 Found 97 hits with Last Name = 'haber' and Initial = 'e'
Found 97 hits with Last Name = 'haber' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50021989
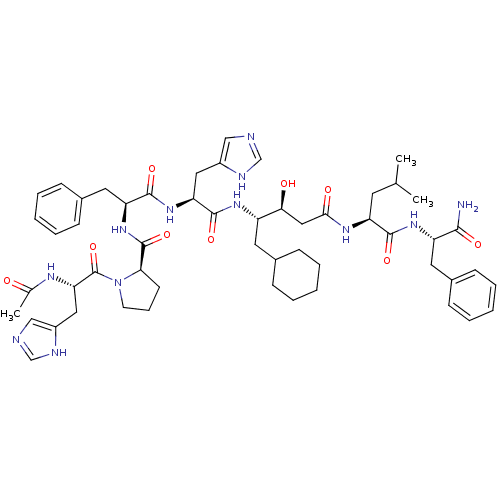
(Ac-His-Pro-Phe-His-ACHPA-Leu-Phe-NH2 | CHEMBL38686...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H74N12O9/c1-33(2)22-42(50(71)63-41(49(55)70)24-36-16-9-5-10-17-36)61-48(69)28-47(68)40(23-35-14-7-4-8-15-35)62-52(73)44(26-38-29-56-31-58-38)64-51(72)43(25-37-18-11-6-12-19-37)65-53(74)46-20-13-21-66(46)54(75)45(60-34(3)67)27-39-30-57-32-59-39/h5-6,9-12,16-19,29-33,35,40-47,68H,4,7-8,13-15,20-28H2,1-3H3,(H2,55,70)(H,56,58)(H,57,59)(H,60,67)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)/t40-,41-,42-,43-,44-,45-,46+,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human plasma renin |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022978
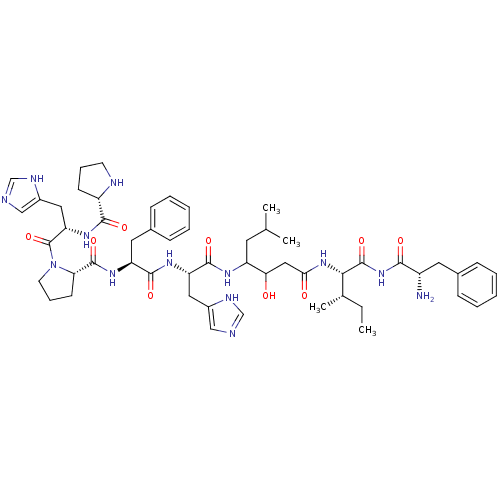
(CHEMBL264339 | Pro-His-Pro-Phe-His-Statine-Ile-Phe...)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1)C(=O)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C54H75N13O9/c1-5-33(4)47(53(75)66-48(70)38(55)23-34-14-8-6-9-15-34)65-46(69)27-45(68)40(22-32(2)3)61-51(73)42(25-36-28-56-30-59-36)62-50(72)41(24-35-16-10-7-11-17-35)63-52(74)44-19-13-21-67(44)54(76)43(26-37-29-57-31-60-37)64-49(71)39-18-12-20-58-39/h6-11,14-17,28-33,38-45,47,58,68H,5,12-13,18-27,55H2,1-4H3,(H,56,59)(H,57,60)(H,61,73)(H,62,72)(H,63,74)(H,64,71)(H,65,69)(H,66,70,75)/t33-,38-,39-,40?,41-,42-,43-,44-,45?,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory potency on plasma renin obtained from cats |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022978

(CHEMBL264339 | Pro-His-Pro-Phe-His-Statine-Ile-Phe...)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1)C(=O)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C54H75N13O9/c1-5-33(4)47(53(75)66-48(70)38(55)23-34-14-8-6-9-15-34)65-46(69)27-45(68)40(22-32(2)3)61-51(73)42(25-36-28-56-30-59-36)62-50(72)41(24-35-16-10-7-11-17-35)63-52(74)44-19-13-21-67(44)54(76)43(26-37-29-57-31-60-37)64-49(71)39-18-12-20-58-39/h6-11,14-17,28-33,38-45,47,58,68H,5,12-13,18-27,55H2,1-4H3,(H,56,59)(H,57,60)(H,61,73)(H,62,72)(H,63,74)(H,64,71)(H,65,69)(H,66,70,75)/t33-,38-,39-,40?,41-,42-,43-,44-,45?,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory potency on plasma renin obtained from mongrel dogs |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022002
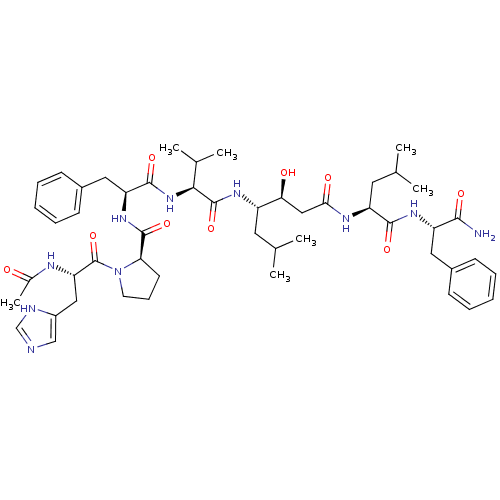
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H72N10O9/c1-29(2)21-36(42(62)26-43(63)55-38(22-30(3)4)46(65)57-37(45(51)64)23-33-15-10-8-11-16-33)56-49(68)44(31(5)6)59-47(66)39(24-34-17-12-9-13-18-34)58-48(67)41-19-14-20-60(41)50(69)40(54-32(7)61)25-35-27-52-28-53-35/h8-13,15-18,27-31,36-42,44,62H,14,19-26H2,1-7H3,(H2,51,64)(H,52,53)(H,54,61)(H,55,63)(H,56,68)(H,57,65)(H,58,67)(H,59,66)/t36-,37-,38-,39-,40-,41+,42-,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human plasma renin |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022977
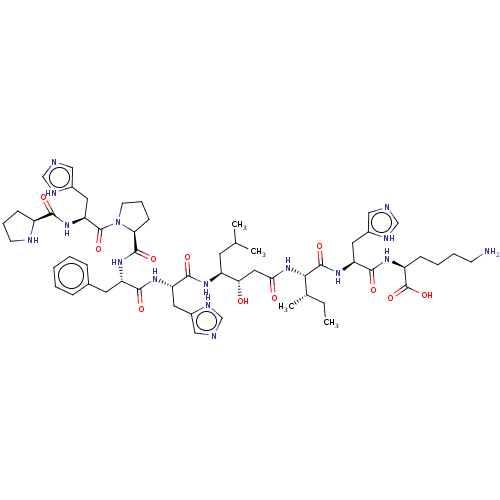
(CHEMBL2371846 | Pro-His-Pro-Phe-His-Statine-Ile-Hi...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C57H84N16O11/c1-5-34(4)49(55(81)70-44(24-37-28-60-31-64-37)52(78)66-40(57(83)84)15-9-10-18-58)72-48(75)26-47(74)41(21-33(2)3)67-53(79)43(23-36-27-59-30-63-36)68-51(77)42(22-35-13-7-6-8-14-35)69-54(80)46-17-12-20-73(46)56(82)45(25-38-29-61-32-65-38)71-50(76)39-16-11-19-62-39/h6-8,13-14,27-34,39-47,49,62,74H,5,9-12,15-26,58H2,1-4H3,(H,59,63)(H,60,64)(H,61,65)(H,66,78)(H,67,79)(H,68,77)(H,69,80)(H,70,81)(H,71,76)(H,72,75)(H,83,84)/t34-,39-,40-,41-,42-,43-,44-,45-,46-,47-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory potency on plasma renin obtained from mongrel dogs |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022977

