 Found 798 hits with Last Name = 'hawkins' and Initial = 'e'
Found 798 hits with Last Name = 'hawkins' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM50045050

(1-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...)Show SMILES CC(=O)C1CCC2C3CC[C@H]4C[C@H](O)CC[C@]4(C)C3CC[C@]12C Show InChI InChI=1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16?,17?,18?,19?,20-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 17.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(RAT) | BDBM50045050

(1-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...)Show SMILES CC(=O)C1CCC2C3CC[C@H]4C[C@H](O)CC[C@]4(C)C3CC[C@]12C Show InChI InChI=1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16?,17?,18?,19?,20-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM50045050

(1-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...)Show SMILES CC(=O)C1CCC2C3CC[C@H]4C[C@H](O)CC[C@]4(C)C3CC[C@]12C Show InChI InChI=1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16?,17?,18?,19?,20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM50045050

(1-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...)Show SMILES CC(=O)C1CCC2C3CC[C@H]4C[C@H](O)CC[C@]4(C)C3CC[C@]12C Show InChI InChI=1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16?,17?,18?,19?,20-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(RAT) | BDBM50045050

(1-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...)Show SMILES CC(=O)C1CCC2C3CC[C@H]4C[C@H](O)CC[C@]4(C)C3CC[C@]12C Show InChI InChI=1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16?,17?,18?,19?,20-,21+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-3
(RAT) | BDBM85683
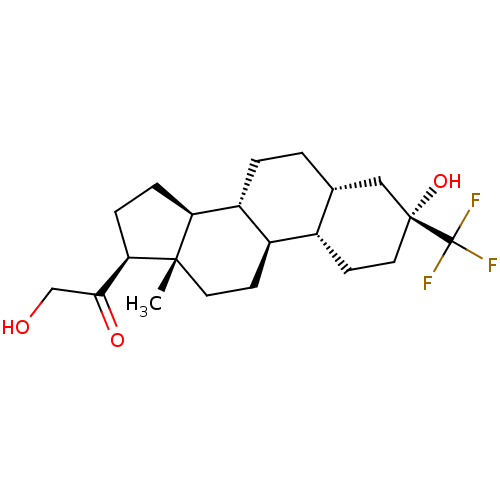
(Co 2-6749 | Co-2-6749 | GMA-839 | WAY-141839)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@](O)(CC[C@H]34)C(F)(F)F)[C@@H]1CC[C@@H]2C(=O)CO Show InChI InChI=1S/C21H31F3O3/c1-19-8-6-14-13-7-9-20(27,21(22,23)24)10-12(13)2-3-15(14)16(19)4-5-17(19)18(26)11-25/h12-17,25,27H,2-11H2,1H3/t12-,13+,14-,15-,16+,17-,19+,20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-2
(Rattus norvegicus (Rat)) | BDBM85683

(Co 2-6749 | Co-2-6749 | GMA-839 | WAY-141839)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@](O)(CC[C@H]34)C(F)(F)F)[C@@H]1CC[C@@H]2C(=O)CO Show InChI InChI=1S/C21H31F3O3/c1-19-8-6-14-13-7-9-20(27,21(22,23)24)10-12(13)2-3-15(14)16(19)4-5-17(19)18(26)11-25/h12-17,25,27H,2-11H2,1H3/t12-,13+,14-,15-,16+,17-,19+,20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-1
(Rattus norvegicus (Rat)) | BDBM85683

(Co 2-6749 | Co-2-6749 | GMA-839 | WAY-141839)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@](O)(CC[C@H]34)C(F)(F)F)[C@@H]1CC[C@@H]2C(=O)CO Show InChI InChI=1S/C21H31F3O3/c1-19-8-6-14-13-7-9-20(27,21(22,23)24)10-12(13)2-3-15(14)16(19)4-5-17(19)18(26)11-25/h12-17,25,27H,2-11H2,1H3/t12-,13+,14-,15-,16+,17-,19+,20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-5
(RAT) | BDBM85683

(Co 2-6749 | Co-2-6749 | GMA-839 | WAY-141839)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@](O)(CC[C@H]34)C(F)(F)F)[C@@H]1CC[C@@H]2C(=O)CO Show InChI InChI=1S/C21H31F3O3/c1-19-8-6-14-13-7-9-20(27,21(22,23)24)10-12(13)2-3-15(14)16(19)4-5-17(19)18(26)11-25/h12-17,25,27H,2-11H2,1H3/t12-,13+,14-,15-,16+,17-,19+,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(RAT) | BDBM50045050

