

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163573 ((R)-3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 7 (Homo sapiens (Human)) | BDBM50533413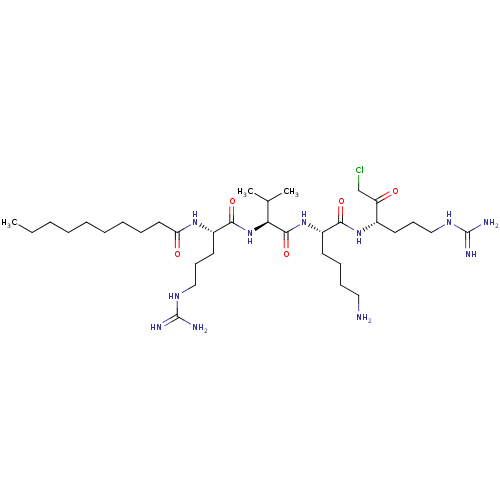 (CHEMBL3126388) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal truncated human SPC7 expressed in drosophila Schneider 2 cells after 1 hr by spectrofluorometry | J Med Chem 59: 7719-37 (2016) Article DOI: 10.1021/acs.jmedchem.5b01516 BindingDB Entry DOI: 10.7270/Q2M048X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163576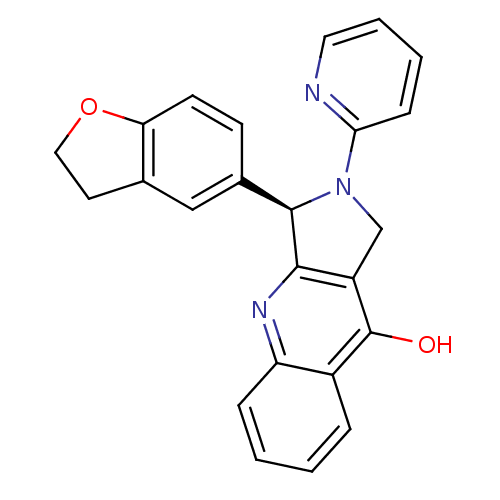 ((R)-3-(2,3-Dihydro-benzofuran-5-yl)-2-pyridin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proprotein convertase subtilisin/kexin type 5 (Homo sapiens (Human)) | BDBM50533413 (CHEMBL3126388) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal truncated human SPC6 expressed in drosophila Schneider 2 cells after 1 hr by spectrofluorometry | J Med Chem 59: 7719-37 (2016) Article DOI: 10.1021/acs.jmedchem.5b01516 BindingDB Entry DOI: 10.7270/Q2M048X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM85335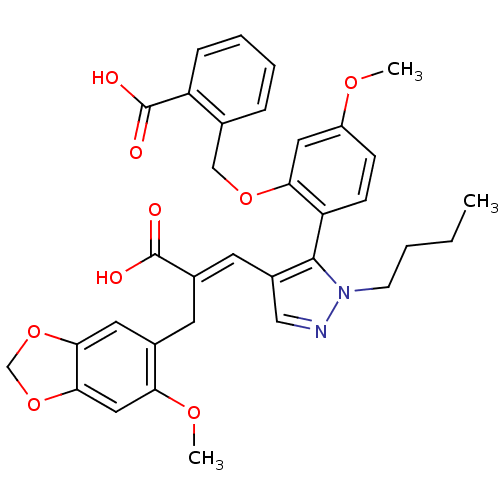 (SB 234551 | SB-234551) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | J Pharmacol Exp Ther 286: 650-6 (1998) BindingDB Entry DOI: 10.7270/Q2V40SSK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163581 (2-[2,3'']Bipyridinyl-6''-yl-3-(2,3-dihydro-benzofu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122970 (3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-3-yl-furan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163570 (2-[5-(3-Benzyl-3H-imidazol-4-yl)-pyridin-2-yl]-3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163577 (3-Benzofuran-5-yl-2-(5-pyridin-2-yl-pyrimidin-2-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122969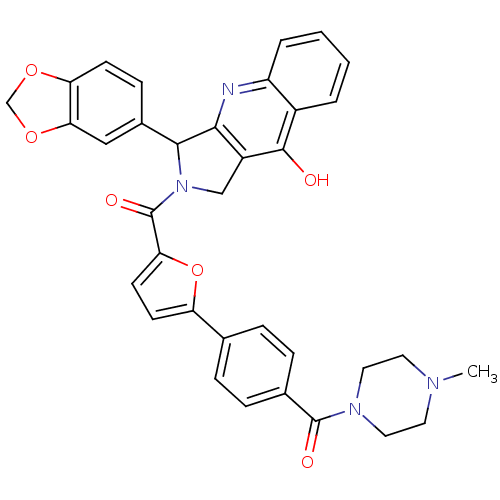 (3-Benzo[1,3]dioxol-5-yl-2-{5-[4-(4-methyl-piperazi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50118249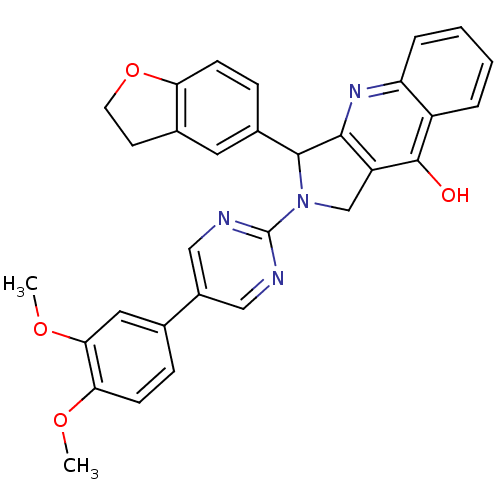 (3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(3,4-dimethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Phosphodiesterase 5 activity of human corpus cavernosum | J Med Chem 45: 4094-6 (2002) BindingDB Entry DOI: 10.7270/Q2R49RGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50370143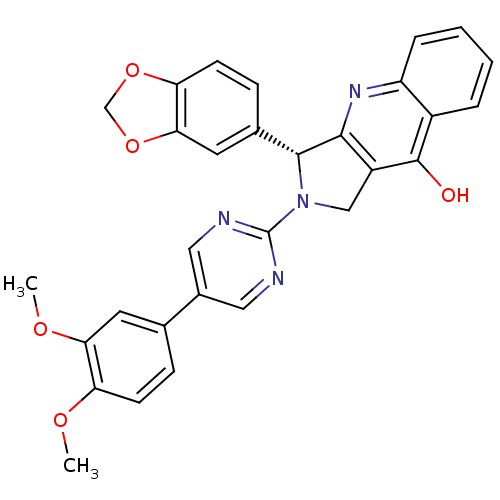 (CHEMBL1744059) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Phosphodiesterase 5 activity of human corpus cavernosum | J Med Chem 45: 4094-6 (2002) BindingDB Entry DOI: 10.7270/Q2R49RGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50138930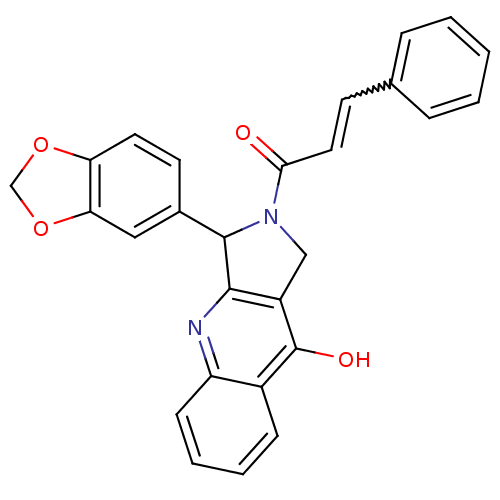 (3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-acryloyl)-1,2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Inhibitory activity against human Phosphodiesterase 5 | J Med Chem 47: 656-62 (2004) Article DOI: 10.1021/jm020521s BindingDB Entry DOI: 10.7270/Q228070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163578 (3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-4-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50118248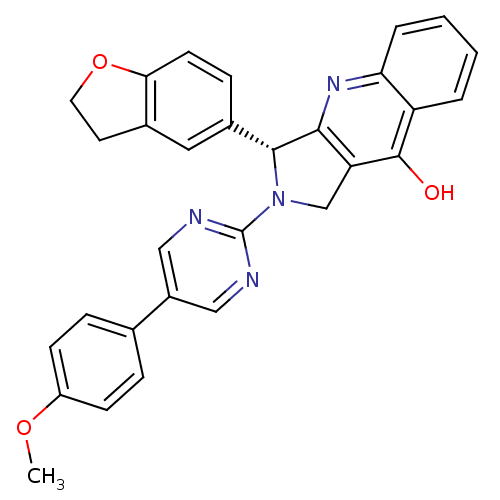 (3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-methoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Phosphodiesterase 5 activity of human corpus cavernosum | J Med Chem 45: 4094-6 (2002) BindingDB Entry DOI: 10.7270/Q2R49RGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50138939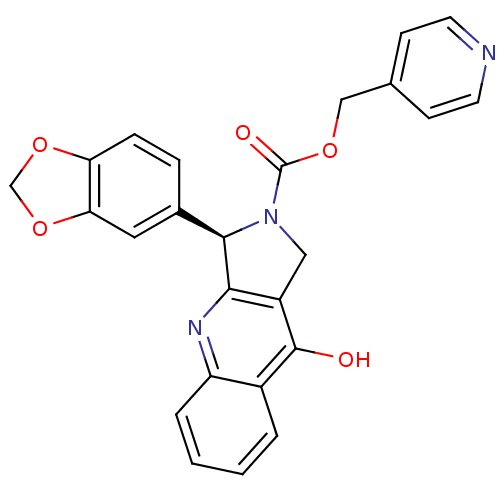 ((R)-3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Inhibitory activity against human Phosphodiesterase 5 | J Med Chem 47: 656-62 (2004) Article DOI: 10.1021/jm020521s BindingDB Entry DOI: 10.7270/Q228070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50071112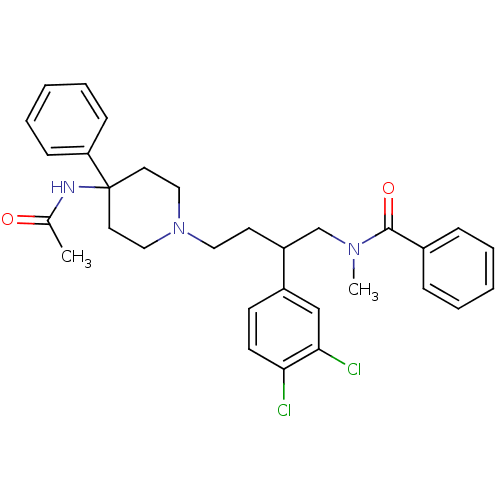 (CHEMBL56835 | N-[4-(4-Acetylamino-4-phenyl-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | J Pharmacol Exp Ther 281: 1303-11 (1997) BindingDB Entry DOI: 10.7270/Q2K35S61 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122990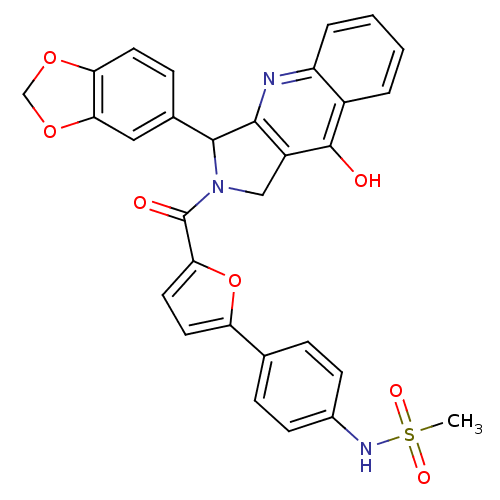 (CHEMBL342159 | N-{4-[5-(3-Benzo[1,3]dioxol-5-yl-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163571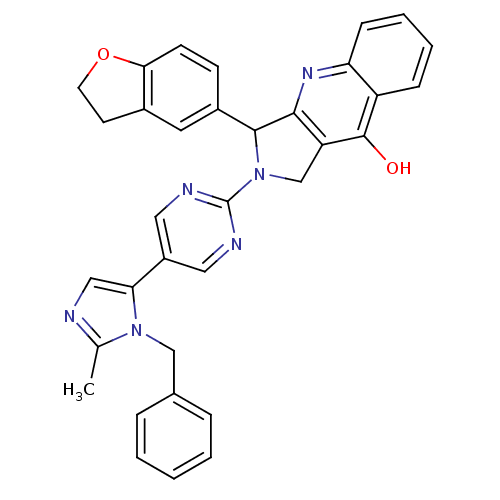 (2-[5-(3-Benzyl-2-methyl-3H-imidazol-4-yl)-pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163574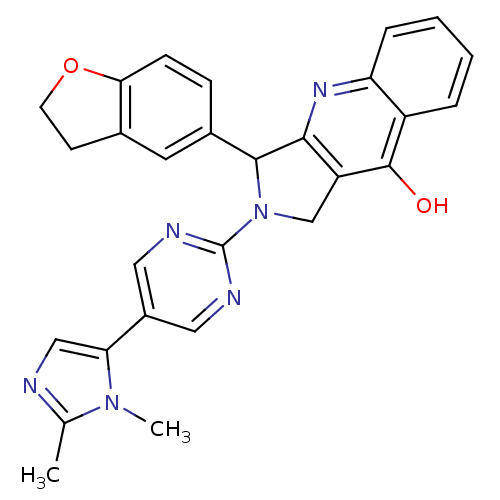 (3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(2,3-dimethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163579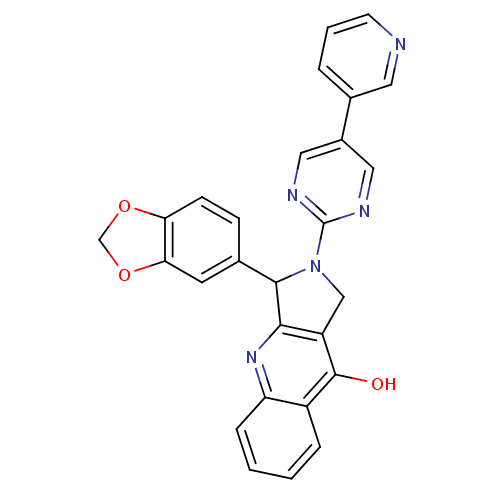 (3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-3-yl-pyrimidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50118250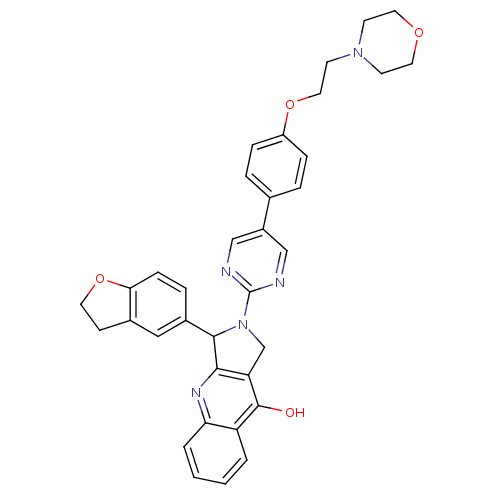 (3-(2,3-Dihydro-benzofuran-5-yl)-2-{5-[4-(2-morphol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Phosphodiesterase 5 activity of human corpus cavernosum | J Med Chem 45: 4094-6 (2002) BindingDB Entry DOI: 10.7270/Q2R49RGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50138929 (3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Inhibitory activity against human Phosphodiesterase 5 | J Med Chem 47: 656-62 (2004) Article DOI: 10.1021/jm020521s BindingDB Entry DOI: 10.7270/Q228070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50118252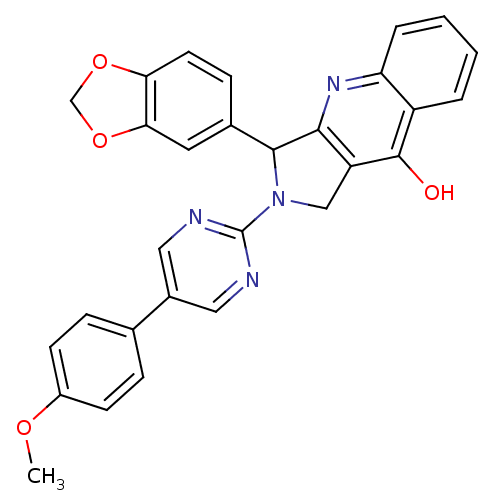 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-methoxy-phenyl)-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Phosphodiesterase 5 activity of human corpus cavernosum | J Med Chem 45: 4094-6 (2002) BindingDB Entry DOI: 10.7270/Q2R49RGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuromedin-K receptor (Homo sapiens (Human)) | BDBM85083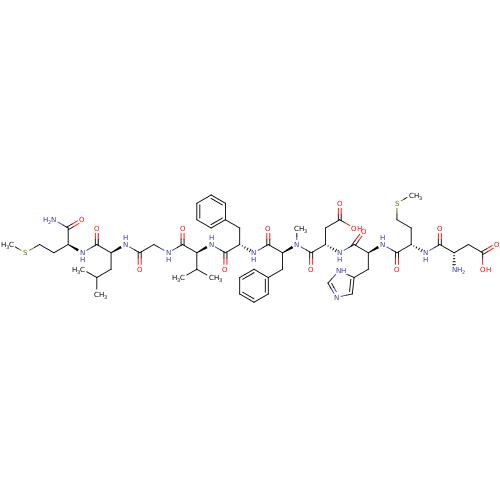 (NKB [MePhe7]) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | J Pharmacol Exp Ther 281: 1303-11 (1997) BindingDB Entry DOI: 10.7270/Q2K35S61 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122974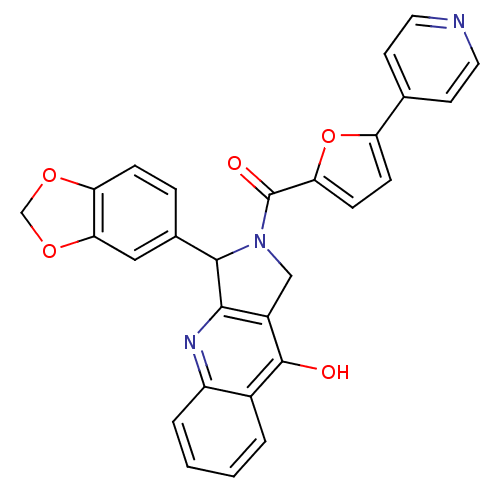 (3-Benzo[1,3]dioxol-5-yl-2-(5-pyridin-4-yl-furan-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163572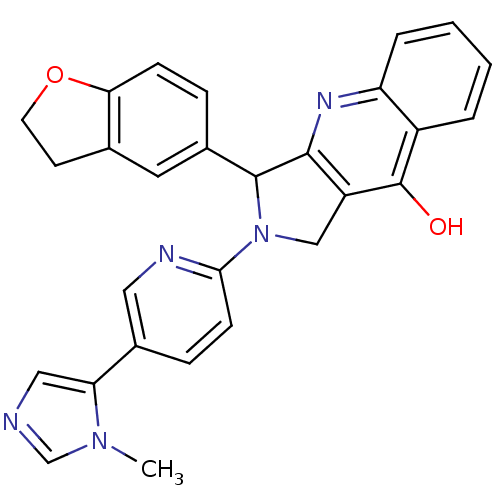 (3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(3-methyl-3H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122964 (3-Benzo[1,3]dioxol-5-yl-2-(6-hydroxy-benzofuran-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50138936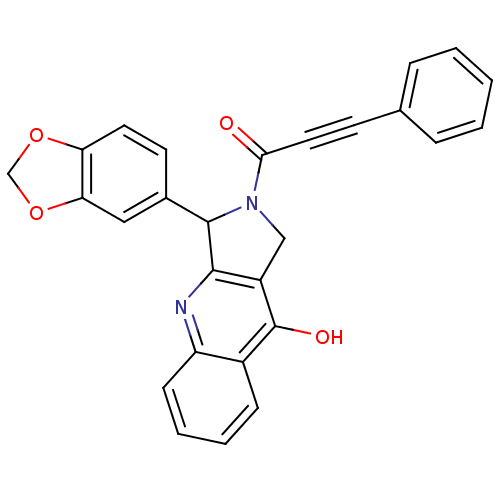 (3-Benzo[1,3]dioxol-5-yl-2-(3-phenyl-propynoyl)-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Inhibitory activity against human Phosphodiesterase 5 | J Med Chem 47: 656-62 (2004) Article DOI: 10.1021/jm020521s BindingDB Entry DOI: 10.7270/Q228070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163568 (3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-3-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuroendocrine convertase 2 (Homo sapiens (Human)) | BDBM50533413 (CHEMBL3126388) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) Curated by ChEMBL | Assay Description Inhibition of recombinant C-terminal truncated human SPC2 expressed in drosophila Schneider 2 cells after 1 hr by spectrofluorometry | J Med Chem 59: 7719-37 (2016) Article DOI: 10.1021/acs.jmedchem.5b01516 BindingDB Entry DOI: 10.7270/Q2M048X4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50118255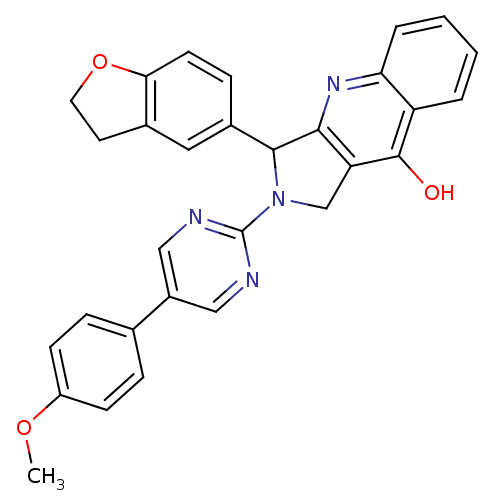 (3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-methoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Phosphodiesterase 5 activity of human corpus cavernosum | J Med Chem 45: 4094-6 (2002) BindingDB Entry DOI: 10.7270/Q2R49RGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-2 angiotensin II receptor (Homo sapiens (Human)) | BDBM50156173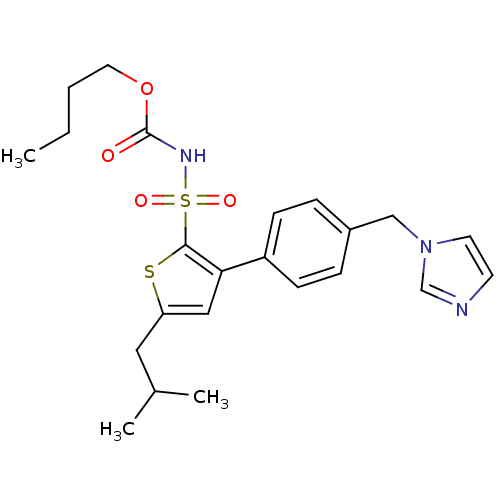 ((1-((2'-(1H-tetrazol-5-yl)biphenyl-4-yl)methyl)-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL) Curated by