(CHEMBL2371846 | Pro-His-Pro-Phe-His-Statine-Ile-Hi...)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC(C)C)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C57H84N16O11/c1-5-34(4)49(55(81)70-44(24-37-28-60-31-64-37)52(78)66-40(57(83)84)15-9-10-18-58)72-48(75)26-47(74)41(21-33(2)3)67-53(79)43(23-36-27-59-30-63-36)68-51(77)42(22-35-13-7-6-8-14-35)69-54(80)46-17-12-20-73(46)56(82)45(25-38-29-61-32-65-38)71-50(76)39-16-11-19-62-39/h6-8,13-14,27-34,39-47,49,62,74H,5,9-12,15-26,58H2,1-4H3,(H,59,63)(H,60,64)(H,61,65)(H,66,78)(H,67,79)(H,68,77)(H,69,80)(H,70,81)(H,71,76)(H,72,75)(H,83,84)/t34-,39-,40-,41-,42-,43-,44-,45-,46-,47-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory potency on plasma renin obtained from hypertensive humans |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022000
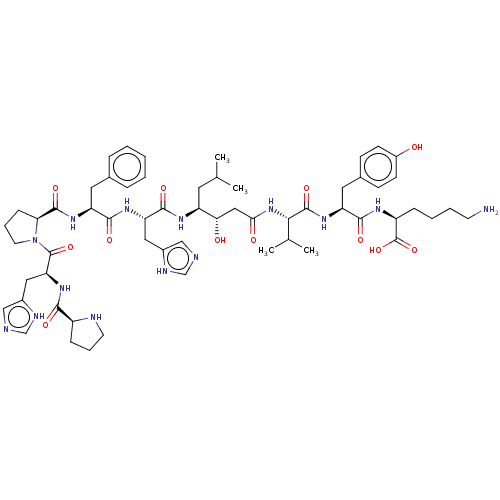
(CHEMBL2371853 | Pro-His-Pro-Phe-His-Statine-Val-Ty...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C59H84N14O12/c1-34(2)24-43(49(75)29-50(76)72-51(35(3)4)57(82)70-45(26-37-17-19-40(74)20-18-37)53(78)66-42(59(84)85)14-8-9-21-60)67-55(80)46(27-38-30-61-32-64-38)68-54(79)44(25-36-12-6-5-7-13-36)69-56(81)48-16-11-23-73(48)58(83)47(28-39-31-62-33-65-39)71-52(77)41-15-10-22-63-41/h5-7,12-13,17-20,30-35,41-49,51,63,74-75H,8-11,14-16,21-29,60H2,1-4H3,(H,61,64)(H,62,65)(H,66,78)(H,67,80)(H,68,79)(H,69,81)(H,70,82)(H,71,77)(H,72,76)(H,84,85)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory potency on plasma renin obtained from mongrel dogs |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022000

(CHEMBL2371853 | Pro-His-Pro-Phe-His-Statine-Val-Ty...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C59H84N14O12/c1-34(2)24-43(49(75)29-50(76)72-51(35(3)4)57(82)70-45(26-37-17-19-40(74)20-18-37)53(78)66-42(59(84)85)14-8-9-21-60)67-55(80)46(27-38-30-61-32-64-38)68-54(79)44(25-36-12-6-5-7-13-36)69-56(81)48-16-11-23-73(48)58(83)47(28-39-31-62-33-65-39)71-52(77)41-15-10-22-63-41/h5-7,12-13,17-20,30-35,41-49,51,63,74-75H,8-11,14-16,21-29,60H2,1-4H3,(H,61,64)(H,62,65)(H,66,78)(H,67,80)(H,68,79)(H,69,81)(H,70,82)(H,71,77)(H,72,76)(H,84,85)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022000

(CHEMBL2371853 | Pro-His-Pro-Phe-His-Statine-Val-Ty...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C59H84N14O12/c1-34(2)24-43(49(75)29-50(76)72-51(35(3)4)57(82)70-45(26-37-17-19-40(74)20-18-37)53(78)66-42(59(84)85)14-8-9-21-60)67-55(80)46(27-38-30-61-32-64-38)68-54(79)44(25-36-12-6-5-7-13-36)69-56(81)48-16-11-23-73(48)58(83)47(28-39-31-62-33-65-39)71-52(77)41-15-10-22-63-41/h5-7,12-13,17-20,30-35,41-49,51,63,74-75H,8-11,14-16,21-29,60H2,1-4H3,(H,61,64)(H,62,65)(H,66,78)(H,67,80)(H,68,79)(H,69,81)(H,70,82)(H,71,77)(H,72,76)(H,84,85)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022005

(Ac-His-Pro-Phe-His-Statine-Leu-Phe-NH2 | CHEMBL266...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H70N12O9/c1-30(2)19-37(44(65)25-45(66)58-39(20-31(3)4)47(68)60-38(46(52)67)21-33-13-8-6-9-14-33)59-49(70)41(23-35-26-53-28-55-35)61-48(69)40(22-34-15-10-7-11-16-34)62-50(71)43-17-12-18-63(43)51(72)42(57-32(5)64)24-36-27-54-29-56-36/h6-11,13-16,26-31,37-44,65H,12,17-25H2,1-5H3,(H2,52,67)(H,53,55)(H,54,56)(H,57,64)(H,58,66)(H,59,70)(H,60,68)(H,61,69)(H,62,71)/t37-,38-,39-,40-,41-,42-,43+,44-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human plasma renin |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022007
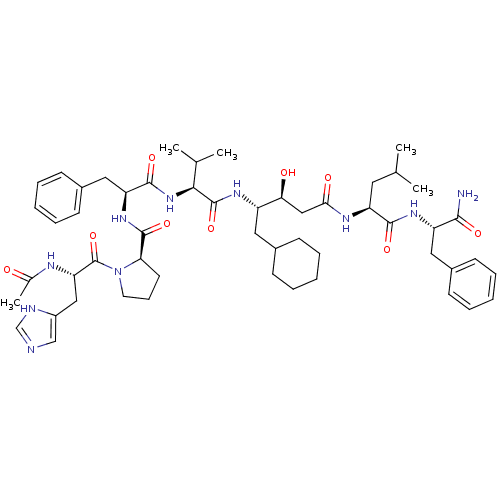
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H76N10O9/c1-32(2)24-41(49(68)60-40(48(54)67)26-36-18-11-7-12-19-36)58-46(66)29-45(65)39(25-35-16-9-6-10-17-35)59-52(71)47(33(3)4)62-50(69)42(27-37-20-13-8-14-21-37)61-51(70)44-22-15-23-63(44)53(72)43(57-34(5)64)28-38-30-55-31-56-38/h7-8,11-14,18-21,30-33,35,39-45,47,65H,6,9-10,15-17,22-29H2,1-5H3,(H2,54,67)(H,55,56)(H,57,64)(H,58,66)(H,59,71)(H,60,68)(H,61,70)(H,62,69)/t39-,40-,41-,42-,43-,44+,45-,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Compound was tested for its inhibitory activity against human plasma renin |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022988
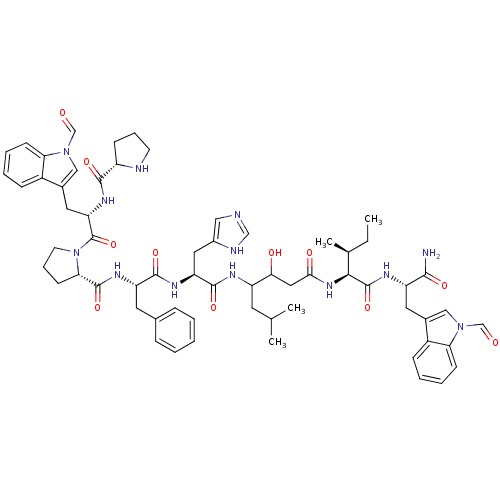
(CHEMBL410352 | Pro-Trp(CHO)-Pro-Phe-His-Statine-Il...)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cn(C=O)c2ccccc12)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1cn(C=O)c2ccccc12)C(N)=O Show InChI InChI=1S/C63H79N13O11/c1-5-38(4)56(62(86)69-47(57(64)81)27-40-32-74(35-77)51-20-11-9-17-43(40)51)73-55(80)30-54(79)46(25-37(2)3)68-60(84)49(29-42-31-65-34-67-42)70-59(83)48(26-39-15-7-6-8-16-39)71-61(85)53-22-14-24-76(53)63(87)50(72-58(82)45-19-13-23-66-45)28-41-33-75(36-78)52-21-12-10-18-44(41)52/h6-12,15-18,20-21,31-38,45-50,53-54,56,66,79H,5,13-14,19,22-30H2,1-4H3,(H2,64,81)(H,65,67)(H,68,84)(H,69,86)(H,70,83)(H,71,85)(H,72,82)(H,73,80)/t38-,45-,46?,47-,48-,49-,50-,53-,54?,56-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022996

