

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM14073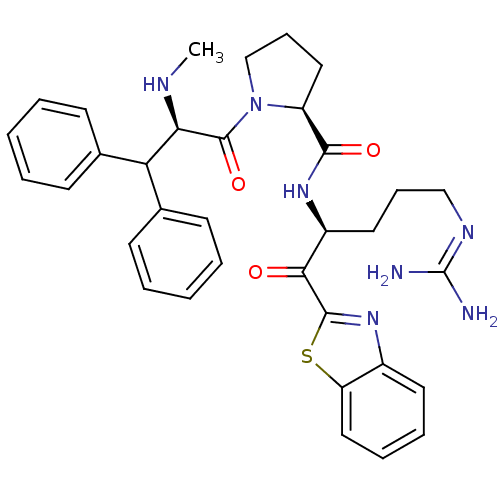 ((2S)-N-[(2S)-1-(1,3-benzothiazol-2-yl)-5-carbamimi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.000650 | -72.4 | 4.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189800 (US10213433, Compound 374 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM356989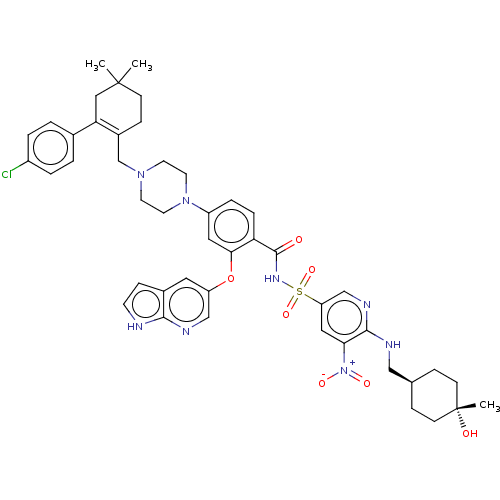 (4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM356990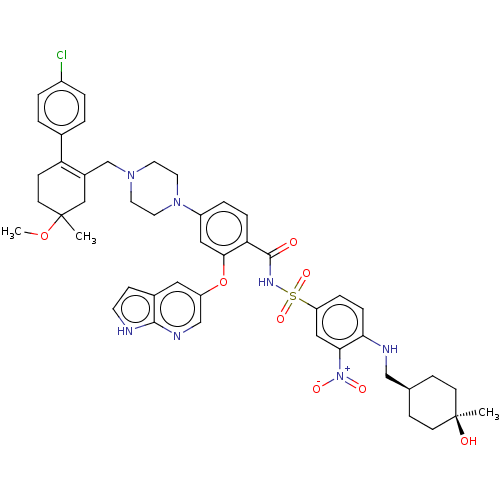 (4-(4-{[2-(4-chlorophenyl)-5-methoxy-5-methylcycloh...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189804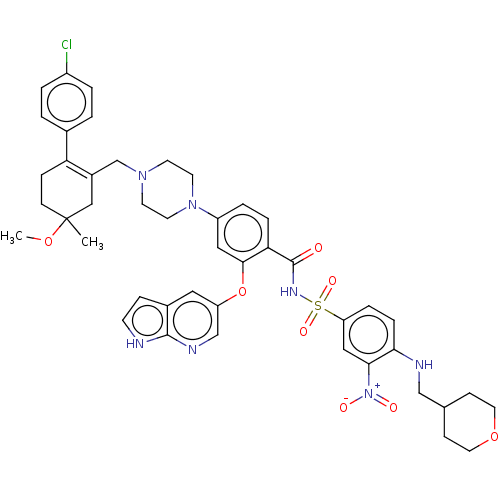 (US10213433, Compound 378 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM356985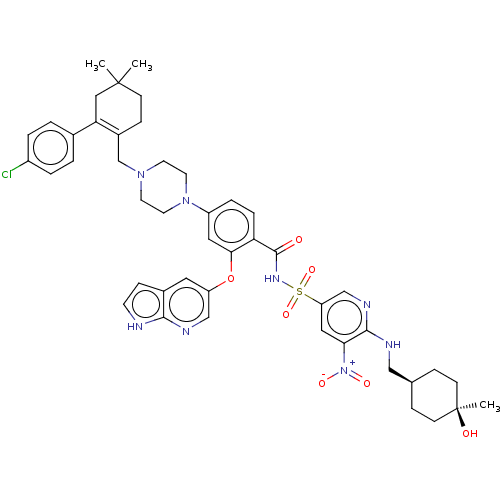 (4-(4-{[2-(4-chlorophenyl)-4,4-dimethylcyclohex-1-e...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189799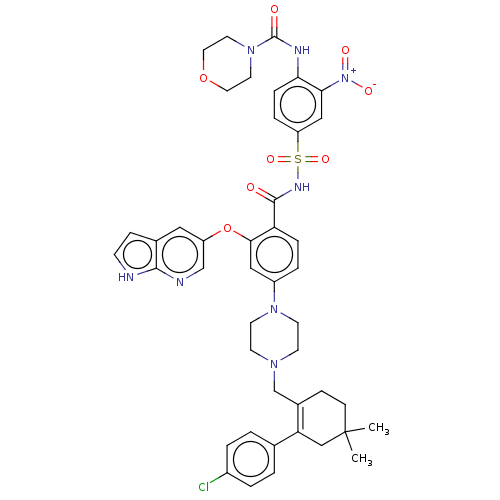 (US10213433, Compound 373 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | <0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM144940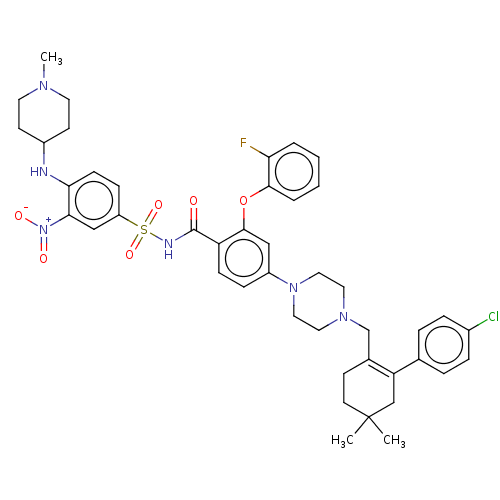 (US8952157, 122 | US9303025, 122) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.; The Walter and Eliza Hall Institute of Medical Research US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US8952157 (2015) BindingDB Entry