(1-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...)Show SMILES CC(=O)C1CCC2C3CC[C@H]4C[C@H](O)CC[C@]4(C)C3CC[C@]12C Show InChI InChI=1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16?,17?,18?,19?,20-,21+/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM50045050

(1-(3-Hydroxy-10,13-dimethyl-hexadecahydro-cyclopen...)Show SMILES CC(=O)C1CCC2C3CC[C@H]4C[C@H](O)CC[C@]4(C)C3CC[C@]12C Show InChI InChI=1S/C21H34O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h14-19,23H,4-12H2,1-3H3/t14-,15+,16?,17?,18?,19?,20-,21+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-6
(RAT) | BDBM85683

(Co 2-6749 | Co-2-6749 | GMA-839 | WAY-141839)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@](O)(CC[C@H]34)C(F)(F)F)[C@@H]1CC[C@@H]2C(=O)CO Show InChI InChI=1S/C21H31F3O3/c1-19-8-6-14-13-7-9-20(27,21(22,23)24)10-12(13)2-3-15(14)16(19)4-5-17(19)18(26)11-25/h12-17,25,27H,2-11H2,1H3/t12-,13+,14-,15-,16+,17-,19+,20-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid receptor subunit alpha-4
(Rattus norvegicus (Rat)) | BDBM85683