(CHEMBL412841 | acetyl-Pro-His-Pro-Phe-His-Statine-...)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C59H86N16O12/c1-6-35(4)51(57(84)71-45(25-39-29-62-32-65-39)53(80)67-41(59(86)87)16-10-11-19-60)73-50(78)27-49(77)42(22-34(2)3)68-54(81)44(24-38-28-61-31-64-38)69-52(79)43(23-37-14-8-7-9-15-37)70-56(83)48-18-13-21-75(48)58(85)46(26-40-30-63-33-66-40)72-55(82)47-17-12-20-74(47)36(5)76/h7-9,14-15,28-35,41-49,51,77H,6,10-13,16-27,60H2,1-5H3,(H,61,64)(H,62,65)(H,63,66)(H,67,80)(H,68,81)(H,69,79)(H,70,83)(H,71,84)(H,72,82)(H,73,78)(H,86,87)/t35-,41-,42?,43-,44-,45-,46-,47-,48-,49?,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022980
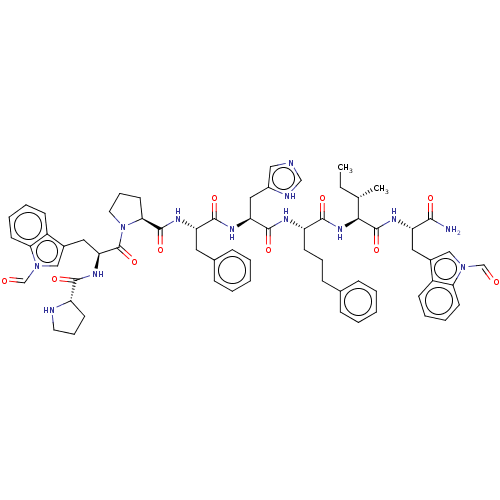
(CHEMBL2371845 | Pro-Trp(CHO)-Pro-Phe-His-4(S)amino...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cn(C=O)c2ccccc12)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1cn(C=O)c2ccccc12)C(N)=O Show InChI InChI=1S/C66H77N13O10/c1-3-41(2)58(65(88)72-51(59(67)82)32-44-36-77(39-80)55-26-12-10-22-47(44)55)76-61(84)50(24-14-21-42-17-6-4-7-18-42)71-63(86)53(34-46-35-68-38-70-46)73-62(85)52(31-43-19-8-5-9-20-43)74-64(87)57-28-16-30-79(57)66(89)54(75-60(83)49-25-15-29-69-49)33-45-37-78(40-81)56-27-13-11-23-48(45)56/h4-13,17-20,22-23,26-27,35-41,49-54,57-58,69H,3,14-16,21,24-25,28-34H2,1-2H3,(H2,67,82)(H,68,70)(H,71,86)(H,72,88)(H,73,85)(H,74,87)(H,75,83)(H,76,84)/t41-,49-,50-,51-,52-,53-,54-,57-,58-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405543
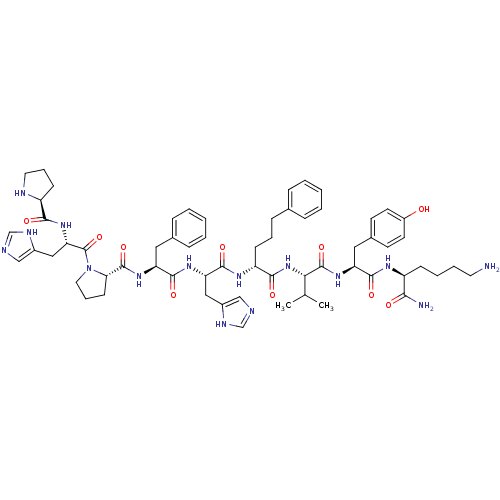
(CHEMBL2028952)Show SMILES CC(C)[C@H](NC(=O)[C@@H](CCCc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(N)=O |r| Show InChI InChI=1S/C62H83N15O10/c1-38(2)53(61(86)74-49(31-41-23-25-44(78)26-24-41)57(82)70-45(54(64)79)19-9-10-27-63)76-56(81)47(20-11-18-39-14-5-3-6-15-39)71-59(84)50(32-42-34-65-36-68-42)72-58(83)48(30-40-16-7-4-8-17-40)73-60(85)52-22-13-29-77(52)62(87)51(33-43-35-66-37-69-43)75-55(80)46-21-12-28-67-46/h3-8,14-17,23-26,34-38,45-53,67,78H,9-13,18-22,27-33,63H2,1-2H3,(H2,64,79)(H,65,68)(H,66,69)(H,70,82)(H,71,84)(H,72,83)(H,73,85)(H,74,86)(H,75,80)(H,76,81)/t45-,46-,47+,48-,49-,50-,51-,52-,53-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022993
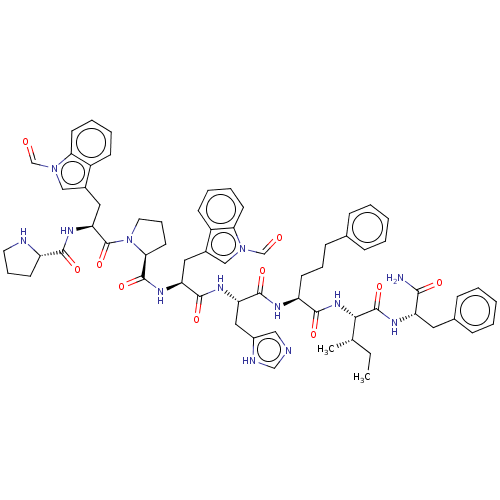
(CHEMBL2371843 | Pro-Trp(CHO)-Pro-Trp(CHO)-His-4(S)...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1cn(C=O)c2ccccc12)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cn(C=O)c2ccccc12)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C66H77N13O10/c1-3-41(2)58(65(88)72-51(59(67)82)31-43-19-8-5-9-20-43)76-61(84)50(24-14-21-42-17-6-4-7-18-42)71-63(86)53(34-46-35-68-38-70-46)73-62(85)52(32-44-36-77(39-80)55-26-12-10-22-47(44)55)74-64(87)57-28-16-30-79(57)66(89)54(75-60(83)49-25-15-29-69-49)33-45-37-78(40-81)56-27-13-11-23-48(45)56/h4-13,17-20,22-23,26-27,35-41,49-54,57-58,69H,3,14-16,21,24-25,28-34H2,1-2H3,(H2,67,82)(H,68,70)(H,71,86)(H,72,88)(H,73,85)(H,74,87)(H,75,83)(H,76,84)/t41-,49-,50-,51-,52-,53-,54-,57-,58-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022991
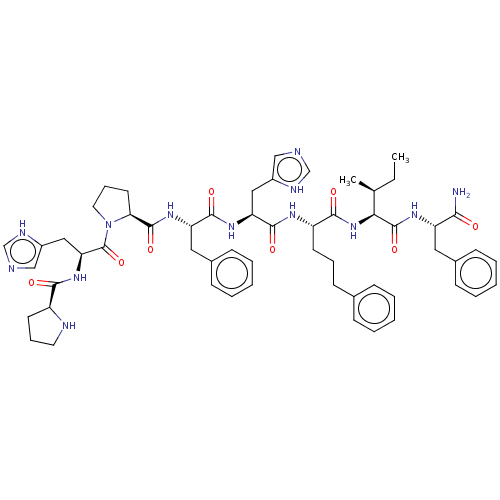
(CHEMBL2371848 | Pro-His-Pro-Phe-His-4(S)amino-3(s)...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C57H73N13O8/c1-3-36(2)49(56(77)65-44(50(58)71)28-38-18-9-5-10-19-38)69-52(73)43(23-13-22-37-16-7-4-8-17-37)64-54(75)46(30-40-32-59-34-62-40)66-53(74)45(29-39-20-11-6-12-21-39)67-55(76)48-25-15-27-70(48)57(78)47(31-41-33-60-35-63-41)68-51(72)42-24-14-26-61-42/h4-12,16-21,32-36,42-49,61H,3,13-15,22-31H2,1-2H3,(H2,58,71)(H,59,62)(H,60,63)(H,64,75)(H,65,77)(H,66,74)(H,67,76)(H,68,72)(H,69,73)/t36-,42-,43-,44-,45-,46-,47-,48-,49-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022981
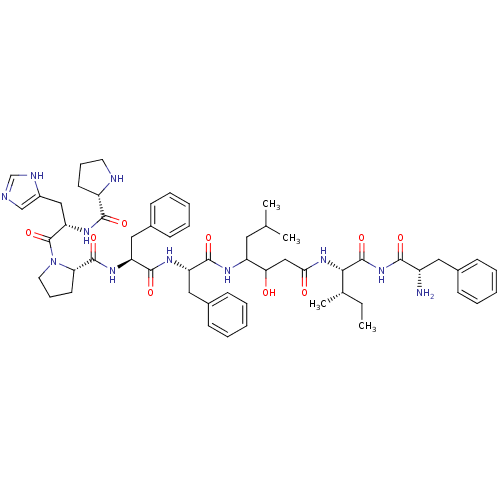
(CHEMBL412209 | Pro-his-Pro-Phe-Phe-Statine-Ile-Phe...)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1)C(=O)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C57H77N11O9/c1-5-36(4)50(56(76)67-51(71)41(58)28-37-17-9-6-10-18-37)66-49(70)32-48(69)43(27-35(2)3)62-53(73)44(29-38-19-11-7-12-20-38)63-54(74)45(30-39-21-13-8-14-22-39)64-55(75)47-24-16-26-68(47)57(77)46(31-40-33-59-34-61-40)65-52(72)42-23-15-25-60-42/h6-14,17-22,33-36,41-48,50,60,69H,5,15-16,23-32,58H2,1-4H3,(H,59,61)(H,62,73)(H,63,74)(H,64,75)(H,65,72)(H,66,70)(H,67,71,76)/t36-,41-,42-,43?,44-,45-,46-,47-,48?,50-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405544
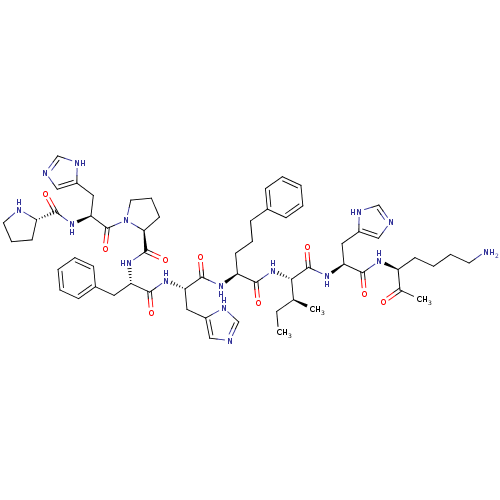
(CHEMBL2028954)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCCCN)C(C)=O |r| Show InChI InChI=1S/C61H84N16O9/c1-4-38(2)53(60(85)74-50(30-43-33-64-36-68-43)57(82)70-45(39(3)78)21-11-12-25-62)76-55(80)47(22-13-20-40-16-7-5-8-17-40)71-58(83)49(29-42-32-63-35-67-42)72-56(81)48(28-41-18-9-6-10-19-41)73-59(84)52-24-15-27-77(52)61(86)51(31-44-34-65-37-69-44)75-54(79)46-23-14-26-66-46/h5-10,16-19,32-38,45-53,66H,4,11-15,20-31,62H2,1-3H3,(H,63,67)(H,64,68)(H,65,69)(H,70,82)(H,71,83)(H,72,81)(H,73,84)(H,74,85)(H,75,79)(H,76,80)/t38-,45-,46-,47-,48-,49-,50-,51-,52-,53-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022002