DOI: 10.7270/Q2QN65G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178562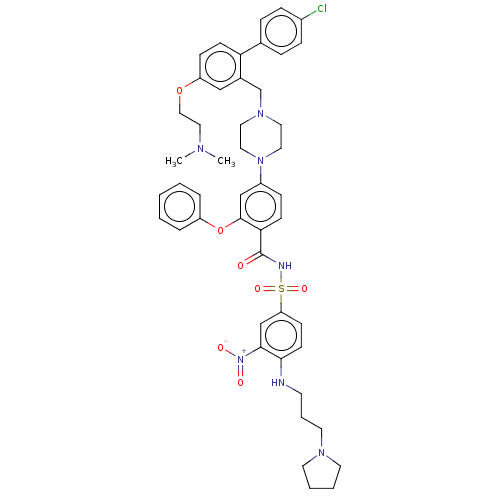 (US9125913, 121) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189804 (US10213433, Compound 378 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189803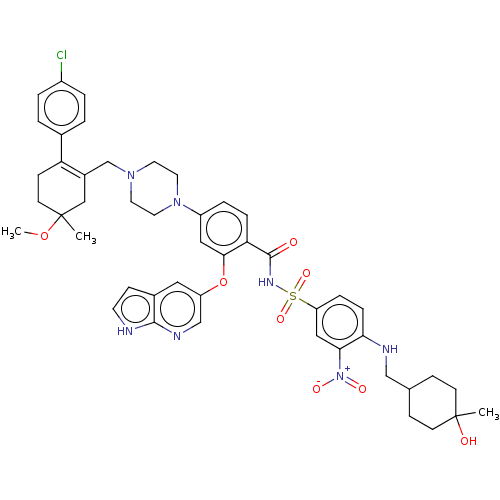 (US11369599, Compound 377 | US9174982, 377) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189797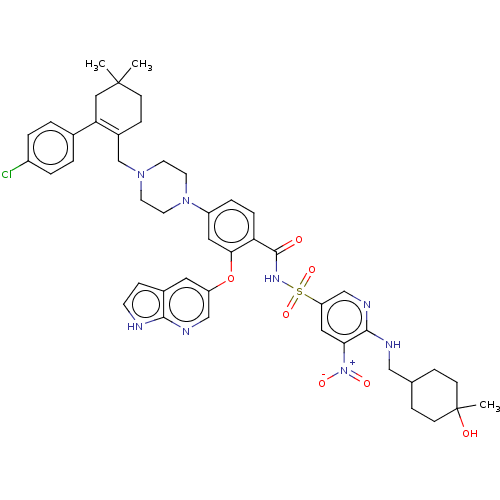 (US11369599, Compound 376 | US9174982, 371 | US9174...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189800 (US10213433, Compound 374 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189799 (US10213433, Compound 373 | US11369599, Compound 37...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189797 (US11369599, Compound 376 | US9174982, 371 | US9174...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM144940 (US8952157, 122 | US9303025, 122) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | <0.00100 | <-68.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... | US Patent US9303025 (2016) BindingDB Entry DOI: 10.7270/Q2XD10JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178855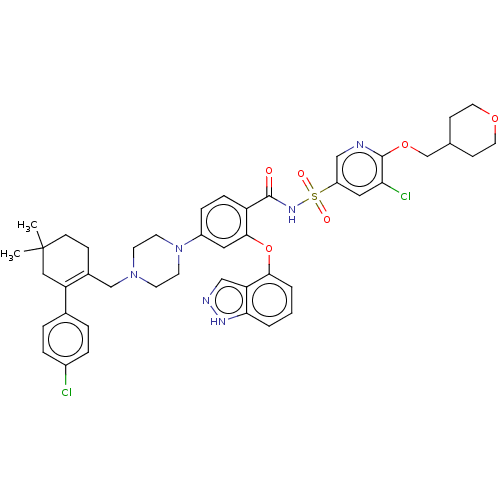 (US9125913, 427) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178843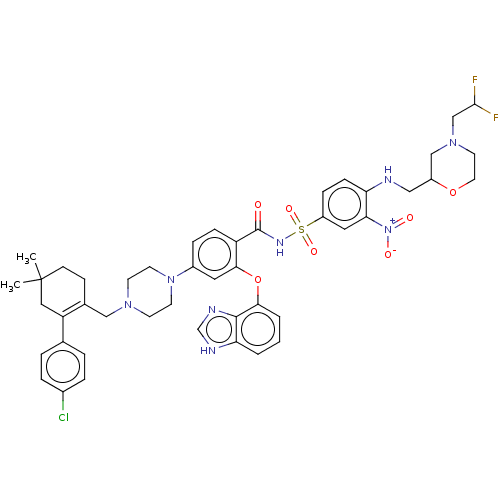 (US9125913, 415) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189567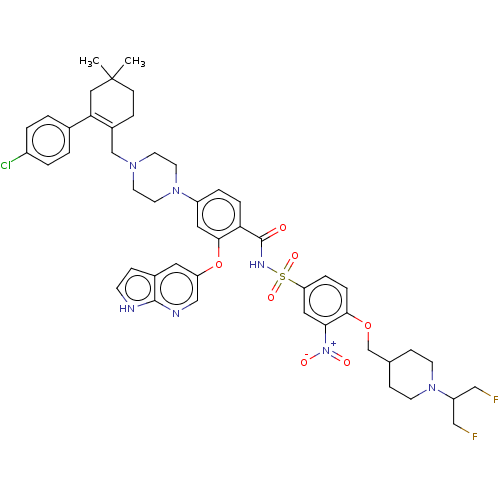 (US10213433, Compound 129 | US11369599, Compound 12...