(Co 2-6749 | Co-2-6749 | GMA-839 | WAY-141839)Show SMILES C[C@]12CC[C@H]3[C@@H](CC[C@@H]4C[C@](O)(CC[C@H]34)C(F)(F)F)[C@@H]1CC[C@@H]2C(=O)CO Show InChI InChI=1S/C21H31F3O3/c1-19-8-6-14-13-7-9-20(27,21(22,23)24)10-12(13)2-3-15(14)16(19)4-5-17(19)18(26)11-25/h12-17,25,27H,2-11H2,1H3/t12-,13+,14-,15-,16+,17-,19+,20-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CoCensys, Inc.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 295: 337-45 (2000)
BindingDB Entry DOI: 10.7270/Q2251GQJ |
More data for this
Ligand-Target Pair | |
Sodium/potassium-transporting ATPase subunit alpha-4/beta-1
(Rattus norvegicus) | BDBM50255109
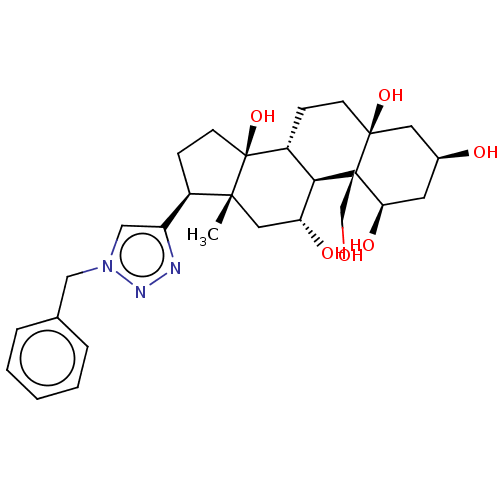
(CHEMBL4081196)Show SMILES [H][C@@]12CC[C@]3(O)C[C@@H](O)C[C@@H](O)[C@]3(CO)[C@@]1([H])[C@H](O)C[C@]1(C)[C@H](CC[C@]21O)c1cn(Cc2ccccc2)nn1 |r| Show InChI InChI=1S/C28H39N3O6/c1-25-13-22(34)24-20(7-9-26(36)12-18(33)11-23(35)27(24,26)16-32)28(25,37)10-8-19(25)21-15-31(30-29-21)14-17-5-3-2-4-6-17/h2-6,15,18-20,22-24,32-37H,7-14,16H2,1H3/t18-,19+,20+,22+,23+,24+,25+,26-,27+,28-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... |
J Med Chem 61: 1800-1820 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00925
BindingDB Entry DOI: 10.7270/Q2T43WJH |
More data for this
Ligand-Target Pair | |
Sodium/potassium-transporting ATPase subunit alpha-4/beta-1
(Rattus norvegicus) | BDBM50255111
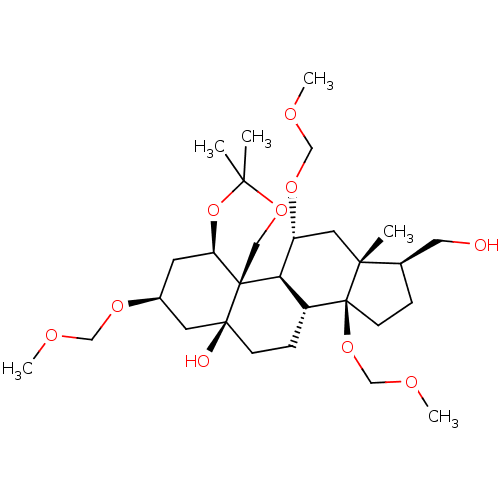
(CHEMBL4092961)Show SMILES [H][C@@]12C[C@@H](C[C@@]3(O)CC[C@]4([H])[C@]([H])([C@@H](C[C@]5(C)[C@@H](CO)CC[C@]45OCOC)OCOC)[C@@]13COC(C)(C)O2)OCOC |r| Show InChI InChI=1S/C29H50O10/c1-25(2)37-15-28-23(39-25)11-20(35-16-32-4)12-27(28,31)9-8-21-24(28)22(36-17-33-5)13-26(3)19(14-30)7-10-29(21,26)38-18-34-6/h19-24,30-31H,7-18H2,1-6H3/t19-,20+,21-,22-,23-,24-,26-,27+,28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... |
J Med Chem 61: 1800-1820 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00925
BindingDB Entry DOI: 10.7270/Q2T43WJH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50474994
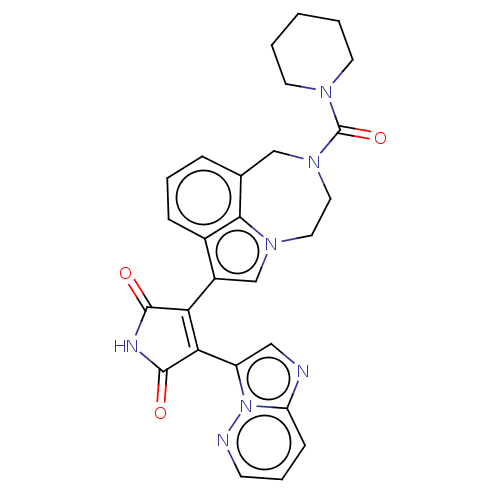
(Bisarylmaleimide 1)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cccnn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-14-28-21-8-5-9-29-34(20)21)19-16-32-12-13-33(27(37)31-10-2-1-3-11-31)15-17-6-4-7-18(19)24(17)32/h4-9,14,16H,1-3,10-13,15H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50020220
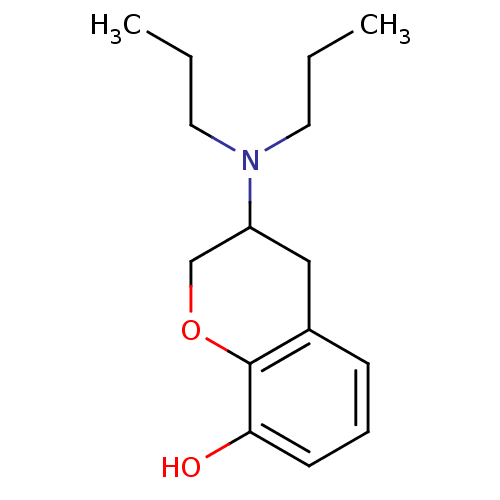
(3-Dipropylamino-chroman-8-ol | CHEMBL38428)Show InChI InChI=1S/C15H23NO2/c1-3-8-16(9-4-2)13-10-12-6-5-7-14(17)15(12)18-11-13/h5-7,13,17H,3-4,8-11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
In vitro affinity to dopamine receptor using [3H]NPAD as radioligand in rat striatal membranes |
J Med Chem 31: 688-91 (1988)
BindingDB Entry DOI: 10.7270/Q2KD2140 |
More data for this
Ligand-Target Pair | |
Sodium/potassium-transporting ATPase subunit alpha-4/beta-1
(Rattus norvegicus) | BDBM50255122
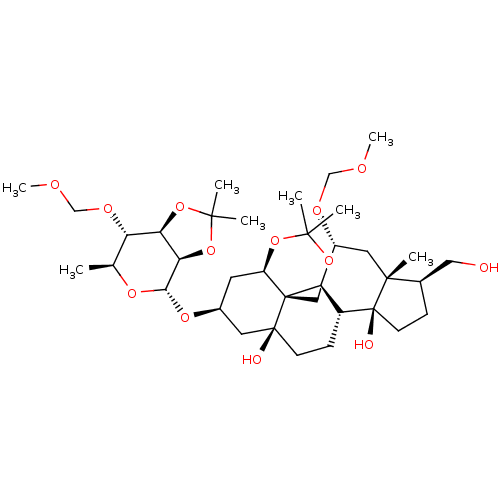
(CHEMBL4059538)Show SMILES [H][C@]12OC(C)(C)O[C@@]1([H])[C@]([H])(O[C@H]1C[C@@]3([H])OC(C)(C)OC[C@]33[C@@]4([H])[C@@H](C[C@]5(C)[C@@H](CO)CC[C@]5(O)[C@]4([H])CC[C@]3(O)C1)OCOC)O[C@@H](C)[C@@H]2OCOC |r| Show InChI InChI=1S/C36H60O13/c1-20-27(43-19-41-8)28-29(49-32(4,5)48-28)30(45-20)46-22-13-25-35(17-44-31(2,3)47-25)26-23(10-11-34(35,38)14-22)36(39)12-9-21(16-37)33(36,6)15-24(26)42-18-40-7/h20-30,37-39H,9-19H2,1-8H3/t20-,21+,22-,23+,24+,25+,26+,27-,28+,29+,30-,33+,34-,35+,36-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... |
J Med Chem 61: 1800-1820 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00925
BindingDB Entry DOI: 10.7270/Q2T43WJH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475007

