

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242333 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260295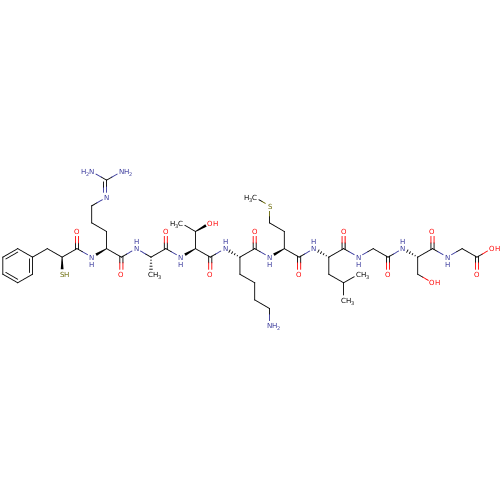 (2-((S)-2-(2-((S)-2-((S)-2-((S)-6-amino-2-((2S,3R)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260294 ((S)-2-((S)-6-amino-2-((2S,3R)-2-((S)-2-((S)-5-guan...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50302031 (2-(4-(4-(N'-(3-(7-chloroquinolin-4-ylamino)propyl)...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50302032 (2-(4-(4-carbamimidoylphenoxy)phenyl)-N'-(3-(7-chlo...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242339 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242337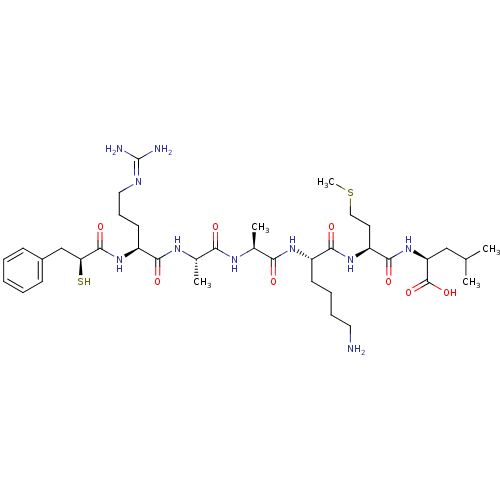 ((S)-2-{(S)-2-[(S)-6-Amino-2-((S)-2-{(S)-2-[(S)-5-g...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242336 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260296 (CHEMBL501525 | CRATKML) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242338 ((S)-2-{(S)-2-[(S)-2-((2S,3R)-2-{(S)-2-[(S)-5-Guani...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50240902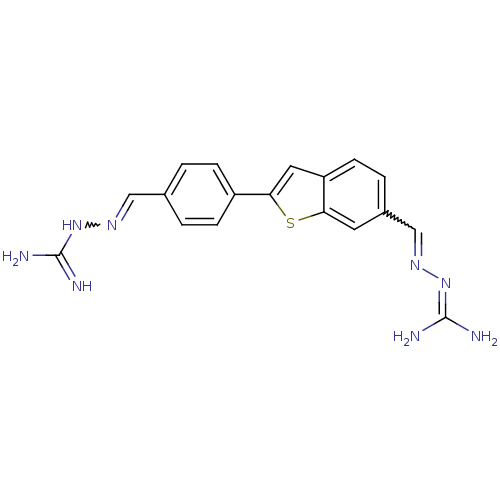 ((2E)-2-{4-[6-((E)-{[(E)-amino(imino)methyl]hydrazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260293 ((S)-6-amino-2-((2S,3R)-2-((S)-2-((S)-5-guanidino-2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50240901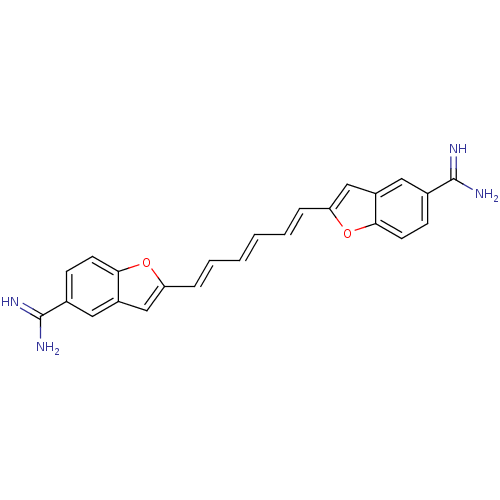 (2-((1E,3E,5E)-6-{5-[(E)-amino(imino)methyl]-1-benz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242334 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50100895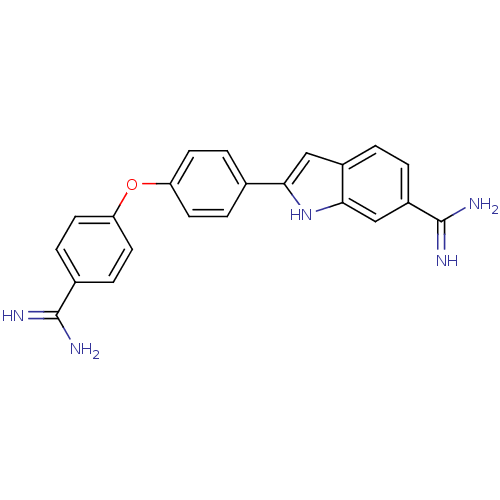 (2-(4-(4-carbamimidoylphenoxy)phenyl)-1H-indole-6-c...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260300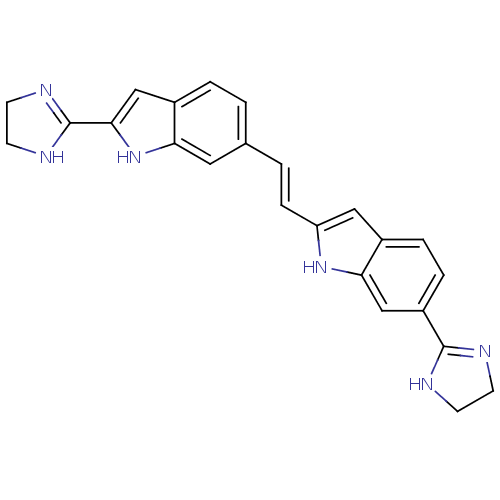 (6-(4,5-dihydro-1H-imidazol-2-yl)-2-(2-(2-(4,5-dihy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260292 ((2S,3R)-2-{(S)-2-[(S)-5-Guanidino-2-((S)-2-mercapt...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242311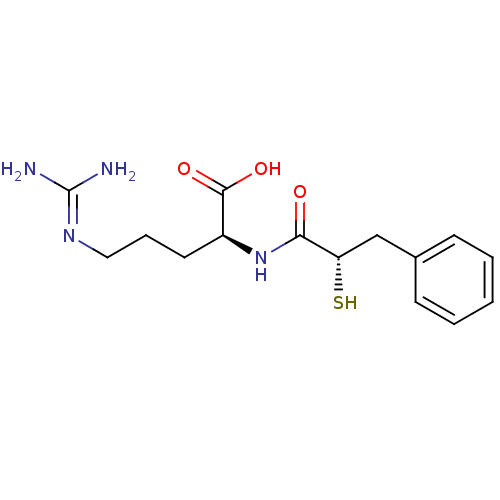 ((S)-5-Guanidino-2-((S)-2-mercapto-3-phenyl-propion...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50260291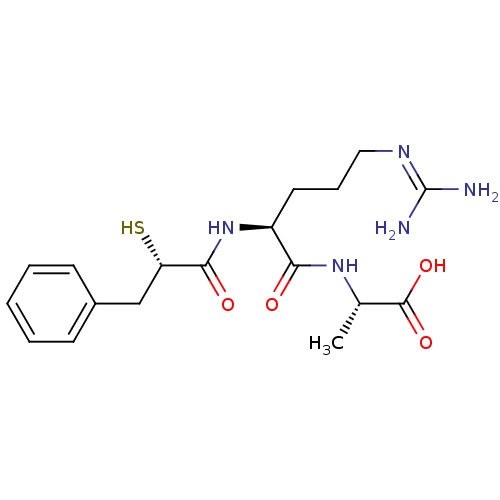 ((S)-2-[(S)-5-Guanidino-2-((S)-2-mercapto-3-phenyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum (strain Hall / ATCC 3502 / N...) | BDBM50242335 ((S)-2-{(S)-2-[(S)-6-Amino-2-((2S,3R)-2-{(S)-2-[(S)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | >3.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SAIC-Frederick, Inc. Curated by ChEMBL | Assay Description Inhibition of BoNT/A light chain metalloprotease activity | J Biol Chem 282: 5004-14 (2007) Article DOI: 10.1074/jbc.M608166200 BindingDB Entry DOI: 10.7270/Q20C4VJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50100895 (2-(4-(4-carbamimidoylphenoxy)phenyl)-1H-indole-6-c...