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | -66.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
ABBVIE INC.; GENENTECH, INC.; THE WALTER AND ELIZA HALL INSTITUTE OF MEDICAL RESEARCH US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US9174982 (2015) BindingDB Entry DOI: 10.7270/Q2RB73D5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178836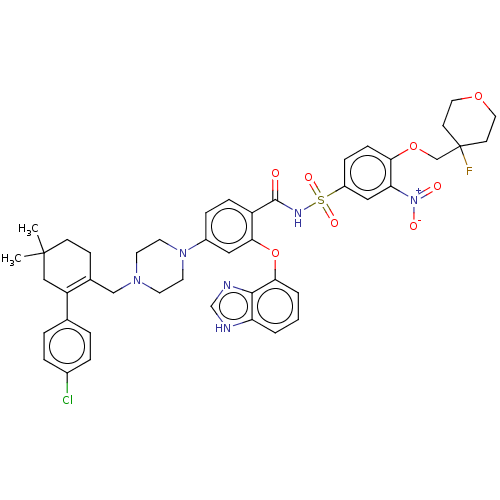 (US9125913, 408) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM189567 (US10213433, Compound 129 | US11369599, Compound 12...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028533 (4-Carbamoyl-2-{4-[(2,4-diamino-pteridin-6-ylmethyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase in mouse L1210 cells | J Med Chem 25: 182-7 (1982) BindingDB Entry DOI: 10.7270/Q2KS6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028539 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.00270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase in mouse L1210 cells | J Med Chem 25: 182-7 (1982) BindingDB Entry DOI: 10.7270/Q2KS6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028542 (CHEMBL293546 | derivative of methotrexate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.00280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase in mouse L1210 cells | J Med Chem 25: 182-7 (1982) BindingDB Entry DOI: 10.7270/Q2KS6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028536 (4-(Carboxymethyl-carbamoyl)-2-{4-[(2,4-diamino-pte...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase in mouse L1210 cells | J Med Chem 25: 182-7 (1982) BindingDB Entry DOI: 10.7270/Q2KS6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178831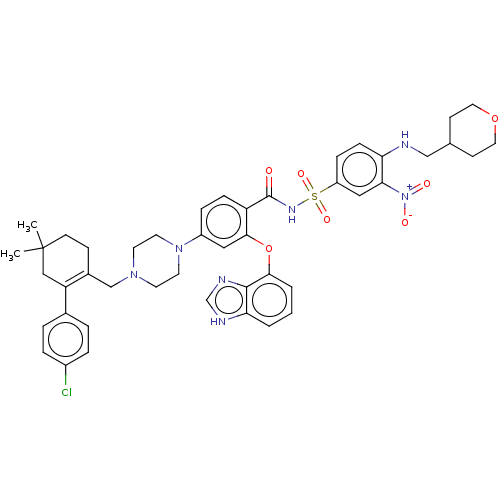 (US9125913, 403) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167296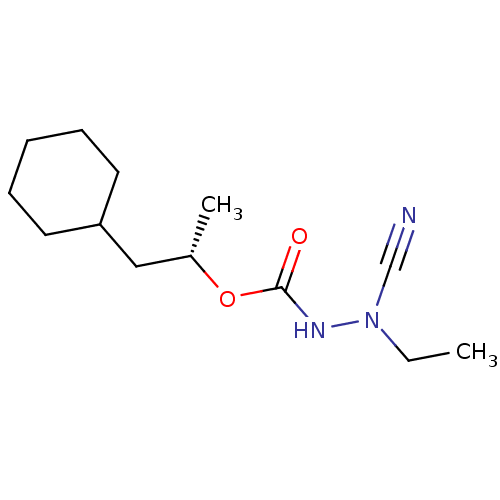 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-ethylhyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178566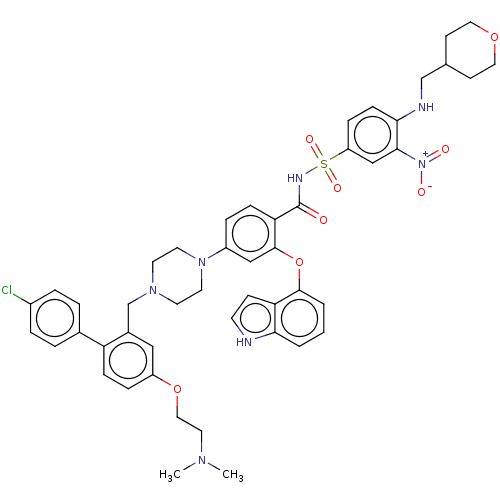 (US9125913, 125) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178856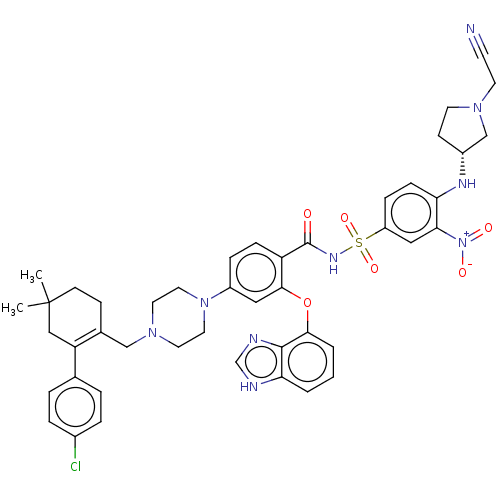 (US9125913, 428) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178853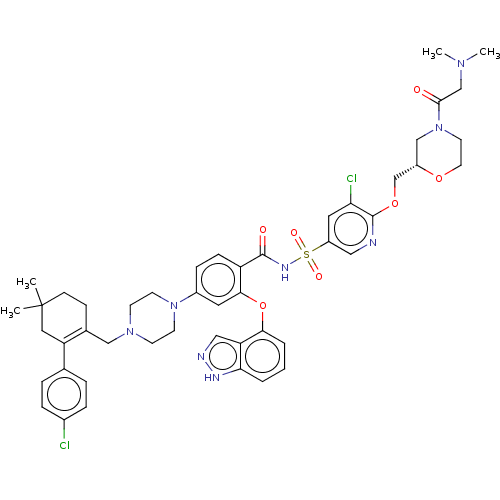 (US9125913, 425) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178839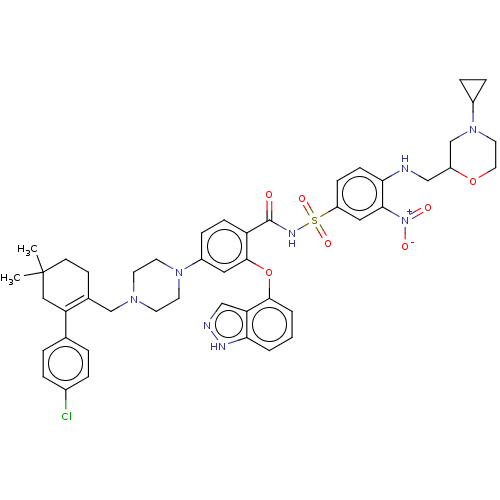 (US9125913, 411) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028541 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase in mouse L1210 cells | J Med Chem 25: 182-7 (1982) BindingDB Entry DOI: 10.7270/Q2KS6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028531 (4-Benzylcarbamoyl-2-{4-[(2,4-diamino-pteridin-6-yl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase in mouse L1210 cells | J Med Chem 25: 182-7 (1982) BindingDB Entry DOI: 10.7270/Q2KS6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028538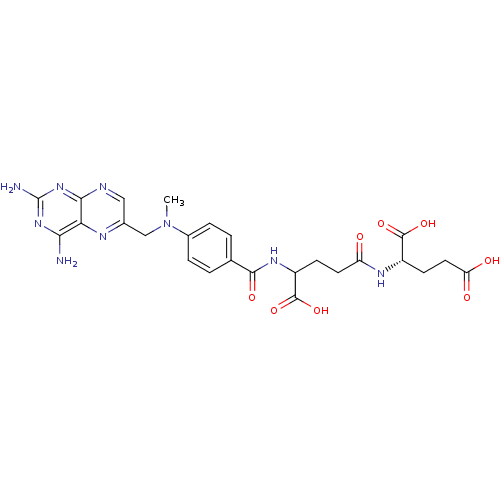 (CHEMBL293147 | derivative of methotrexate) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.00370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase in mouse L1210 cells | J Med Chem 25: 182-7 (1982) BindingDB Entry DOI: 10.7270/Q2KS6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50028540 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.00390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase in mouse L1210 cells | J Med Chem 25: 182-7 (1982) BindingDB Entry DOI: 10.7270/Q2KS6QJZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167295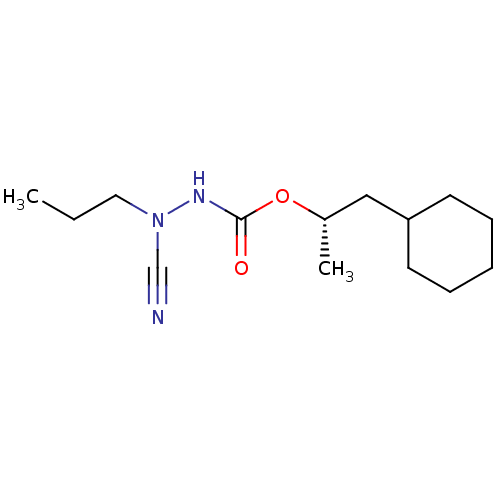 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-propylhy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50167302 ((1S)-2-cyclohexyl-1-methylethyl 2-cyano-2-isobutyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition constant against human cathepsin K in fluorescence assay using Cbz-Phe-Arg-AMC | Bioorg Med Chem Lett 15: 3039-43 (2005) Article DOI: 10.1016/j.bmcl.2005.04.032 BindingDB Entry DOI: 