(Bisarylmaleimide 2)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H23N5O5/c33-25-21(19-13-28-12-16-4-9-37-24(16)19)22(26(34)29-25)20-15-31-5-6-32(27(35)30-7-10-36-11-8-30)14-17-2-1-3-18(20)23(17)31/h1-4,9,12-13,15H,5-8,10-11,14H2,(H,29,33,34) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475025

(CHEMBL181339)Show SMILES O=C(C1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H25N5O4/c34-26-23(24(27(35)30-26)21-14-29-22-6-1-2-9-33(21)22)20-16-31-10-11-32(28(36)17-7-12-37-13-8-17)15-18-4-3-5-19(20)25(18)31/h1-6,9,14,16-17H,7-8,10-13,15H2,(H,30,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475024

(CHEMBL181371)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H18N4O4/c1-13(29)27-6-7-28-12-18(16-4-2-3-15(11-27)21(16)28)20-19(23(30)26-24(20)31)17-10-25-9-14-5-8-32-22(14)17/h2-5,8-10,12H,6-7,11H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475018
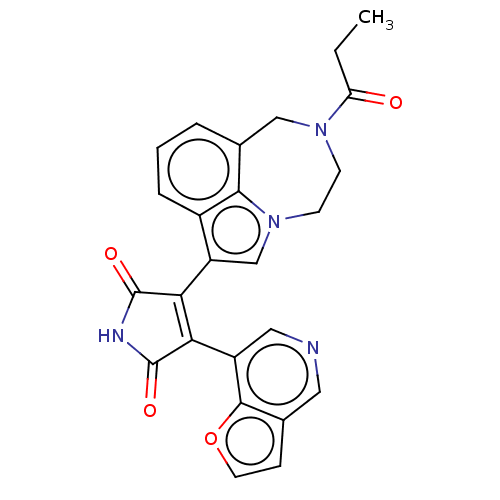
(CHEMBL181518)Show SMILES CCC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:10| Show InChI InChI=1S/C25H20N4O4/c1-2-19(30)28-7-8-29-13-18(16-5-3-4-15(12-28)22(16)29)21-20(24(31)27-25(21)32)17-11-26-10-14-6-9-33-23(14)17/h3-6,9-11,13H,2,7-8,12H2,1H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150699

(3-(9-fluoro-2-(piperidine-1-carbonyl)-1,2,3,4-tetr...)Show SMILES Fc1cc2CN(CCn3cc(C4=C(C(=O)NC4=O)c4cnc5ccccn45)c(c1)c23)C(=O)N1CCCCC1 |t:11| Show InChI InChI=1S/C28H25FN6O3/c29-18-12-17-15-34(28(38)32-7-3-1-4-8-32)11-10-33-16-20(19(13-18)25(17)33)23-24(27(37)31-26(23)36)21-14-30-22-6-2-5-9-35(21)22/h2,5-6,9,12-14,16H,1,3-4,7-8,10-11,15H2,(H,31,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475031