ChEMBL | Assay Description Binding affinity to AT2 receptor (unknown origin) | J Med Chem 61: 9811-9840 (2018) Article DOI: 10.1021/acs.jmedchem.8b00294 BindingDB Entry DOI: 10.7270/Q2XK8J7N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50041617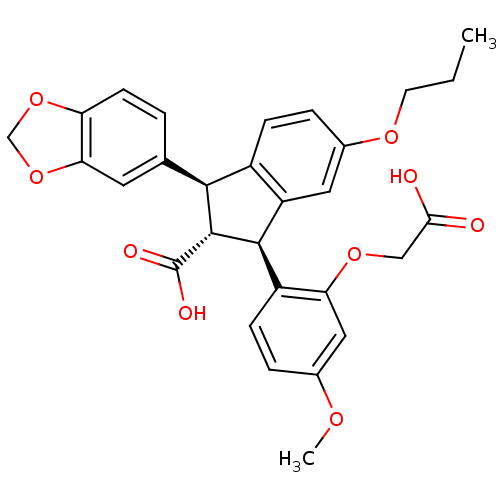 ((1S,2R,3S)-1-Benzo[1,3]dioxol-5-yl-3-(2-carboxymet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by PDSP Ki Database | J Pharmacol Exp Ther 286: 650-6 (1998) BindingDB Entry DOI: 10.7270/Q2V40SSK | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122966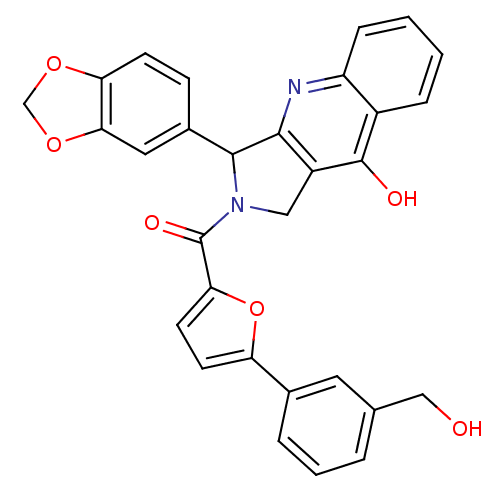 (3-Benzo[1,3]dioxol-5-yl-2-[5-(3-hydroxymethyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Rattus norvegicus (Rat)) | BDBM50291261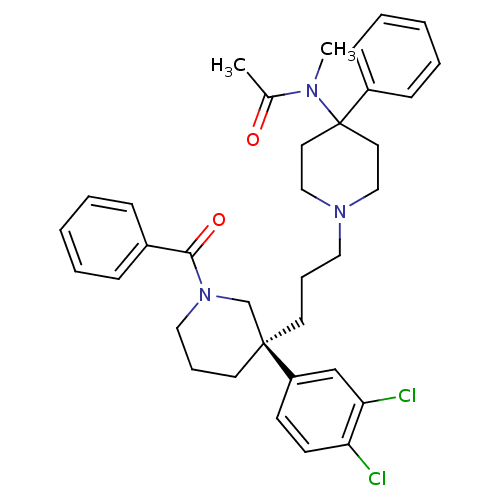 ((S)-(+)-N-((3-[1-Benzoyl-3-(3,4-dichlorophenyl)pip...) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham S.p.A. Curated by ChEMBL | Assay Description Ability to displace [125I]NKA from Tachykinin receptor 2 in rat deodenum membrane | J Med Chem 39: 2281-4 (1996) Article DOI: 10.1021/jm9602423 BindingDB Entry DOI: 10.7270/Q22J6CJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163580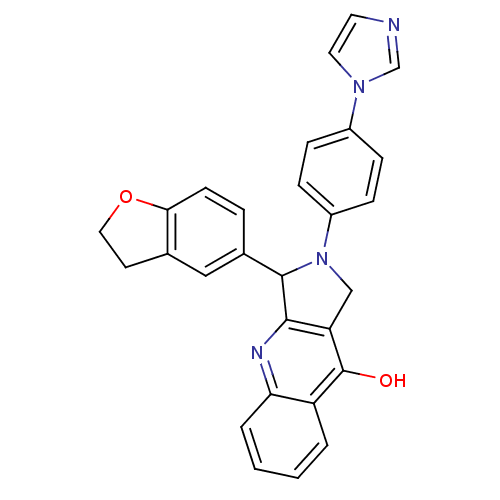 (3-(2,3-Dihydro-benzofuran-5-yl)-2-(4-imidazol-1-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50118251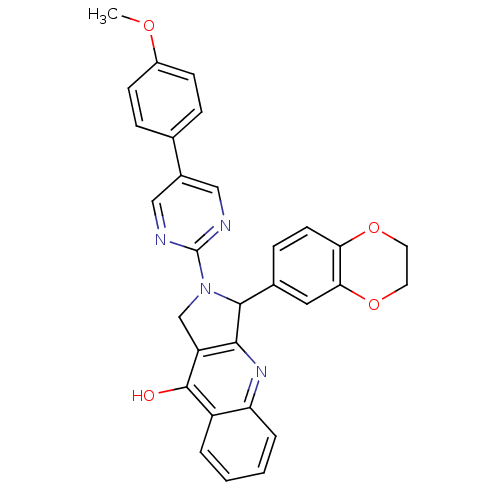 (3-(2,3-Dihydro-benzo[1,4]dioxin-6-yl)-2-[5-(4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Phosphodiesterase 5 activity of human corpus cavernosum | J Med Chem 45: 4094-6 (2002) BindingDB Entry