10.7270/Q2MS3S8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178883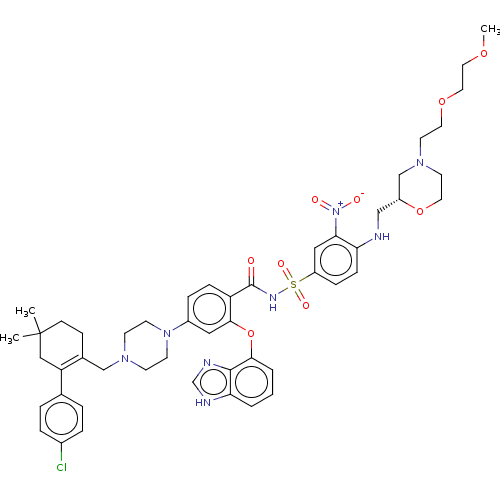 (US9125913, 455) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178846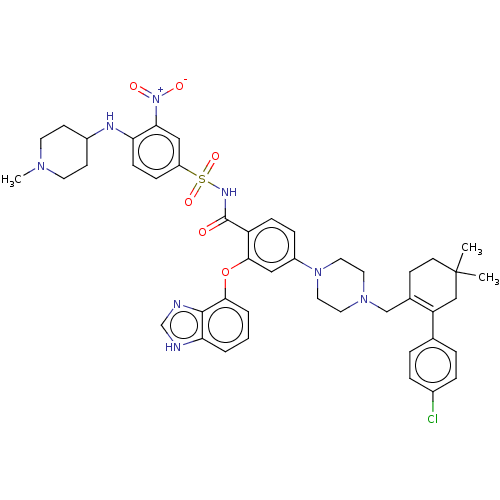 (US9125913, 418) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Lactobacillus casei) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of compound against L. casei Dihydrofolate reductase | J Med Chem 27: 1672-6 (1985) BindingDB Entry DOI: 10.7270/Q2G73CQG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB PubMed | 0.00430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Dihydrofolate reductase in mouse L1210 cells | J Med Chem 25: 182-7 (1982) BindingDB Entry DOI: 10.7270/Q2KS6QJZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178876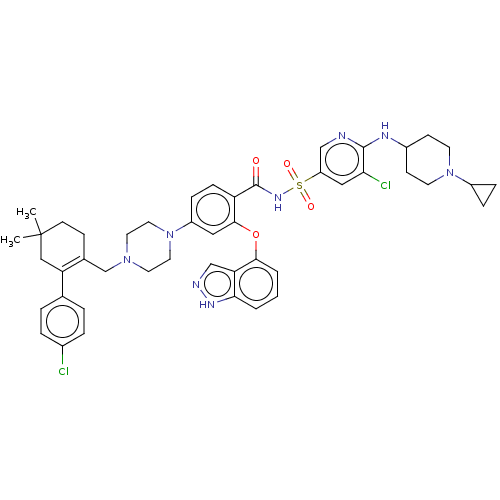 (US9125913, 448) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM145154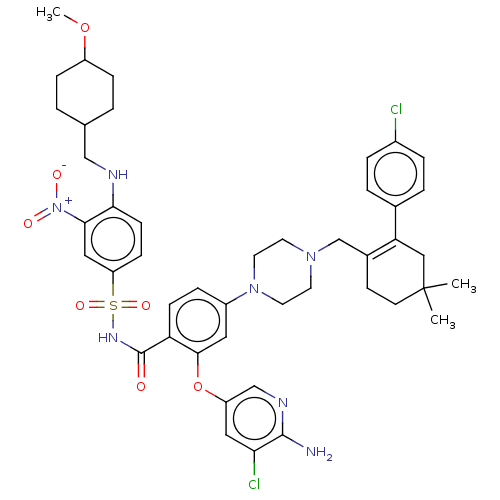 (US8952157, 345 | US9303025, 345) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.; The Walter and Eliza Hall Institute of Medical Research US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US8952157 (2015) BindingDB Entry DOI: 10.7270/Q2QN65G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM144855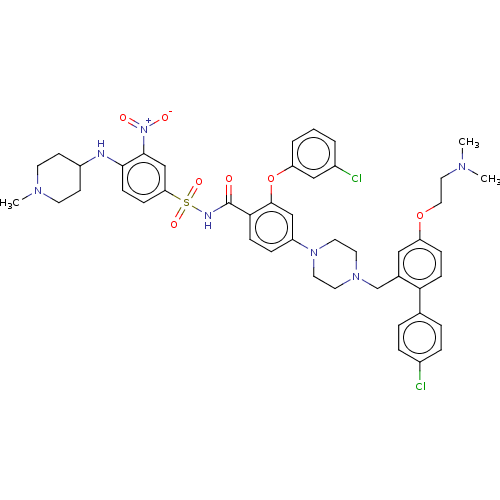 (US8952157, 37 | US9303025, 37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc.; The Walter and