016/j.bmcl.2007.06.081 BindingDB Entry DOI: 10.7270/Q27D2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319869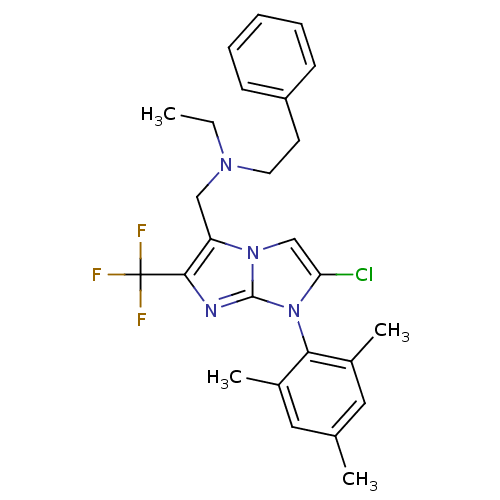 (CHEMBL1086504 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50165017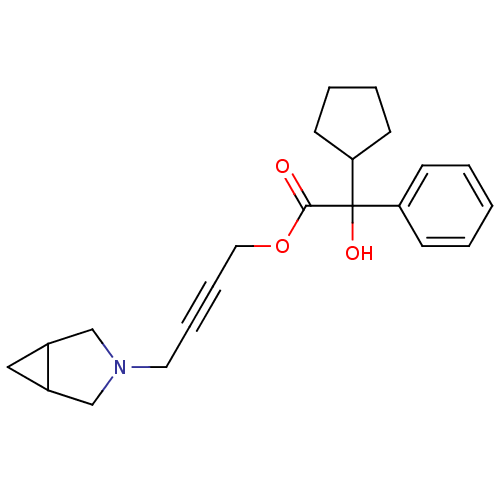 (CHEMBL192485 | Cyclopentyl-hydroxy-phenyl-acetic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Ki value for rat Muscarinic acetylcholine receptor M3 | Bioorg Med Chem Lett 15: 2093-6 (2005) Article DOI: 10.1016/j.bmcl.2005.02.036 BindingDB Entry DOI: 10.7270/Q2DJ5F4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50165017 (CHEMBL192485 | Cyclopentyl-hydroxy-phenyl-acetic a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Ki value for rat Muscarinic acetylcholine receptor M3 | Bioorg Med Chem Lett 15: 2093-6 (2005) Article DOI: 10.1016/j.bmcl.2005.02.036 BindingDB Entry DOI: 10.7270/Q2DJ5F4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319890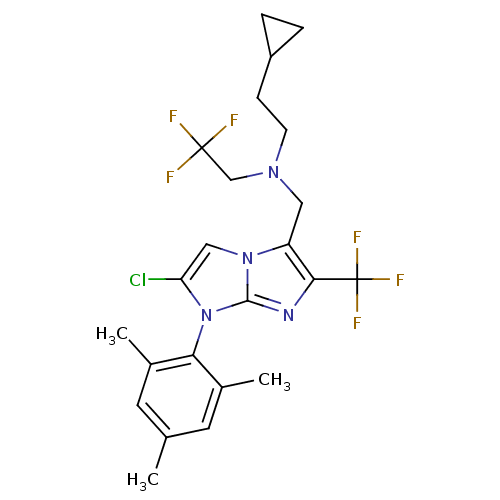 (CHEMBL1082696 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50218061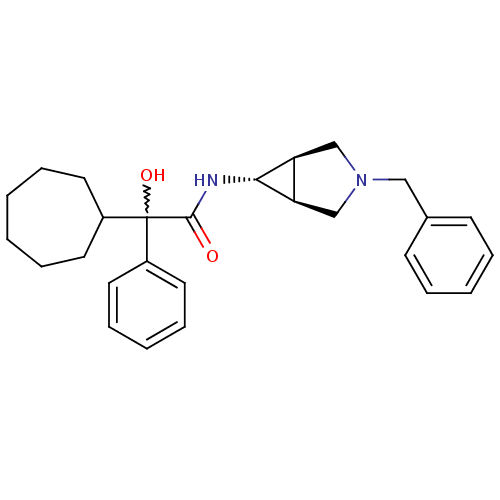 ((R)-N-((1S,5R,6s)-3-benzyl-3-aza-bicyclo[3.1.0]hex...