(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C50H72N10O9/c1-29(2)21-36(42(62)26-43(63)55-38(22-30(3)4)46(65)57-37(45(51)64)23-33-15-10-8-11-16-33)56-49(68)44(31(5)6)59-47(66)39(24-34-17-12-9-13-18-34)58-48(67)41-19-14-20-60(41)50(69)40(54-32(7)61)25-35-27-52-28-53-35/h8-13,15-18,27-31,36-42,44,62H,14,19-26H2,1-7H3,(H2,51,64)(H,52,53)(H,54,61)(H,55,63)(H,56,68)(H,57,65)(H,58,67)(H,59,66)/t36-,37-,38-,39-,40-,41+,42-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022007

(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H76N10O9/c1-32(2)24-41(49(68)60-40(48(54)67)26-36-18-11-7-12-19-36)58-46(66)29-45(65)39(25-35-16-9-6-10-17-35)59-52(71)47(33(3)4)62-50(69)42(27-37-20-13-8-14-21-37)61-51(70)44-22-15-23-63(44)53(72)43(57-34(5)64)28-38-30-55-31-56-38/h7-8,11-14,18-21,30-33,35,39-45,47,65H,6,9-10,15-17,22-29H2,1-5H3,(H2,54,67)(H,55,56)(H,57,64)(H,58,66)(H,59,71)(H,60,68)(H,61,70)(H,62,69)/t39-,40-,41-,42-,43-,44+,45-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021993

(1-[2-Acetylamino-3-(4-methoxy-phenyl)-propionyl]-p...)Show SMILES COc1ccc(C[C@H](NC(C)=O)C(=O)N2CCC[C@@H]2C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](CC(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc2ccccc2)C(N)=O)cc1 Show InChI InChI=1S/C54H76N8O10/c1-32(2)26-40(46(64)31-47(65)57-42(27-33(3)4)50(67)59-41(49(55)66)28-36-16-11-9-12-17-36)58-53(70)48(34(5)6)61-51(68)43(29-37-18-13-10-14-19-37)60-52(69)45-20-15-25-62(45)54(71)44(56-35(7)63)30-38-21-23-39(72-8)24-22-38/h9-14,16-19,21-24,32-34,40-46,48,64H,15,20,25-31H2,1-8H3,(H2,55,66)(H,56,63)(H,57,65)(H,58,70)(H,59,67)(H,60,69)(H,61,68)/t40-,41-,42-,43-,44-,45+,46-,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022000