Eliza Hall Institute of Medical Research US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 uM (2x starting concentration; 10% DMSO) and 10 uL were tr... | US Patent US8952157 (2015) BindingDB Entry DOI: 10.7270/Q2QN65G9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM145154 (US8952157, 345 | US9303025, 345) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... | US Patent US9303025 (2016) BindingDB Entry DOI: 10.7270/Q2XD10JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM144855 (US8952157, 37 | US9303025, 37) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | -64.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
AbbVie Inc. US Patent | Assay Description Representative compounds were serially diluted in dimethyl sulfoxide (DMSO) starting at 50 μM (2× starting concentration; 10% DMSO) and 10 μ... | US Patent US9303025 (2016) BindingDB Entry DOI: 10.7270/Q2XD10JP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM17135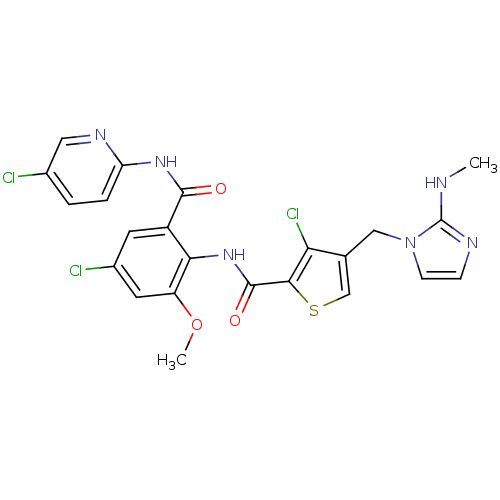 (3-chloro-N-{4-chloro-2-[(5-chloropyridin-2-yl)carb...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of factor 10a | J Med Chem 53: 6243-74 (2010) Article DOI: 10.1021/jm100146h BindingDB Entry DOI: 10.7270/Q2CR5VBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Apoptosis regulator Bcl-2 (Mus musculus (Mouse)) | BDBM178639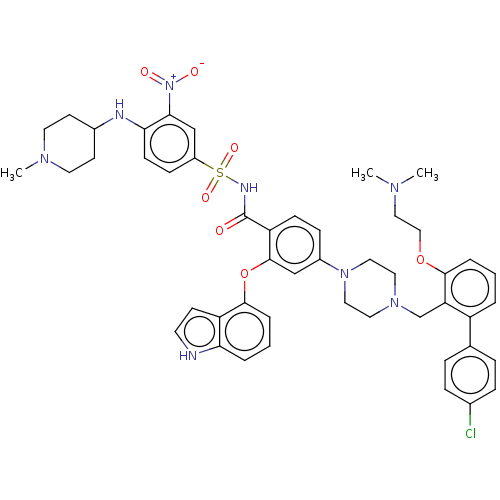 (US9125913, 205) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Inc. US Patent | Assay Description The inhibition constant (Ki) is the dissociation constant of an enzyme-inhibitor complex or a protein/small molecule complex, wherein the small molec... | US Patent US9125913 (2015) BindingDB Entry DOI: 10.7270/Q29Z93PP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50377655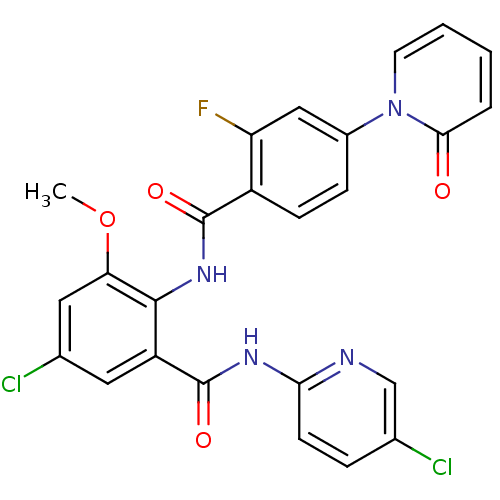 (CHEMBL260160) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development Curated by ChEMBL | Assay Description Inhibition of human factor 10a | Bioorg Med Chem Lett 18: 2845-9 (2008) Article DOI: 10.1016/j.bmcl.2008.03.092 BindingDB Entry DOI: 10.7270/Q2611169 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM14065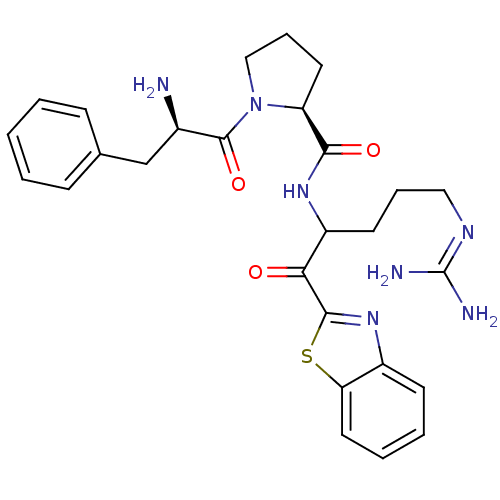 ((2S)-1-[(2R)-2-amino-3-phenylpropanoyl]-N-[1-(1,3-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.00550 | -66.9 | 21 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Johnson & Johnson Pharmaceutical | Assay Description Thrombin-catalyzed hydrolysis rates were measured spectrophotometrically using human alpha-thrombin, a chromogenic substrate in aqueous buffer, and a... | J Med Chem 48: 1984-2008 (2005) Article DOI: 10.1021/jm0303857 BindingDB Entry DOI: 10.7270/Q2X0658X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 87853 total ) | Next | Last >> |