DOI: 10.7270/Q2R49RGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50118255 (3-(2,3-Dihydro-benzofuran-5-yl)-2-[5-(4-methoxy-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50118258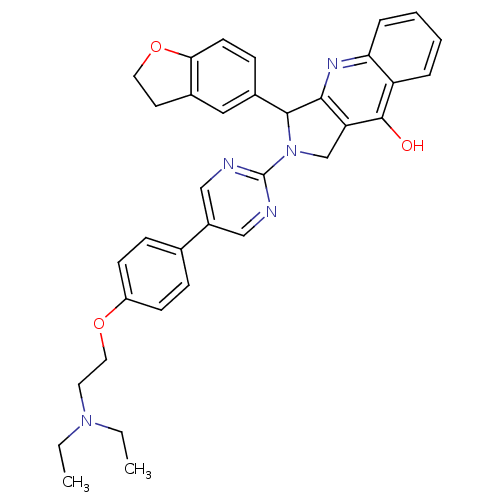 (2-{5-[4-(2-Diethylamino-ethoxy)-phenyl]-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122971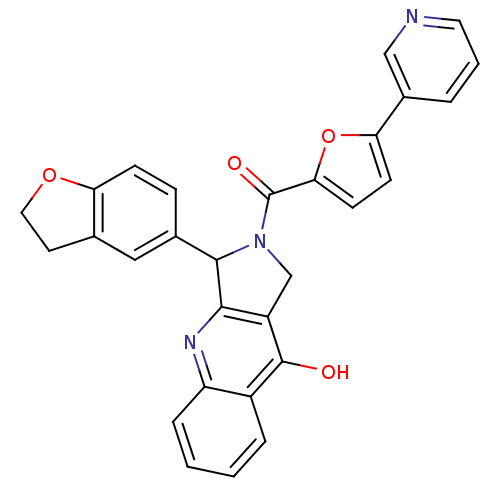 (3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-3-yl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50118258 (2-{5-[4-(2-Diethylamino-ethoxy)-phenyl]-pyrimidin-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Phosphodiesterase 5 activity of human corpus cavernosum | J Med Chem 45: 4094-6 (2002) BindingDB Entry DOI: 10.7270/Q2R49RGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50138927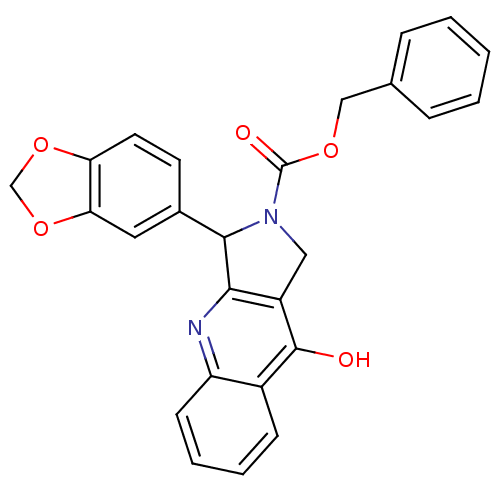 (3-Benzo[1,3]dioxol-5-yl-9-oxo-1,3,4,9-tetrahydro-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development LLC Curated by ChEMBL | Assay Description Inhibitory activity against human Phosphodiesterase 5 | J Med Chem 47: 656-62 (2004) Article DOI: 10.1021/jm020521s BindingDB Entry DOI: 10.7270/Q228070W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50099628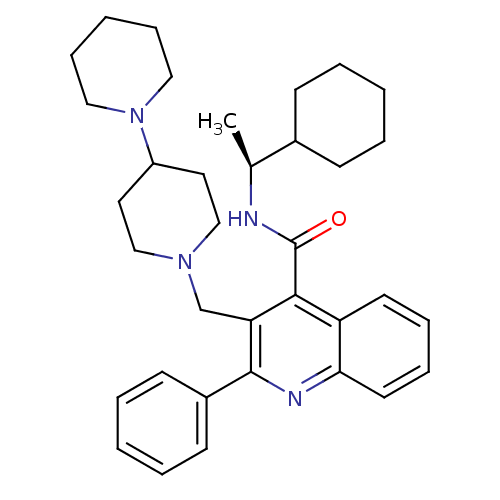 (3-[1,4']Bipiperidinyl-1'-ylmethyl-2-phenyl-quinoli...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125 I]-NKA binding to tachykinin receptor 2 in CHO membranes | J Med Chem 44: 1675-89 (2001) BindingDB Entry DOI: 10.7270/Q29C6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122983 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-nitro-phenyl)-fura...