(CHEMBL359871)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4CCOc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C25H21N3O4/c1-14(29)27-9-10-28-13-19(17-6-3-5-16(12-27)22(17)28)21-20(24(30)26-25(21)31)18-7-2-4-15-8-11-32-23(15)18/h2-7,13H,8-12H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475029

(CHEMBL180779)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H21N5O4/c1-28(2)25(33)30-8-7-29-13-18(16-5-3-4-15(12-30)21(16)29)20-19(23(31)27-24(20)32)17-11-26-10-14-6-9-34-22(14)17/h3-6,9-11,13H,7-8,12H2,1-2H3,(H,27,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150698
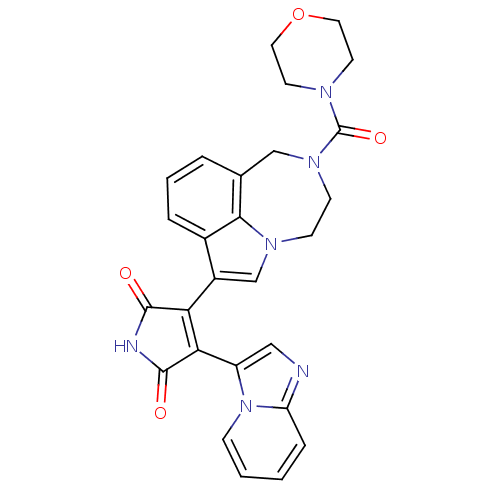
(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475008

(CHEMBL369090)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3ccn4ncccc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(19-6-8-33-21(19)5-2-7-28-33)23(26(35)29-25)20-16-31-9-10-32(27(36)30-11-13-37-14-12-30)15-17-3-1-4-18(20)24(17)31/h1-8,16H,9-15H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150700
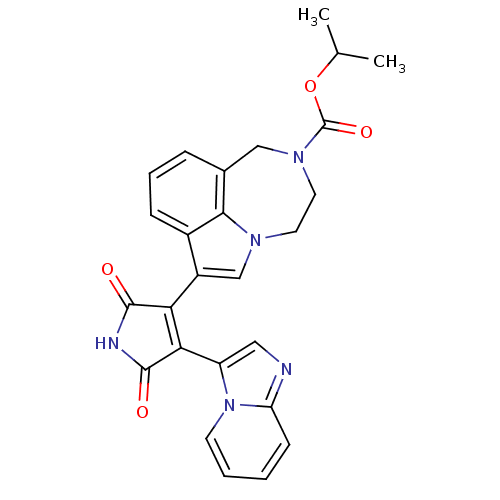
(7-(4-Imidazo[1,2-a]pyridin-3-yl-2,5-dioxo-2,5-dihy...)Show SMILES CC(C)OC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:12| Show InChI InChI=1S/C26H23N5O4/c1-15(2)35-26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Sodium/potassium-transporting ATPase subunit alpha-4/beta-1
(Rattus norvegicus) | BDBM50255138

(CHEMBL4066361)Show SMILES [H][C@@]12C[C@@H](C[C@@]3(O)CC[C@]4([H])[C@]([H])([C@@H](C[C@]5(C)[C@H](CC[C@]45OCOC)\C=N\O)OCOC)[C@@]13COC(C)(C)O2)OCOC |r| Show InChI InChI=1S/C29H49NO10/c1-25(2)38-15-28-23(40-25)11-20(36-16-33-4)12-27(28,31)9-8-21-24(28)22(37-17-34-5)13-26(3)19(14-30-32)7-10-29(21,26)39-18-35-6/h14,19-24,31-32H,7-13,15-18H2,1-6H3/b30-14+/t19-,20+,21-,22-,23-,24-,26-,27+,28-,29+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... |
J Med Chem 61: 1800-1820 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00925
BindingDB Entry DOI: 10.7270/Q2T43WJH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475022