(CHEMBL2371853 | Pro-His-Pro-Phe-His-Statine-Val-Ty...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C59H84N14O12/c1-34(2)24-43(49(75)29-50(76)72-51(35(3)4)57(82)70-45(26-37-17-19-40(74)20-18-37)53(78)66-42(59(84)85)14-8-9-21-60)67-55(80)46(27-38-30-61-32-64-38)68-54(79)44(25-36-12-6-5-7-13-36)69-56(81)48-16-11-23-73(48)58(83)47(28-39-31-62-33-65-39)71-52(77)41-15-10-22-63-41/h5-7,12-13,17-20,30-35,41-49,51,63,74-75H,8-11,14-16,21-29,60H2,1-4H3,(H,61,64)(H,62,65)(H,66,78)(H,67,80)(H,68,79)(H,69,81)(H,70,82)(H,71,77)(H,72,76)(H,84,85)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory potency on plasma renin obtained from hypertensive humans |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022986
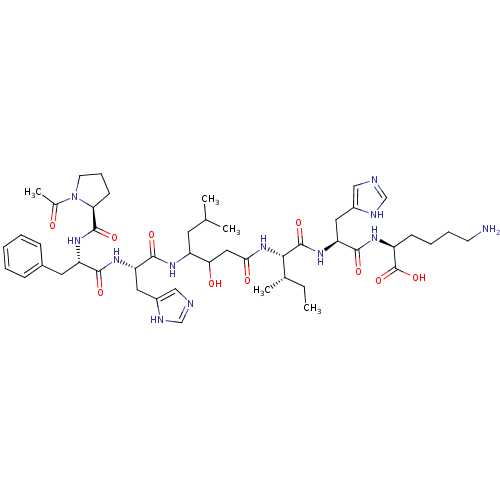
(Acetyl-Pro-Phe-His-Statine-Ile-His-Lys | CHEMBL408...)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C48H72N12O10/c1-6-29(4)42(47(68)58-38(22-33-25-51-27-53-33)44(65)54-34(48(69)70)15-10-11-17-49)59-41(63)23-40(62)35(19-28(2)3)55-45(66)37(21-32-24-50-26-52-32)56-43(64)36(20-31-13-8-7-9-14-31)57-46(67)39-16-12-18-60(39)30(5)61/h7-9,13-14,24-29,34-40,42,62H,6,10-12,15-23,49H2,1-5H3,(H,50,52)(H,51,53)(H,54,65)(H,55,66)(H,56,64)(H,57,67)(H,58,68)(H,59,63)(H,69,70)/t29-,34-,35?,36-,37-,38-,39-,40?,42-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022021
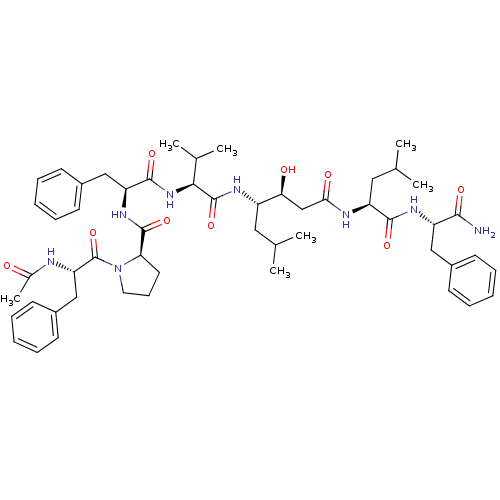
(1-(2-Acetylamino-3-phenyl-propionyl)-pyrrolidine-2...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1ccccc1)NC(C)=O)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H74N8O9/c1-32(2)26-39(45(63)31-46(64)56-41(27-33(3)4)49(66)58-40(48(54)65)28-36-18-11-8-12-19-36)57-52(69)47(34(5)6)60-50(67)42(29-37-20-13-9-14-21-37)59-51(68)44-24-17-25-61(44)53(70)43(55-35(7)62)30-38-22-15-10-16-23-38/h8-16,18-23,32-34,39-45,47,63H,17,24-31H2,1-7H3,(H2,54,65)(H,55,62)(H,56,64)(H,57,69)(H,58,66)(H,59,68)(H,60,67)/t39-,40-,41-,42-,43-,44+,45-,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50405542
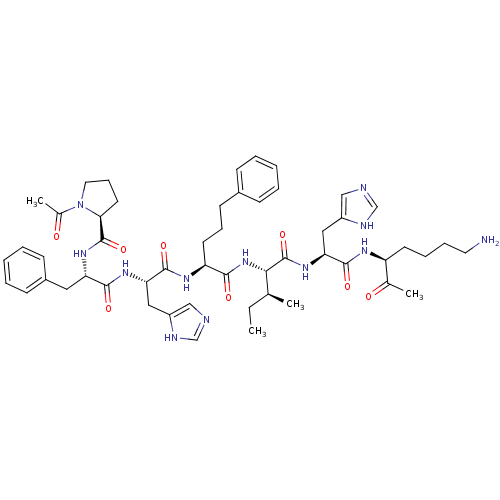
(CHEMBL2028955)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(C)=O)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCCCN)C(C)=O |r| Show InChI InChI=1S/C52H72N12O8/c1-5-33(2)46(52(72)62-44(28-39-30-55-32-57-39)49(69)58-40(34(3)65)21-12-13-24-53)63-47(67)41(22-14-20-36-16-8-6-9-17-36)59-50(70)43(27-38-29-54-31-56-38)60-48(68)42(26-37-18-10-7-11-19-37)61-51(71)45-23-15-25-64(45)35(4)66/h6-11,16-19,29-33,40-46H,5,12-15,20-28,53H2,1-4H3,(H,54,56)(H,55,57)(H,58,69)(H,59,70)(H,60,68)(H,61,71)(H,62,72)(H,63,67)/t33-,40-,41-,42-,43-,44-,45-,46-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022972
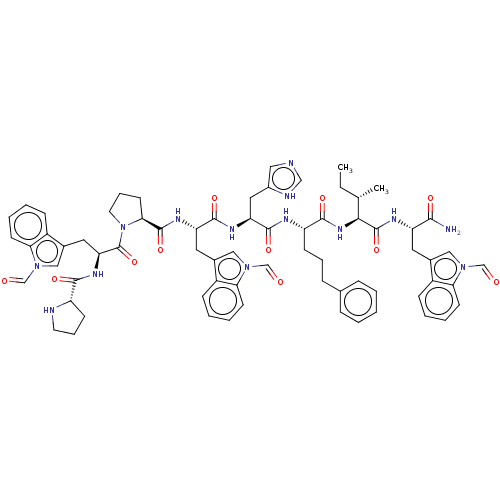
(CHEMBL2371844 | Pro-Trp(CHO)-Pro-Trp(CHO)-His-4(S)...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1cn(C=O)c2ccccc12)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cn(C=O)c2ccccc12)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1cn(C=O)c2ccccc12)C(N)=O Show InChI InChI=1S/C69H78N14O11/c1-3-42(2)61(68(93)75-53(62(70)87)30-44-35-80(39-84)57-24-10-7-19-48(44)57)79-64(89)52(22-13-18-43-16-5-4-6-17-43)74-66(91)55(33-47-34-71-38-73-47)76-65(90)54(31-45-36-81(40-85)58-25-11-8-20-49(45)58)77-67(92)60-27-15-29-83(60)69(94)56(78-63(88)51-23-14-28-72-51)32-46-37-82(41-86)59-26-12-9-21-50(46)59/h4-12,16-17,19-21,24-26,34-42,51-56,60-61,72H,3,13-15,18,22-23,27-33H2,1-2H3,(H2,70,87)(H,71,73)(H,74,91)(H,75,93)(H,76,90)(H,77,92)(H,78,88)(H,79,89)/t42-,51-,52-,53-,54-,55-,56-,60-,61-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021989

(Ac-His-Pro-Phe-His-ACHPA-Leu-Phe-NH2 | CHEMBL38686...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H74N12O9/c1-33(2)22-42(50(71)63-41(49(55)70)24-36-16-9-5-10-17-36)61-48(69)28-47(68)40(23-35-14-7-4-8-15-35)62-52(73)44(26-38-29-56-31-58-38)64-51(72)43(25-37-18-11-6-12-19-37)65-53(74)46-20-13-21-66(46)54(75)45(60-34(3)67)27-39-30-57-32-59-39/h5-6,9-12,16-19,29-33,35,40-47,68H,4,7-8,13-15,20-28H2,1-3H3,(H2,55,70)(H,56,58)(H,57,59)(H,60,67)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)/t40-,41-,42-,43-,44-,45-,46+,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022015

(1-(2-Acetylamino-3-naphthalen-1-yl-propionyl)-pyrr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cccc2ccccc12)NC(C)=O)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C57H76N8O9/c1-34(2)28-43(49(67)33-50(68)60-45(29-35(3)4)53(70)62-44(52(58)69)30-38-18-10-8-11-19-38)61-56(73)51(36(5)6)64-54(71)46(31-39-20-12-9-13-21-39)63-55(72)48-26-17-27-65(48)57(74)47(59-37(7)66)32-41-24-16-23-40-22-14-15-25-42(40)41/h8-16,18-25,34-36,43-49,51,67H,17,26-33H2,1-7H3,(H2,58,69)(H,59,66)(H,60,68)(H,61,73)(H,62,70)(H,63,72)(H,64,71)/t43-,44-,45-,46-,47-,48+,49-,51-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022978

(CHEMBL264339 | Pro-His-Pro-Phe-His-Statine-Ile-Phe...)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1)C(=O)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C54H75N13O9/c1-5-33(4)47(53(75)66-48(70)38(55)23-34-14-8-6-9-15-34)65-46(69)27-45(68)40(22-32(2)3)61-51(73)42(25-36-28-56-30-59-36)62-50(72)41(24-35-16-10-7-11-17-35)63-52(74)44-19-13-21-67(44)54(76)43(26-37-29-57-31-60-37)64-49(71)39-18-12-20-58-39/h6-11,14-17,28-33,38-45,47,58,68H,5,12-13,18-27,55H2,1-4H3,(H,56,59)(H,57,60)(H,61,73)(H,62,72)(H,63,74)(H,64,71)(H,65,69)(H,66,70,75)/t33-,38-,39-,40?,41-,42-,43-,44-,45?,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory potency on plasma renin obtained from hypertensive humans |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50367761