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine from muscarinic M3 receptor in rat submandibular gland | Bioorg Med Chem Lett 17: 5256-60 (2007) Article DOI: 10.1016/j.bmcl.2007.06.081 BindingDB Entry DOI: 10.7270/Q27D2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 6.91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Affinity for rat Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 15: 2093-6 (2005) Article DOI: 10.1016/j.bmcl.2005.02.036 BindingDB Entry DOI: 10.7270/Q2DJ5F4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (RAT) | BDBM50165019 (4-(Diethylamino)-2-butynyl alpha-phenylcyclohexane...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Affinity for rat Muscarinic acetylcholine receptor M2 | Bioorg Med Chem Lett 15: 2093-6 (2005) Article DOI: 10.1016/j.bmcl.2005.02.036 BindingDB Entry DOI: 10.7270/Q2DJ5F4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50165008 ((+)-(R)-2-(alpha-(2-(Diisopropylamino)ethyl)benzyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7.07 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Affinity for rat Muscarinic acetylcholine receptor M3 | Bioorg Med Chem Lett 15: 2093-6 (2005) Article DOI: 10.1016/j.bmcl.2005.02.036 BindingDB Entry DOI: 10.7270/Q2DJ5F4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50314008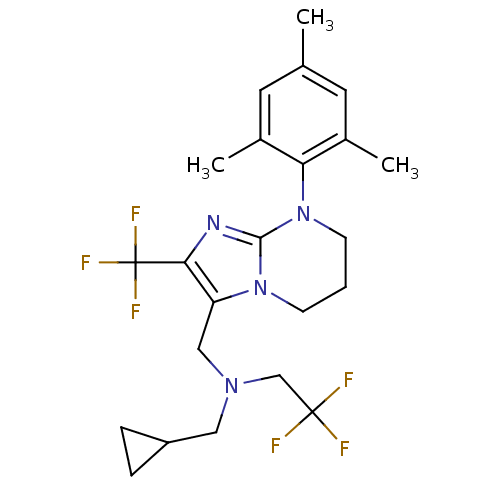 (CHEMBL1087165 | N-(cyclopropylmethyl)-2,2,2-triflu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from human CRFR1 expressed in human