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23299 (4-amino-7-chloroquinoline (ACQ)-based compound, 4 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23298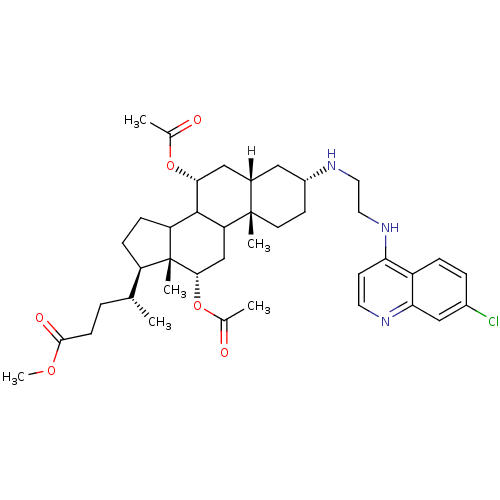 (4-amino-7-chloroquinoline (ACQ)-based compound, 3 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23296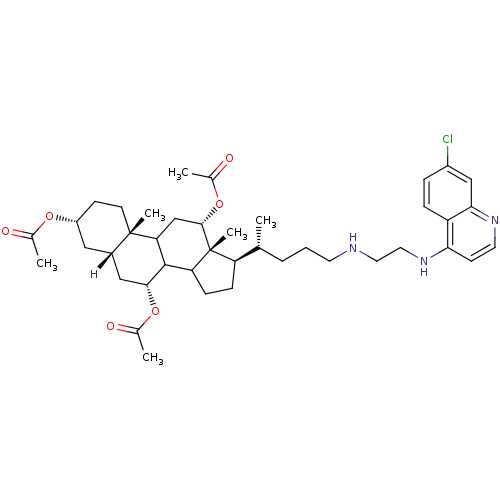 ((2S,5R,7S,9R,14R,15R,16S)-14-[(1R)-4-({2-[(7-chlor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM50067697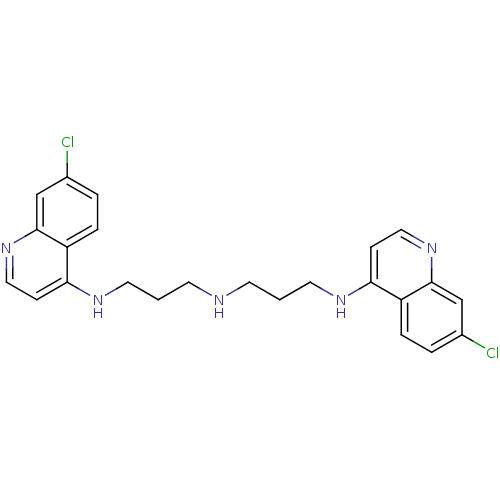 (7-chloro-N-(3-(3-(7-chloroquinolin-4-ylamino)propy...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23297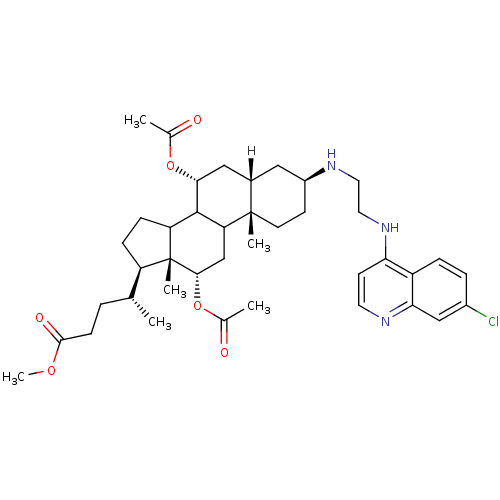 (4-amino-7-chloroquinoline (ACQ)-based compound, 2 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A (Clostridium botulinum) | BDBM23274 ((2E)-3-(2,4-dichlorophenyl)-N-hydroxyprop-2-enamid...) | PDB MMDB NCI pathway Reactome pathway UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | >2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Inhibition of Clostridium botulinum neurotoxin A light chain by HPLC-based assay | Bioorg Med Chem Lett 19: 5811-3 (2009) Article DOI: 10.1016/j.bmcl.2009.01.111 BindingDB Entry DOI: 10.7270/Q2988720 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23300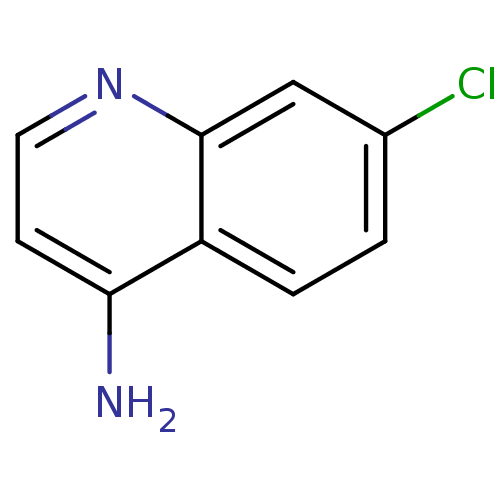 (4-amino-7-chloroquinoline (ACQ)-based compound, 5 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23301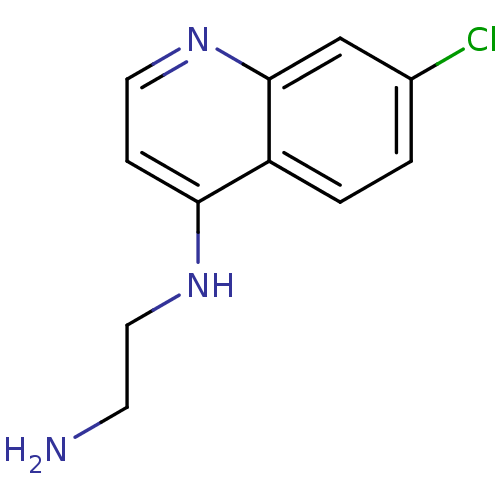 (4-amino-7-chloroquinoline (ACQ)-based compound, 6 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23302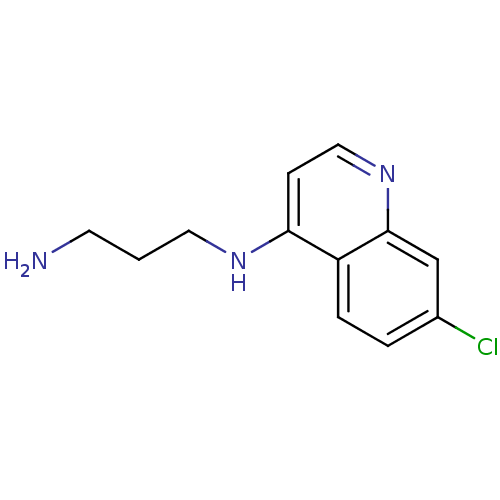 (4-amino-7-chloroquinoline (ACQ)-based compound, 7 ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23303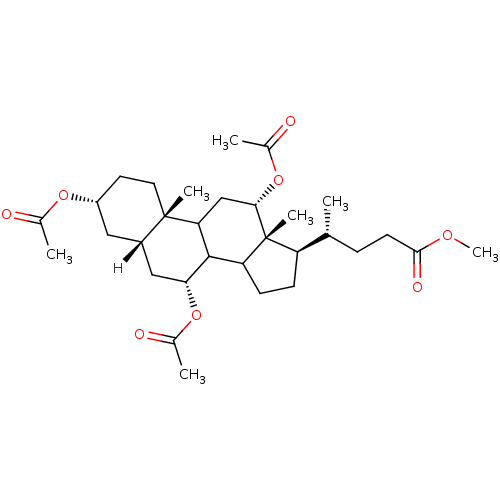 (methyl (4R)-4-[(2S,5R,7S,9R,14R,15R,16S)-5,9,16-tr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23304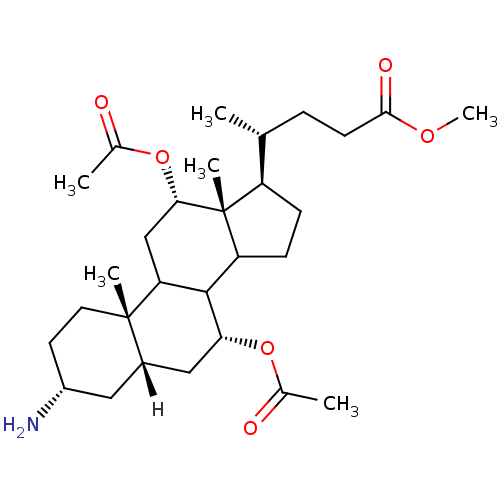 (bis(acetyloxy)-3-aminocholan-24-oate | bis(acetylo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23306 (4-amino-7-chloroquinoline (ACQ)-based compound, 11...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Botulinum neurotoxin type A2 [1-425] (Clostridium botulinum) | BDBM23305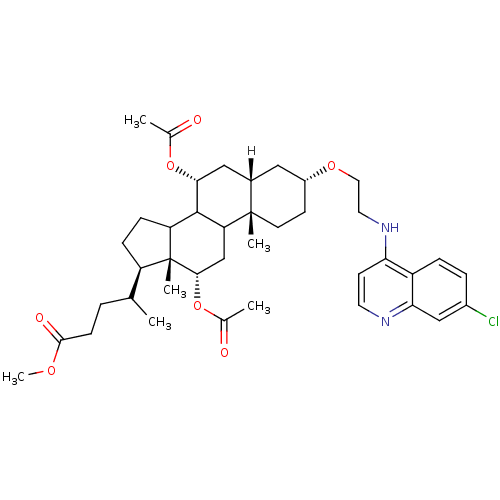 (4-amino-7-chloroquinoline (ACQ)-based compound, 10...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 7.3 | 22 |
National Cancer Institute at Frederick | Assay Description Botox A catalyzed the hydrolysis of substrate peptide between residues 11 (glutamine) and 12 (arginine), corresponding to residues 197 and 198 of SNA... | J Med Chem 50: 2127-36 (2007) Article DOI: 10.1021/jm061446e BindingDB Entry DOI: 10.7270/Q2VQ3105 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||