(CHEMBL1790252)Show SMILES CC[C@@H](C)[C@H](NC(=O)C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CCCN1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C57H79N13O9/c1-3-35(2)50(56(78)65-43(51(58)73)26-37-17-9-5-10-18-37)69-49(72)30-48(71)42(25-36-15-7-4-8-16-36)64-54(76)45(28-39-31-59-33-62-39)66-53(75)44(27-38-19-11-6-12-20-38)67-55(77)47-22-14-24-70(47)57(79)46(29-40-32-60-34-63-40)68-52(74)41-21-13-23-61-41/h5-6,9-12,17-20,31-36,41-48,50,61,71H,3-4,7-8,13-16,21-30H2,1-2H3,(H2,58,73)(H,59,62)(H,60,63)(H,64,76)(H,65,78)(H,66,75)(H,67,77)(H,68,74)(H,69,72)/t35-,41-,42+,43+,44+,45+,46+,47-,48+,50+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022987
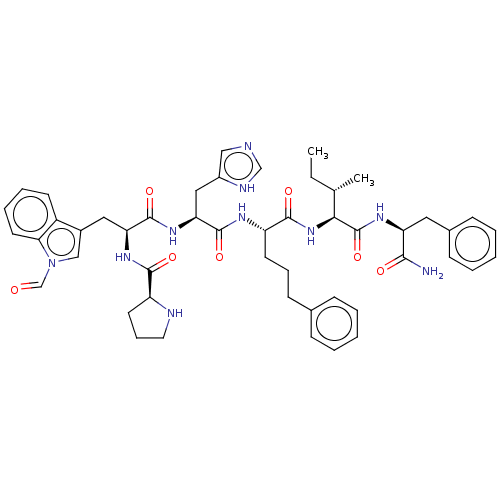
(CHEMBL2371841 | Pro-Trp(CHO)-His-4(S)amino-3(s)-hy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1cn(C=O)c2ccccc12)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C49H60N10O7/c1-3-31(2)43(49(66)55-39(44(50)61)24-33-16-8-5-9-17-33)58-46(63)38(20-12-18-32-14-6-4-7-15-32)54-48(65)41(26-35-27-51-29-53-35)57-47(64)40(56-45(62)37-21-13-23-52-37)25-34-28-59(30-60)42-22-11-10-19-36(34)42/h4-11,14-17,19,22,27-31,37-41,43,52H,3,12-13,18,20-21,23-26H2,1-2H3,(H2,50,61)(H,51,53)(H,54,65)(H,55,66)(H,56,62)(H,57,64)(H,58,63)/t31-,37-,38-,39-,40-,41-,43-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022978

(CHEMBL264339 | Pro-His-Pro-Phe-His-Statine-Ile-Phe...)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1)C(=O)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C54H75N13O9/c1-5-33(4)47(53(75)66-48(70)38(55)23-34-14-8-6-9-15-34)65-46(69)27-45(68)40(22-32(2)3)61-51(73)42(25-36-28-56-30-59-36)62-50(72)41(24-35-16-10-7-11-17-35)63-52(74)44-19-13-21-67(44)54(76)43(26-37-29-57-31-60-37)64-49(71)39-18-12-20-58-39/h6-11,14-17,28-33,38-45,47,58,68H,5,12-13,18-27,55H2,1-4H3,(H,56,59)(H,57,60)(H,61,73)(H,62,72)(H,63,74)(H,64,71)(H,65,69)(H,66,70,75)/t33-,38-,39-,40?,41-,42-,43-,44-,45?,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory potency on plasma renin obtained from cats |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022000

(CHEMBL2371853 | Pro-His-Pro-Phe-His-Statine-Val-Ty...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C59H84N14O12/c1-34(2)24-43(49(75)29-50(76)72-51(35(3)4)57(82)70-45(26-37-17-19-40(74)20-18-37)53(78)66-42(59(84)85)14-8-9-21-60)67-55(80)46(27-38-30-61-32-64-38)68-54(79)44(25-36-12-6-5-7-13-36)69-56(81)48-16-11-23-73(48)58(83)47(28-39-31-62-33-65-39)71-52(77)41-15-10-22-63-41/h5-7,12-13,17-20,30-35,41-49,51,63,74-75H,8-11,14-16,21-29,60H2,1-4H3,(H,61,64)(H,62,65)(H,66,78)(H,67,80)(H,68,79)(H,69,81)(H,70,82)(H,71,77)(H,72,76)(H,84,85)/t41-,42-,43-,44-,45-,46-,47-,48-,49-,51-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory potency on plasma renin obtained from New Zealand white rabbits |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM31123
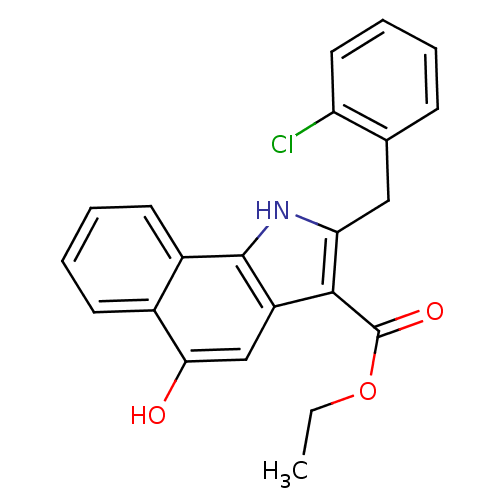
(5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11b)Show SMILES CCOC(=O)c1c(Cc2ccccc2Cl)[nH]c2c1cc(O)c1ccccc21 Show InChI InChI=1S/C22H18ClNO3/c1-2-27-22(26)20-16-12-19(25)14-8-4-5-9-15(14)21(16)24-18(20)11-13-7-3-6-10-17(13)23/h3-10,12,24-25H,2,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 |
Bioorg Med Chem 17: 7924-32 (2009)
Article DOI: 10.1016/j.bmc.2009.10.025
BindingDB Entry DOI: 10.7270/Q25T3KK7 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021994
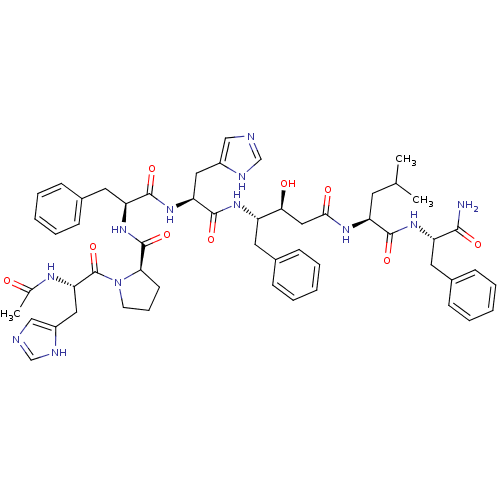
(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H68N12O9/c1-33(2)22-42(50(71)63-41(49(55)70)24-36-16-9-5-10-17-36)61-48(69)28-47(68)40(23-35-14-7-4-8-15-35)62-52(73)44(26-38-29-56-31-58-38)64-51(72)43(25-37-18-11-6-12-19-37)65-53(74)46-20-13-21-66(46)54(75)45(60-34(3)67)27-39-30-57-32-59-39/h4-12,14-19,29-33,40-47,68H,13,20-28H2,1-3H3,(H2,55,70)(H,56,58)(H,57,59)(H,60,67)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)/t40-,41-,42-,43-,44-,45-,46+,47-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022006

(Ac-His-Pro-Phe-His-Statine-Leu-CHA-NH2 | CHEMBL217...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C1CCCCC1)C(N)=O Show InChI InChI=1S/C50H74N12O9/c1-29(2)19-36(42(64)24-43(65)57-37(20-30(3)4)48(69)61-44(45(51)66)33-15-10-7-11-16-33)58-47(68)39(22-34-25-52-27-54-34)59-46(67)38(21-32-13-8-6-9-14-32)60-49(70)41-17-12-18-62(41)50(71)40(56-31(5)63)23-35-26-53-28-55-35/h6,8-9,13-14,25-30,33,36-42,44,64H,7,10-12,15-24H2,1-5H3,(H2,51,66)(H,52,54)(H,53,55)(H,56,63)(H,57,65)(H,58,68)(H,59,67)(H,60,70)(H,61,69)/t36-,37-,38-,39-,40-,41+,42-,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022978