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122973 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-hydroxymethyl-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Rattus norvegicus) | BDBM50163575 (3-(2,3-Dihydro-benzofuran-5-yl)-2-pyridin-2-yl-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells | J Med Chem 48: 2126-33 (2005) Article DOI: 10.1021/jm0401098 BindingDB Entry DOI: 10.7270/Q2WH2QR5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50099638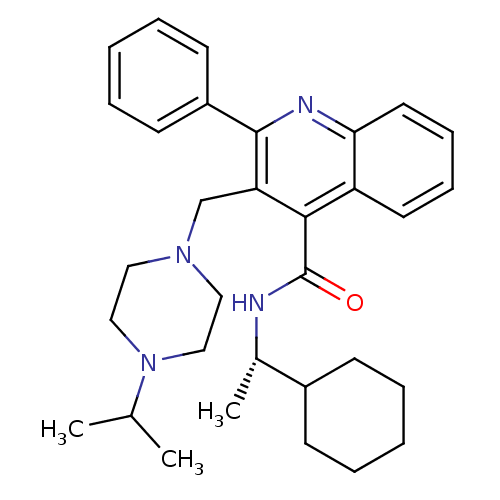 (3-(4-Isopropyl-piperazin-1-ylmethyl)-2-phenyl-quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Displacement of [125 I]-NKA binding to tachykinin receptor 2 in CHO membranes | J Med Chem 44: 1675-89 (2001) BindingDB Entry DOI: 10.7270/Q29C6WQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122980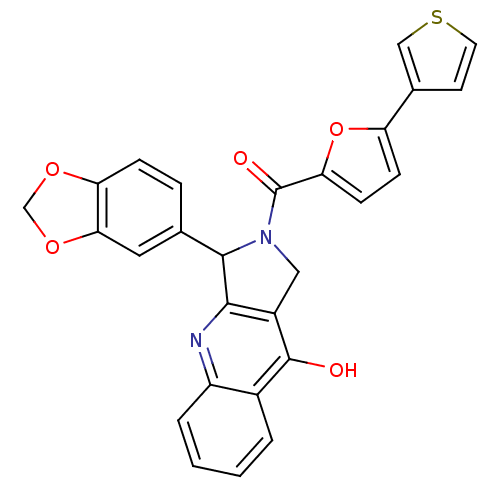 (3-Benzo[1,3]dioxol-5-yl-2-(5-thiophen-3-yl-furan-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| cGMP-specific 3',5'-cyclic phosphodiesterase (Homo sapiens (Human)) | BDBM50122981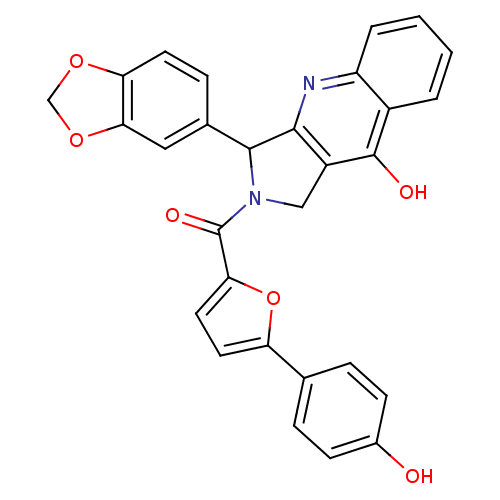 (3-Benzo[1,3]dioxol-5-yl-2-[5-(4-hydroxy-phenyl)-fu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research & Development L.L.C. Curated by ChEMBL | Assay Description Inhibitory activity against Phosphodiesterase 5 (PDE5) was evaluated | J Med Chem 46: 441-4 (2003) Article DOI: 10.1021/jm0202573 BindingDB Entry DOI: 10.7270/Q2QR4WHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2105 total ) | Next | Last >> |