(CHEMBL361765)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4ccoc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C25H19N3O4/c1-14(29)27-9-10-28-13-19(17-6-3-5-16(12-27)22(17)28)21-20(24(30)26-25(21)31)18-7-2-4-15-8-11-32-23(15)18/h2-8,11,13H,9-10,12H2,1H3,(H,26,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150701
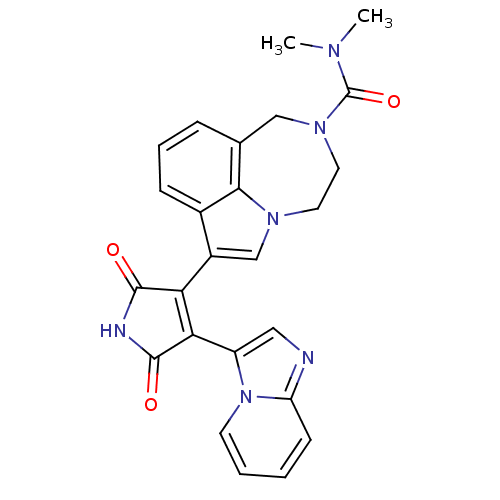
(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150701

(7-(4-(H-imidazo[1,2-a]pyridin-3-yl)-2,5-dioxo-2,5-...)Show SMILES CN(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C25H22N6O3/c1-28(2)25(34)30-11-10-29-14-17(16-7-5-6-15(13-30)22(16)29)20-21(24(33)27-23(20)32)18-12-26-19-8-3-4-9-31(18)19/h3-9,12,14H,10-11,13H2,1-2H3,(H,27,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50474994

(Bisarylmaleimide 1)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cccnn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-14-28-21-8-5-9-29-34(20)21)19-16-32-12-13-33(27(37)31-10-2-1-3-11-31)15-17-6-4-7-18(19)24(17)32/h4-9,14,16H,1-3,10-13,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration to inhibit Ser396 phosphorylation of tau, a natural substrate of GSK-3 in SY5Y cells |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475010
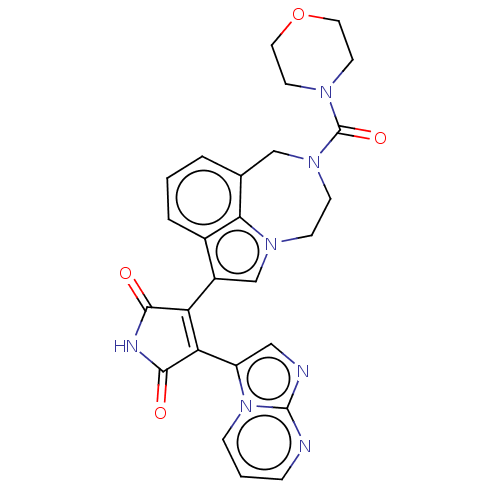
(CHEMBL369316)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ncccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C26H23N7O4/c34-23-20(21(24(35)29-23)19-13-28-25-27-5-2-6-33(19)25)18-15-31-7-8-32(26(36)30-9-11-37-12-10-30)14-16-3-1-4-17(18)22(16)31/h1-6,13,15H,7-12,14H2,(H,29,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475001
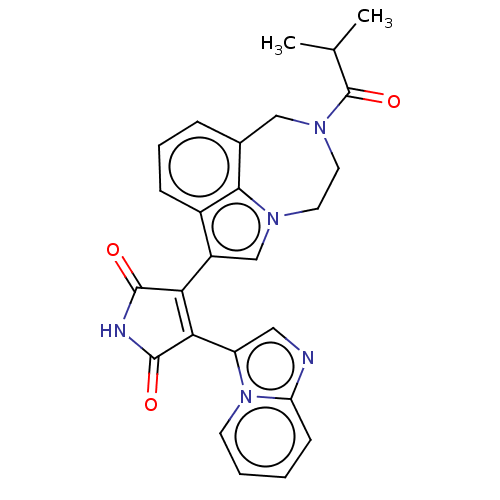
(CHEMBL368246)Show SMILES CC(C)C(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:11| Show InChI InChI=1S/C26H23N5O3/c1-15(2)26(34)30-11-10-29-14-18(17-7-5-6-16(13-30)23(17)29)21-22(25(33)28-24(21)32)19-12-27-20-8-3-4-9-31(19)20/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,28,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Sodium/potassium-transporting ATPase subunit alpha-4/beta-3
(Rattus norvegicus) | BDBM50286739
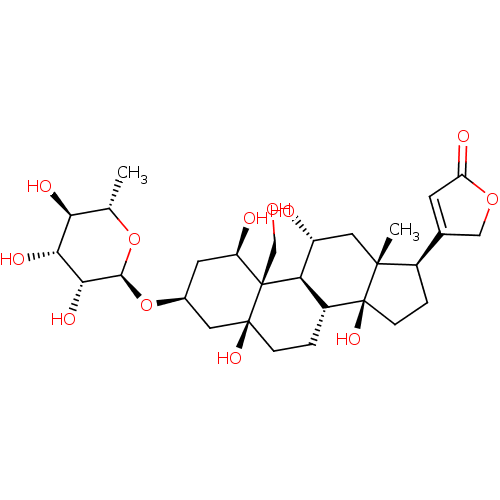
(4-((1R,3S,5S,8R,10R,11R,13R,14S,17R)-1,5,11,14-tet...)Show SMILES C[C@@H]1O[C@@H](O[C@H]2C[C@@H](O)[C@]3(CO)[C@H]4[C@H](O)C[C@]5(C)[C@H](CC[C@]5(O)[C@@H]4CC[C@]3(O)C2)C2=CC(=O)OC2)[C@H](O)[C@H](O)[C@H]1O |r,t:33| Show InChI InChI=1S/C29H44O12/c1-13-22(34)23(35)24(36)25(40-13)41-15-8-19(32)28(12-30)21-17(3-5-27(28,37)9-15)29(38)6-4-16(14-7-20(33)39-11-14)26(29,2)10-18(21)31/h7,13,15-19,21-25,30-32,34-38H,3-6,8-12H2,1-2H3/t13-,15-,16+,17+,18+,19+,21+,22-,23+,24+,25-,26+,27-,28+,29-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta3 expressed in baculovirus infected Sf9 cell membranes using [gamma-32P]ATP as substrate prein... |
J Med Chem 61: 1800-1820 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00925
BindingDB Entry DOI: 10.7270/Q2T43WJH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475004