(CHEMBL264339 | Pro-His-Pro-Phe-His-Statine-Ile-Phe...)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCCN1)C(=O)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C54H75N13O9/c1-5-33(4)47(53(75)66-48(70)38(55)23-34-14-8-6-9-15-34)65-46(69)27-45(68)40(22-32(2)3)61-51(73)42(25-36-28-56-30-59-36)62-50(72)41(24-35-16-10-7-11-17-35)63-52(74)44-19-13-21-67(44)54(76)43(26-37-29-57-31-60-37)64-49(71)39-18-12-20-58-39/h6-11,14-17,28-33,38-45,47,58,68H,5,12-13,18-27,55H2,1-4H3,(H,56,59)(H,57,60)(H,61,73)(H,62,72)(H,63,74)(H,64,71)(H,65,69)(H,66,70,75)/t33-,38-,39-,40?,41-,42-,43-,44-,45?,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory potency on plasma renin obtained from hypertensive humans |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Prostaglandin E synthase
(Homo sapiens (Human)) | BDBM31127
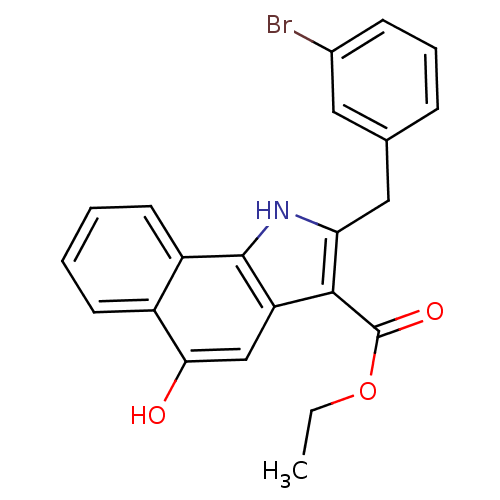
(5-hydroxy-1H-benzo[g]indole-3-carboxylate, 11f)Show SMILES CCOC(=O)c1c(Cc2cccc(Br)c2)[nH]c2c1cc(O)c1ccccc21 Show InChI InChI=1S/C22H18BrNO3/c1-2-27-22(26)20-17-12-19(25)15-8-3-4-9-16(15)21(17)24-18(20)11-13-6-5-7-14(23)10-13/h3-10,12,24-25H,2,11H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Eberhard Karls University Tuebingen
Curated by ChEMBL
| Assay Description
Inhibition of mPGES1 in IL1beta induced human A549 cell microsomal membrane assessed as blockade of conversion of PGH2 to PGE2 |
Bioorg Med Chem 17: 7924-32 (2009)
Article DOI: 10.1016/j.bmc.2009.10.025
BindingDB Entry DOI: 10.7270/Q25T3KK7 |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022005

(Ac-His-Pro-Phe-His-Statine-Leu-Phe-NH2 | CHEMBL266...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H70N12O9/c1-30(2)19-37(44(65)25-45(66)58-39(20-31(3)4)47(68)60-38(46(52)67)21-33-13-8-6-9-14-33)59-49(70)41(23-35-26-53-28-55-35)61-48(69)40(22-34-15-10-7-11-16-34)62-50(71)43-17-12-18-63(43)51(72)42(57-32(5)64)24-36-27-54-29-56-36/h6-11,13-16,26-31,37-44,65H,12,17-25H2,1-5H3,(H2,52,67)(H,53,55)(H,54,56)(H,57,64)(H,58,66)(H,59,70)(H,60,68)(H,61,69)(H,62,71)/t37-,38-,39-,40-,41-,42-,43+,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022971
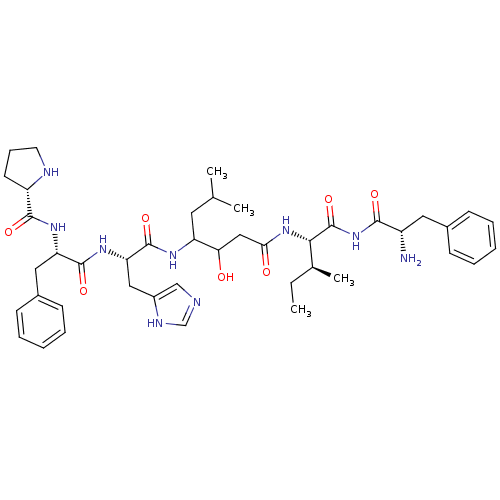
(CHEMBL284701 | Pro-Phe-His-Statine-Ile-Phe-NH2)Show SMILES CC[C@H](C)[C@H](NC(=O)CC(O)C(CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1)C(=O)NC(=O)[C@@H](N)Cc1ccccc1 Show InChI InChI=1S/C43H61N9O7/c1-5-27(4)38(43(59)52-39(55)31(44)20-28-13-8-6-9-14-28)51-37(54)23-36(53)33(19-26(2)3)48-42(58)35(22-30-24-45-25-47-30)50-41(57)34(21-29-15-10-7-11-16-29)49-40(56)32-17-12-18-46-32/h6-11,13-16,24-27,31-36,38,46,53H,5,12,17-23,44H2,1-4H3,(H,45,47)(H,48,58)(H,49,56)(H,50,57)(H,51,54)(H,52,55,59)/t27-,31-,32-,33?,34-,35-,36?,38-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022001
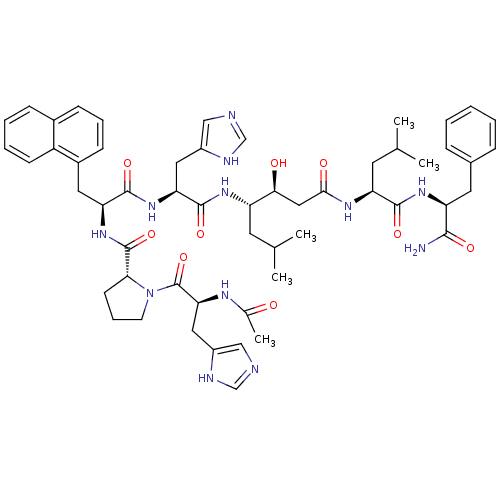
(Ac-His-Pro-Napa-His-Statine-Leu-Phe-NH2 | CHEMBL27...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1cccc2ccccc12)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C55H72N12O9/c1-32(2)21-41(48(69)27-49(70)62-43(22-33(3)4)51(72)64-42(50(56)71)23-35-13-7-6-8-14-35)63-53(74)45(25-38-28-57-30-59-38)65-52(73)44(24-37-17-11-16-36-15-9-10-18-40(36)37)66-54(75)47-19-12-20-67(47)55(76)46(61-34(5)68)26-39-29-58-31-60-39/h6-11,13-18,28-33,41-48,69H,12,19-27H2,1-5H3,(H2,56,71)(H,57,59)(H,58,60)(H,61,68)(H,62,70)(H,63,74)(H,64,72)(H,65,73)(H,66,75)/t41-,42-,43-,44-,45-,46-,47+,48-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022008

(Ac-His-Pro-Trp-His-Statine-Leu-Phe-NH2 | CHEMBL384...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C53H71N13O9/c1-30(2)18-39(46(68)24-47(69)61-41(19-31(3)4)49(71)63-40(48(54)70)20-33-12-7-6-8-13-33)62-51(73)43(22-35-26-55-28-58-35)64-50(72)42(21-34-25-57-38-15-10-9-14-37(34)38)65-52(74)45-16-11-17-66(45)53(75)44(60-32(5)67)23-36-27-56-29-59-36/h6-10,12-15,25-31,39-46,57,68H,11,16-24H2,1-5H3,(H2,54,70)(H,55,58)(H,56,59)(H,60,67)(H,61,69)(H,62,73)(H,63,71)(H,64,72)(H,65,74)/t39-,40-,41-,42-,43-,44-,45+,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022017
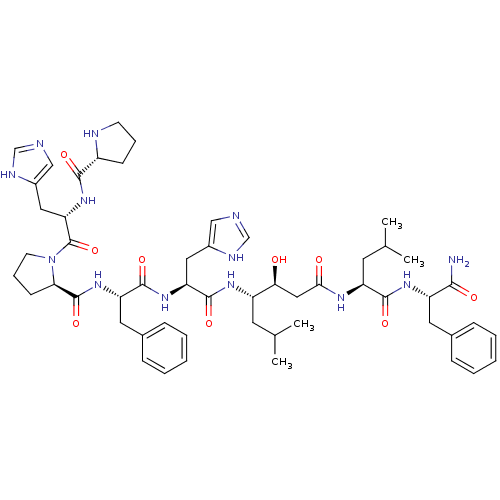
(CHEMBL414537 | Pro-His-Pro-Phe-His-Statine-Leu-Phe...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H]1CCCN1)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C54H75N13O9/c1-32(2)21-39(46(68)27-47(69)61-41(22-33(3)4)50(72)63-40(48(55)70)23-34-13-7-5-8-14-34)62-52(74)43(25-36-28-56-30-59-36)64-51(73)42(24-35-15-9-6-10-16-35)65-53(75)45-18-12-20-67(45)54(76)44(26-37-29-57-31-60-37)66-49(71)38-17-11-19-58-38/h5-10,13-16,28-33,38-46,58,68H,11-12,17-27H2,1-4H3,(H2,55,70)(H,56,59)(H,57,60)(H,61,69)(H,62,74)(H,63,72)(H,64,73)(H,65,75)(H,66,71)/t38-,39+,40+,41+,42+,43+,44+,45-,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022974
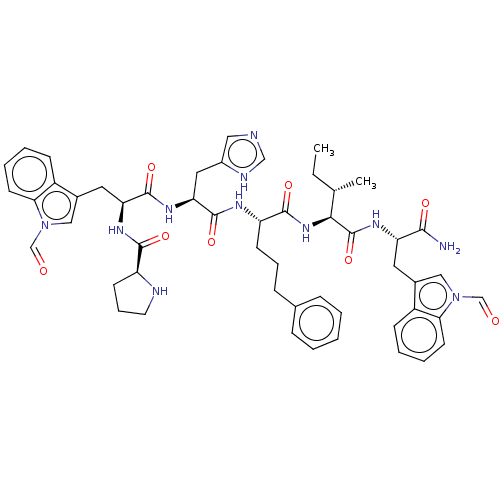
(CHEMBL2371840 | Pro-Trp(CHO)-His-4(S)amino-3(s)-hy...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CCCc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1cn(C=O)c2ccccc12)NC(=O)[C@@H]1CCCN1)C(=O)N[C@@H](Cc1cn(C=O)c2ccccc12)C(N)=O Show InChI InChI=1S/C52H61N11O8/c1-3-32(2)46(52(71)58-41(47(53)66)23-34-27-62(30-64)44-20-9-7-16-37(34)44)61-49(68)40(18-11-15-33-13-5-4-6-14-33)57-51(70)43(25-36-26-54-29-56-36)60-50(69)42(59-48(67)39-19-12-22-55-39)24-35-28-63(31-65)45-21-10-8-17-38(35)45/h4-10,13-14,16-17,20-21,26-32,39-43,46,55H,3,11-12,15,18-19,22-25H2,1-2H3,(H2,53,66)(H,54,56)(H,57,70)(H,58,71)(H,59,67)(H,60,69)(H,61,68)/t32-,39-,40-,41-,42-,43-,46-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021996