IMR32 cells | Bioorg Med Chem Lett 20: 1905-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.127 BindingDB Entry DOI: 10.7270/Q24Q7V3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319903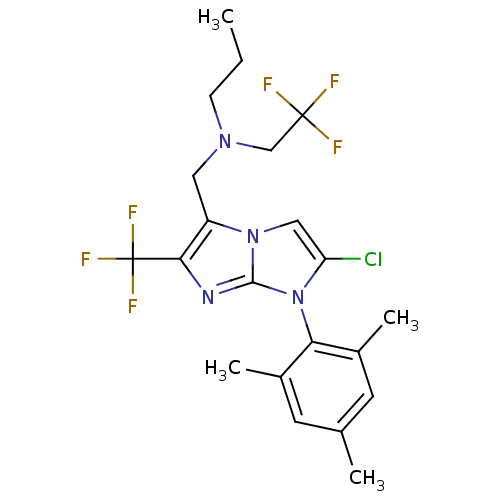 (CHEMBL1082302 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [3H]Haloperidol from sigma 1 receptor in human jurkat cells after 4 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319905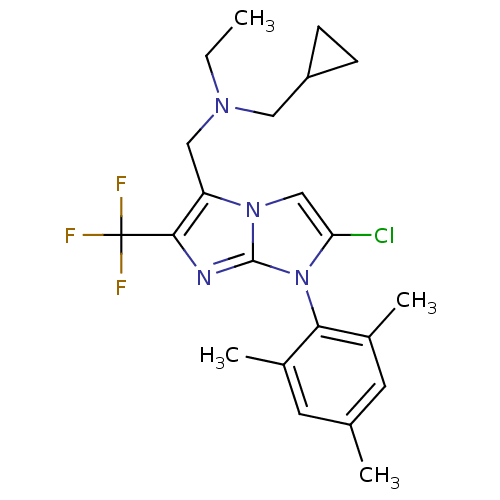 (CHEMBL1083319 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319907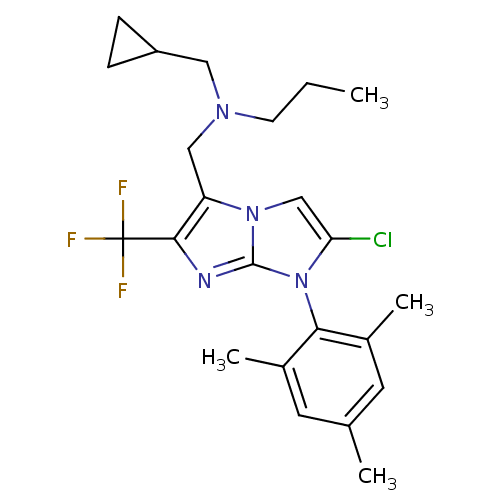 (CHEMBL1086468 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50218043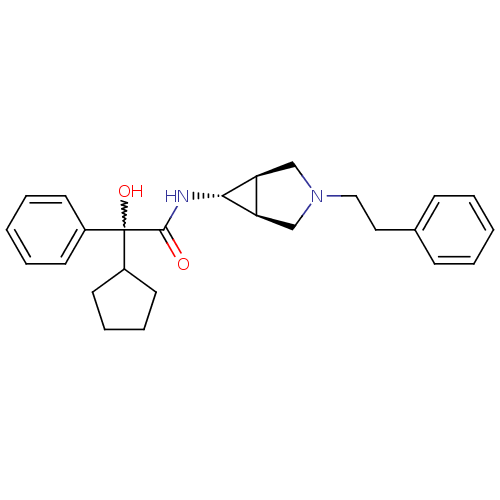 ((R)-2-cyclopentyl-2-hydroxy-N-((1S,5R,6s)-3-phenet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine from muscarinic M3 receptor in rat submandibular gland | Bioorg Med Chem Lett 17: 5256-60 (2007) Article DOI: 10.1016/j.bmcl.2007.06.081 BindingDB Entry DOI: 10.7270/Q27D2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319902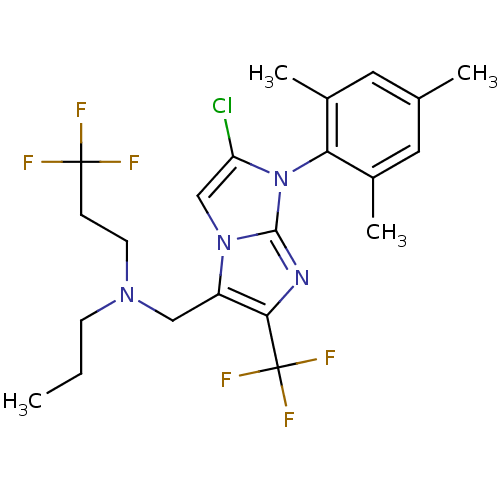 (CHEMBL1083935 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319882 (CHEMBL1082338 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50218057 ((R)-N-((1S,5R,6s)-3-(2-(benzo[d][1,3]dioxol-5-yl)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine from muscarinic M3 receptor in rat submandibular gland | Bioorg Med Chem Lett 17: 5256-60 (2007) Article DOI: 10.1016/j.bmcl.2007.06.081 BindingDB Entry DOI: 10.7270/Q27D2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50218051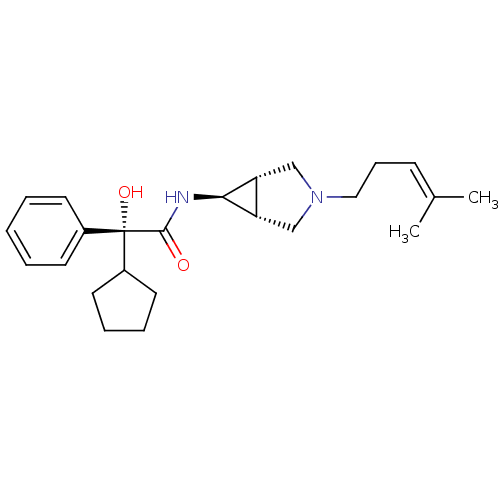 ((R)-2-cyclopentyl-2-hydroxy-N-((1S,5R,6s)-3-(4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine from muscarinic M3 receptor in rat submandibular gland | Bioorg Med Chem Lett 17: 5256-60 (2007) Article DOI: 10.1016/j.bmcl.2007.06.081 BindingDB Entry DOI: 10.7270/Q27D2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319895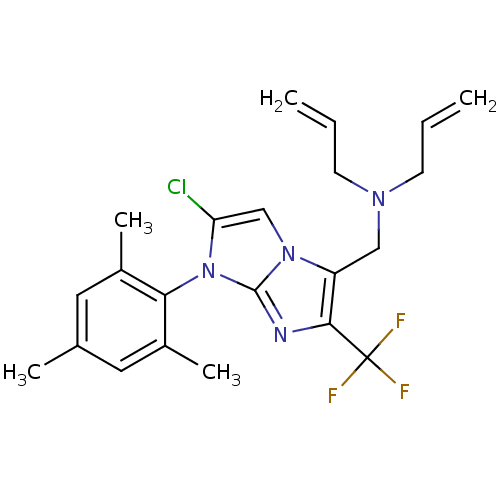 (CHEMBL1085265 | N-allyl-N-((2-chloro-1-mesityl-6-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319892 (CHEMBL1084235 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319848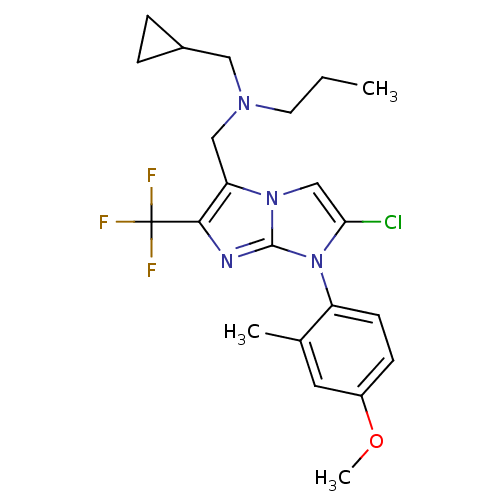 (CHEMBL1083922 | N-((2-chloro-1-(4-methoxy-2-methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319889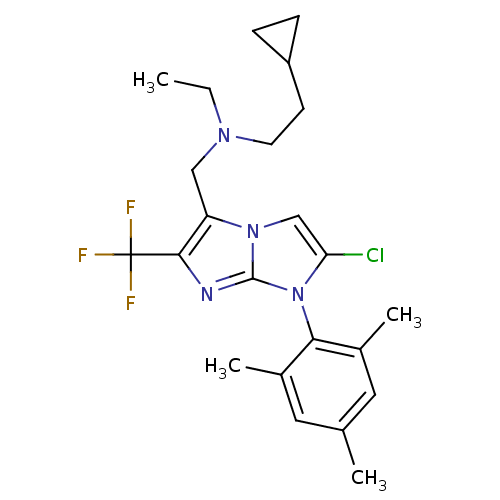 (CHEMBL1086594 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50165012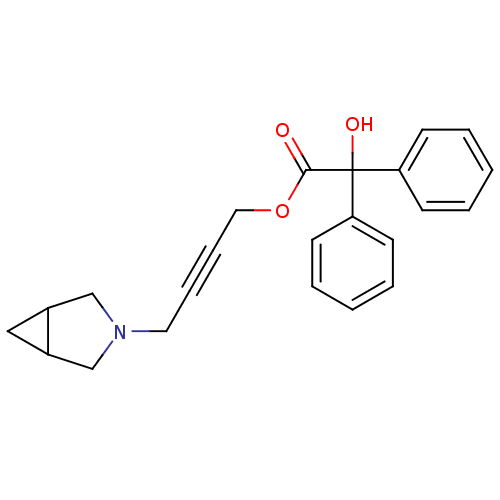 (CHEMBL192065 | Hydroxy-diphenyl-acetic acid 4-(3-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Affinity for rat Muscarinic acetylcholine receptor M3 | Bioorg