(CHEMBL369572)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ncccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-24-21(22(25(36)30-24)20-14-29-26-28-8-5-11-34(20)26)19-16-32-12-13-33(27(37)31-9-2-1-3-10-31)15-17-6-4-7-18(19)23(17)32/h4-8,11,14,16H,1-3,9-10,12-13,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475014
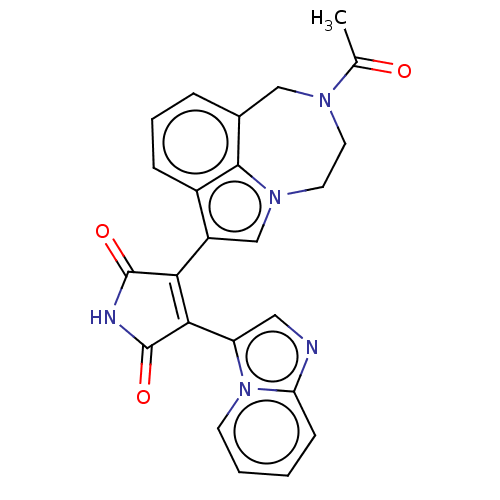
(CHEMBL361948)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H19N5O3/c1-14(30)27-9-10-28-13-17(16-6-4-5-15(12-27)22(16)28)20-21(24(32)26-23(20)31)18-11-25-19-7-2-3-8-29(18)19/h2-8,11,13H,9-10,12H2,1H3,(H,26,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human Glycogen synthase kinase-3 beta |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50150702

(3-Imidazo[1,2-a]pyridin-3-yl-4-[2-(piperidine-1-ca...)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H26N6O3/c35-26-23(24(27(36)30-26)21-15-29-22-9-2-5-12-34(21)22)20-17-32-13-14-33(28(37)31-10-3-1-4-11-31)16-18-7-6-8-19(20)25(18)32/h2,5-9,12,15,17H,1,3-4,10-11,13-14,16H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475006

(CHEMBL178851)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4cnccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H25N7O3/c35-25-22(23(26(36)30-25)20-13-29-21-14-28-7-10-34(20)21)19-16-32-11-12-33(27(37)31-8-2-1-3-9-31)15-17-5-4-6-18(19)24(17)32/h4-7,10,13-14,16H,1-3,8-9,11-12,15H2,(H,30,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50474996