(Ac-His-Pro-Phe-His-Statine-Leu-Val-NH2 | CHEMBL295...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](C(C)C)C(N)=O Show InChI InChI=1S/C47H70N12O9/c1-26(2)16-33(39(61)21-40(62)54-34(17-27(3)4)45(66)58-41(28(5)6)42(48)63)55-44(65)36(19-31-22-49-24-51-31)56-43(64)35(18-30-12-9-8-10-13-30)57-46(67)38-14-11-15-59(38)47(68)37(53-29(7)60)20-32-23-50-25-52-32/h8-10,12-13,22-28,33-39,41,61H,11,14-21H2,1-7H3,(H2,48,63)(H,49,51)(H,50,52)(H,53,60)(H,54,62)(H,55,65)(H,56,64)(H,57,67)(H,58,66)/t33-,34-,35-,36-,37-,38+,39-,41-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50022014
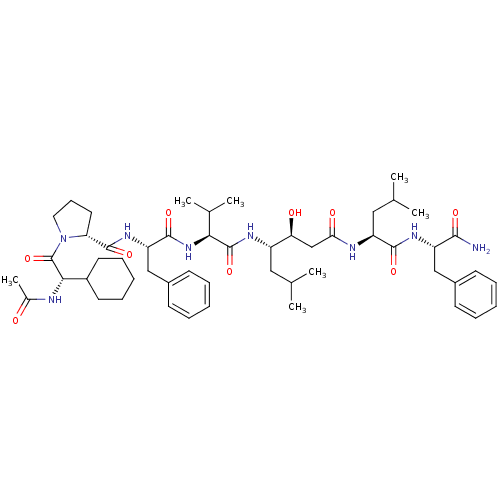
(1-(2-Acetylamino-2-cyclohexyl-acetyl)-pyrrolidine-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@@H](NC(C)=O)C1CCCCC1)C(C)C)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C52H78N8O9/c1-31(2)26-38(43(62)30-44(63)55-40(27-32(3)4)48(65)57-39(47(53)64)28-35-18-11-8-12-19-35)56-51(68)45(33(5)6)59-49(66)41(29-36-20-13-9-14-21-36)58-50(67)42-24-17-25-60(42)52(69)46(54-34(7)61)37-22-15-10-16-23-37/h8-9,11-14,18-21,31-33,37-43,45-46,62H,10,15-17,22-30H2,1-7H3,(H2,53,64)(H,54,61)(H,55,63)(H,56,68)(H,57,65)(H,58,67)(H,59,66)/t38-,39-,40-,41-,42+,43-,45-,46-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50021997

(1-[2-Acetylamino-3-(3H-imidazol-4-yl)-propionyl]-p...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1cnc[nH]1)NC(C)=O)[C@@H](O)CC(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C51H74N10O9/c1-30(2)21-37(44(63)27-45(64)56-39(22-31(3)4)47(66)58-38(46(52)65)24-34-15-10-8-11-16-34)57-48(67)40(23-32(5)6)59-49(68)41(25-35-17-12-9-13-18-35)60-50(69)43-19-14-20-61(43)51(70)42(55-33(7)62)26-36-28-53-29-54-36/h8-13,15-18,28-32,37-44,63H,14,19-27H2,1-7H3,(H2,52,65)(H,53,54)(H,55,62)(H,56,64)(H,57,67)(H,58,66)(H,59,68)(H,60,69)/t37-,38-,39-,40-,41-,42-,43+,44-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Rattus norvegicus) | BDBM50367760

(CHEMBL2367544)Show SMILES CC[C@H](C)[C@H](NC(=O)C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H]1CCCN1C(=O)[C@H](Cc1c[nH]cn1)NC(=O)[C@H]1CCCN1)C(=O)N[C@@H](Cc1ccccc1)C(N)=O Show InChI InChI=1S/C57H73N13O9/c1-3-35(2)50(56(78)65-43(51(58)73)26-37-17-9-5-10-18-37)69-49(72)30-48(71)42(25-36-15-7-4-8-16-36)64-54(76)45(28-39-31-59-33-62-39)66-53(75)44(27-38-19-11-6-12-20-38)67-55(77)47-22-14-24-70(47)57(79)46(29-40-32-60-34-63-40)68-52(74)41-21-13-23-61-41/h4-12,15-20,31-35,41-48,50,61,71H,3,13-14,21-30H2,1-2H3,(H2,58,73)(H,59,62)(H,60,63)(H,64,76)(H,65,78)(H,66,75)(H,67,77)(H,68,74)(H,69,72)/t35-,41+,42-,43-,44-,45-,46-,47+,48-,50-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Massachusetts General Hospital
Curated by ChEMBL
| Assay Description
Inhibitory activity against rat plasma renin in vitro. |
J Med Chem 31: 1679-86 (1988)
BindingDB Entry DOI: 10.7270/Q2NC61TR |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50022990
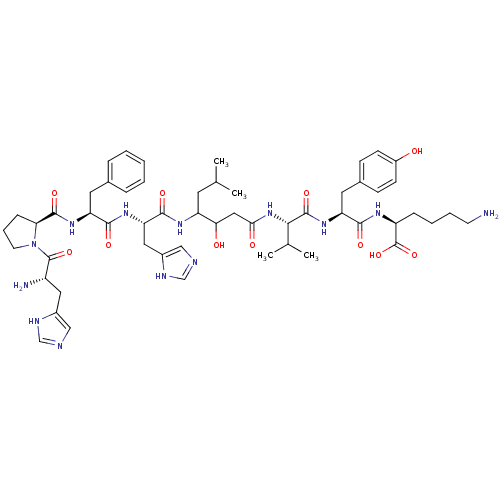
(CHEMBL406168 | His-Pro-Phe-His-Statine-Val-Tyr-Lys)Show SMILES CC(C)CC(NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H]1CCCN1C(=O)[C@@H](N)Cc1cnc[nH]1)C(O)CC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](CCCCN)C(O)=O Show InChI InChI=1S/C54H77N13O11/c1-31(2)21-40(45(69)26-46(70)66-47(32(3)4)52(75)65-42(23-34-15-17-37(68)18-16-34)48(71)61-39(54(77)78)13-8-9-19-55)62-50(73)43(25-36-28-58-30-60-36)63-49(72)41(22-33-11-6-5-7-12-33)64-51(74)44-14-10-20-67(44)53(76)38(56)24-35-27-57-29-59-35/h5-7,11-12,15-18,27-32,38-45,47,68-69H,8-10,13-14,19-26,55-56H2,1-4H3,(H,57,59)(H,58,60)(H,61,71)(H,62,73)(H,63,72)(H,64,74)(H,65,75)(H,66,70)(H,77,78)/t38-,39-,40?,41-,42-,43-,44-,45?,47-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against human plasma renin |
J Med Chem 30: 1287-95 (1987)
BindingDB Entry DOI: 10.7270/Q24J0D3P |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