Med Chem Lett 15: 2093-6 (2005) Article DOI: 10.1016/j.bmcl.2005.02.036 BindingDB Entry DOI: 10.7270/Q2DJ5F4X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50314009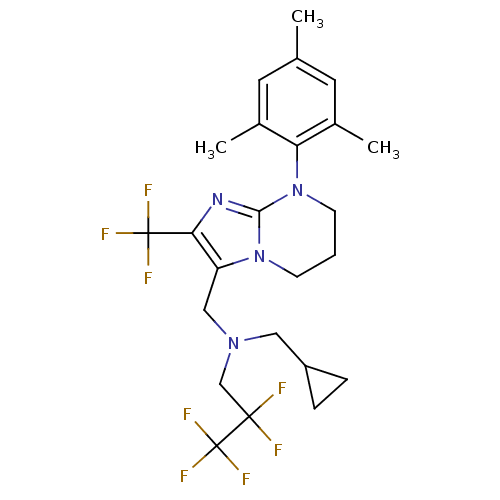 (CHEMBL1087166 | N-(cyclopropylmethyl)-2,2,3,3,3-pe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from human CRFR1 expressed in human IMR32 cells | Bioorg Med Chem Lett 20: 1905-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.127 BindingDB Entry DOI: 10.7270/Q24Q7V3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319904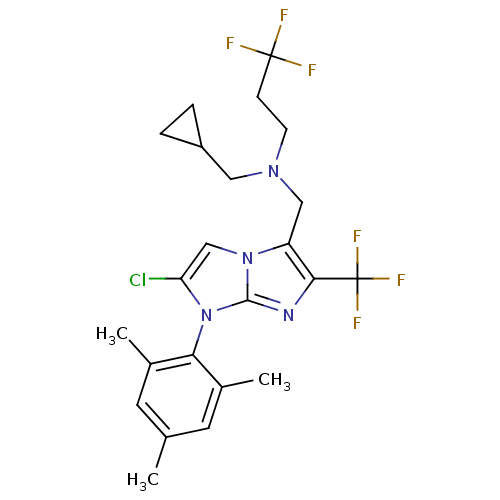 (CHEMBL1083320 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319891 (CHEMBL1084511 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM22568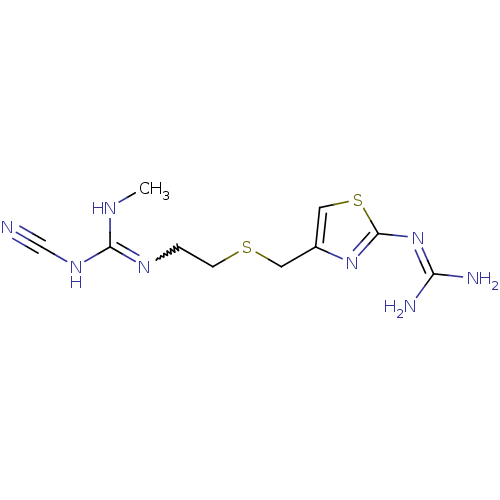 (1-cyano-3-{2-[({2-[(diaminomethylidene)amino]-1,3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Broad Institute of MIT and Harvard Curated by ChEMBL | Assay Description Displacement of [125I]Aminopotentidine from human recombinant histamine H2 receptor expressed in CHOK1 cells after 2 hrs | Bioorg Med Chem Lett 23: 1834-8 (2013) Article DOI: 10.1016/j.bmcl.2013.01.025 BindingDB Entry DOI: 10.7270/Q2P55PWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50314010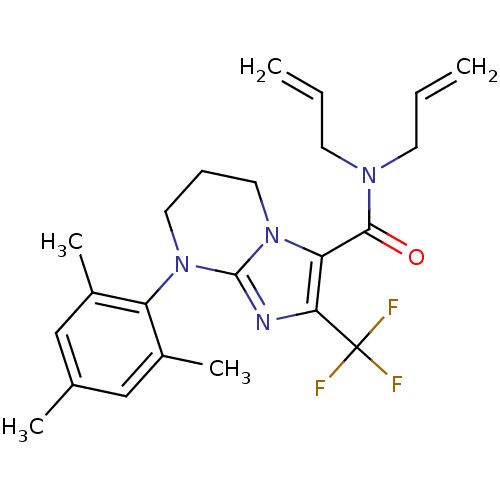 (CHEMBL1087167 | N,N-diallyl-8-mesityl-2-(trifluoro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development Curated by ChEMBL | Assay Description Displacement of [125I]Tyr-o-CRF from human CRFR1 expressed in human IMR32 cells | Bioorg Med Chem Lett 20: 1905-9 (2010) Article DOI: 10.1016/j.bmcl.2010.01.127 BindingDB Entry DOI: 10.7270/Q24Q7V3F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50218058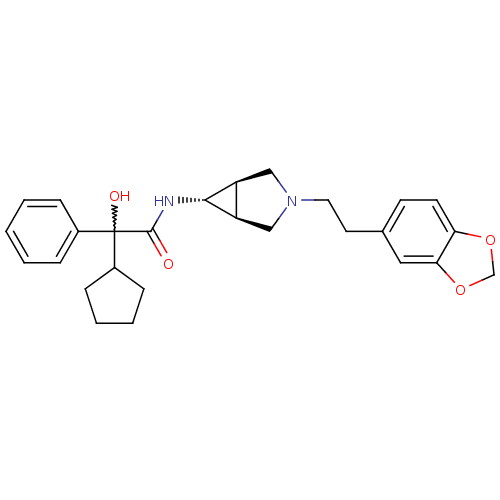 ((R)-N-((1S,5R,6s)-3-(2-(benzo[d][1,3]dioxol-5-yl)e...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]N-methylscopolamine from muscarinic M3 receptor in rat submandibular gland | Bioorg Med Chem Lett 17: 5256-60 (2007) Article DOI: 10.1016/j.bmcl.2007.06.081 BindingDB Entry DOI: 10.7270/Q27D2W0F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Corticotropin-releasing factor receptor 1 (Homo sapiens (Human)) | BDBM50319870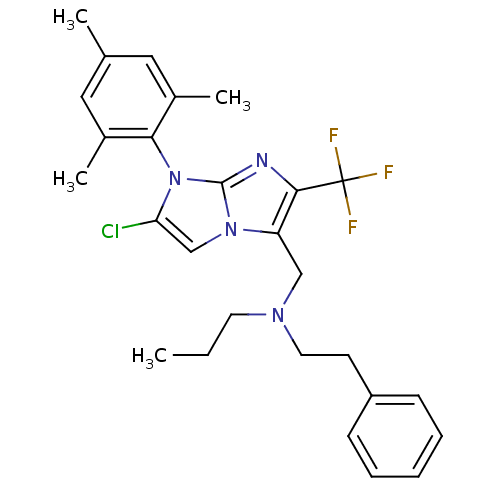 (CHEMBL1083102 | N-((2-chloro-1-mesityl-6-(trifluor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Displacement of [125I] Tyr-o-CRF from human CRF[125I]receptor expressed in human IMR-3[125I]cells after 100 mins | Bioorg Med Chem Lett 20: 3669-74 (2010) Article DOI: 10.1016/j.bmcl.2010.04.094 BindingDB Entry DOI: 10.7270/Q2VX0GPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2509 total ) | Next | Last >> |