(CHEMBL178646)Show SMILES O=C(N1CCCCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cncc4ccoc34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C28H25N5O4/c34-26-22(20-14-29-13-17-7-12-37-25(17)20)23(27(35)30-26)21-16-32-10-11-33(28(36)31-8-2-1-3-9-31)15-18-5-4-6-19(21)24(18)32/h4-7,12-14,16H,1-3,8-11,15H2,(H,30,34,35) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
Sodium/potassium-transporting ATPase subunit alpha-4/beta-1
(Rattus norvegicus) | BDBM50255120
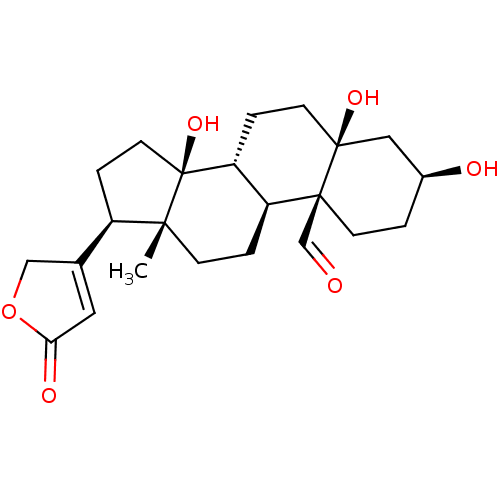
(CHEBI:38178 | STROPHANTHIDIN)Show SMILES [H][C@@]12CC[C@]3(O)C[C@@H](O)CC[C@]3(C=O)[C@@]1([H])CC[C@]1(C)[C@H](CC[C@]21O)C1=CC(=O)OC1 |r,t:29| Show InChI InChI=1S/C23H32O6/c1-20-6-3-17-18(4-8-22(27)11-15(25)2-7-21(17,22)13-24)23(20,28)9-5-16(20)14-10-19(26)29-12-14/h10,13,15-18,25,27-28H,2-9,11-12H2,1H3/t15-,16+,17-,18+,20+,21-,22-,23-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Medicinal Chemistry and Institute for Therapeutics Discovery and Development, College of Pharmacy , University of Minnesota , Minneapolis , Minnesota 55414 , United States.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant rat Na+/K+-ATPase alpha4/beta1 expressed in baculovirus infected insect Sf9 cell membranes using [gamma-32P]ATP as substrat... |
J Med Chem 61: 1800-1820 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00925
BindingDB Entry DOI: 10.7270/Q2T43WJH |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3
(Homo sapiens (Human)) | BDBM50475000

(CHEMBL181296)Show SMILES CC(=O)N1CCn2cc(C3=C(C(=O)NC3=O)c3cccc4OCOc34)c3cccc(C1)c23 |t:9| Show InChI InChI=1S/C24H19N3O5/c1-13(28)26-8-9-27-11-17(15-5-2-4-14(10-26)21(15)27)20-19(23(29)25-24(20)30)16-6-3-7-18-22(16)32-12-31-18/h2-7,11H,8-10,12H2,1H3,(H,25,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory concentration against the Glycogen synthase kinase-3 |
Bioorg Med Chem Lett 15: 899-903 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.063
BindingDB Entry DOI: 10.7270/Q2H70JJG |
More data for this
Ligand-Target Pair | |
D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor
(Rattus norvegicus (rat)-RAT-Rattus norvegicus (Rat...) | BDBM50001955

((-)6-Methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]qu...)Show InChI InChI=1S/C17H17NO2/c1-18-8-7-10-3-2-4-12-15(10)13(18)9-11-5-6-14(19)17(20)16(11)12/h2-6,13,19-20H,7-9H2,1H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
In vitro affinity to dopamine receptor using [3H]NPAD as radioligand in rat striatal membranes |
J Med Chem 31: 688-91 (1988)
BindingDB Entry DOI: 10.7270/Q2KD2140 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50150698

(3-(imidazo[1,2-a]pyridin-3-yl)-4-(2-(morpholine-4-...)Show SMILES O=C(N1CCOCC1)N1CCn2cc(C3=C(C(=O)NC3=O)c3cnc4ccccn34)c3cccc(C1)c23 |t:15| Show InChI InChI=1S/C27H24N6O4/c34-25-22(23(26(35)29-25)20-14-28-21-6-1-2-7-33(20)21)19-16-31-8-9-32(27(36)30-10-12-37-13-11-30)15-17-4-3-5-18(19)24(17)31/h1-7,14,16H,8-13,15H2,(H,29,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta dependent Tau protein serine-396 phosphorylation in human SY5Y cells |
J Med Chem 47: 3934-7 (2004)
Article DOI: 10.1021/jm049768a
BindingDB Entry DOI: 10.7270/Q2B56KG1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data