 Found 118 hits with Last Name = 'herrera' and Initial = 'fj'
Found 118 hits with Last Name = 'herrera' and Initial = 'fj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240209

(CHEMBL4068526)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(NC(=O)c3cccc(c3)-c3cn[nH]c3)c2)CC1 Show InChI InChI=1S/C31H31F3N6O2/c1-20-6-9-26(16-28(20)38-30(42)22-5-3-4-21(14-22)25-17-35-36-18-25)37-29(41)23-7-8-24(27(15-23)31(32,33)34)19-40-12-10-39(2)11-13-40/h3-9,14-18H,10-13,19H2,1-2H3,(H,35,36)(H,37,41)(H,38,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240227

(CHEMBL4084391)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1NC(=O)c1cncc(c1)-c1cnn(C)c1 Show InChI InChI=1S/C25H20F3N5O2/c1-15-6-7-21(31-23(34)16-4-3-5-20(9-16)25(26,27)28)10-22(15)32-24(35)18-8-17(11-29-12-18)19-13-30-33(2)14-19/h3-14H,1-2H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HSV-1 Ribonucleoside diphosphate reductase |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240213

(CHEMBL4085366)Show SMILES Cc1ncc(NC(=O)c2cccc(c2)C(F)(F)F)cc1NC(=O)c1cncc(c1)-c1cnn(C)c1 Show InChI InChI=1S/C24H19F3N6O2/c1-14-21(32-23(35)17-6-16(9-28-10-17)18-11-30-33(2)13-18)8-20(12-29-14)31-22(34)15-4-3-5-19(7-15)24(25,26)27/h3-13H,1-2H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240224
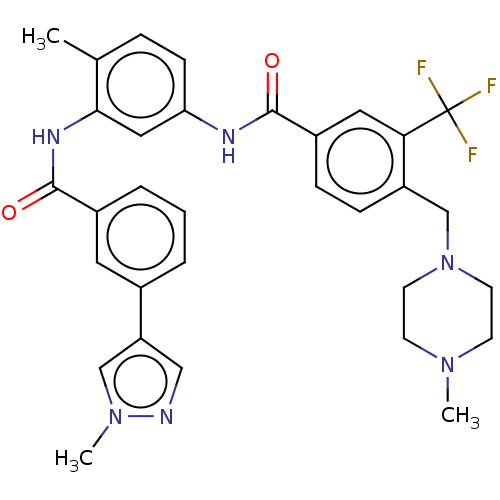
(CHEMBL4095856)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(NC(=O)c3cccc(c3)-c3cnn(C)c3)c2)CC1 Show InChI InChI=1S/C32H33F3N6O2/c1-21-7-10-27(17-29(21)38-31(43)23-6-4-5-22(15-23)26-18-36-40(3)19-26)37-30(42)24-8-9-25(28(16-24)32(33,34)35)20-41-13-11-39(2)12-14-41/h4-10,15-19H,11-14,20H2,1-3H3,(H,37,42)(H,38,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240216
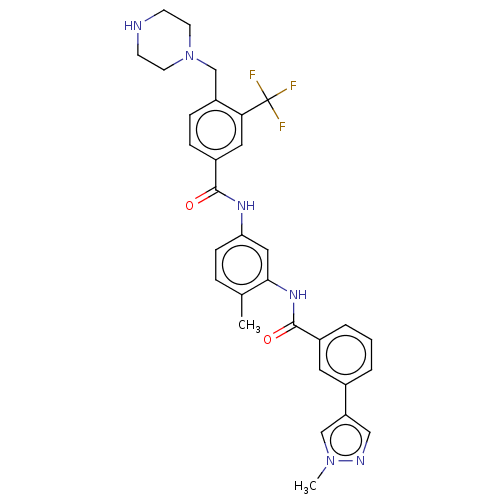
(CHEMBL4074076)Show SMILES Cc1ccc(NC(=O)c2ccc(CN3CCNCC3)c(c2)C(F)(F)F)cc1NC(=O)c1cccc(c1)-c1cnn(C)c1 Show InChI InChI=1S/C31H31F3N6O2/c1-20-6-9-26(16-28(20)38-30(42)22-5-3-4-21(14-22)25-17-36-39(2)18-25)37-29(41)23-7-8-24(27(15-23)31(32,33)34)19-40-12-10-35-11-13-40/h3-9,14-18,35H,10-13,19H2,1-2H3,(H,37,41)(H,38,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240226
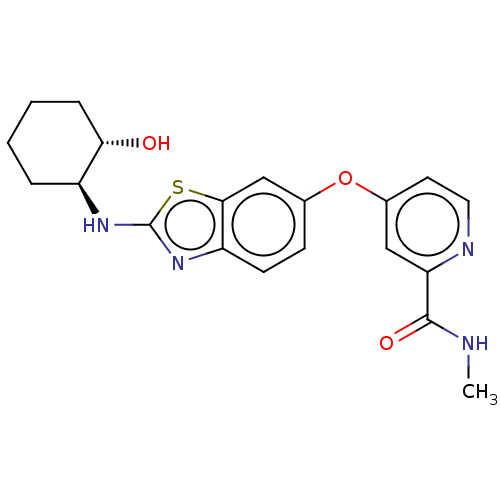
(CHEMBL4065459)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(N[C@H]4CCCC[C@@H]4O)sc3c2)ccn1 |r| Show InChI InChI=1S/C20H22N4O3S/c1-21-19(26)16-10-13(8-9-22-16)27-12-6-7-15-18(11-12)28-20(24-15)23-14-4-2-3-5-17(14)25/h6-11,14,17,25H,2-5H2,1H3,(H,21,26)(H,23,24)/t14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against HSV-1 Ribonucleoside diphosphate reductase was determined |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405407

(CHEMBL5285361)Show SMILES COc1ccc(CCNC[C@H](O)COc2ccc(Cc3nc(c[nH]3)C(C)=O)cc2)cc1OC Show InChI InChI=1S/C25H31N3O5/c1-17(29)22-15-27-25(28-22)13-18-4-7-21(8-5-18)33-16-20(30)14-26-11-10-19-6-9-23(31-2)24(12-19)32-3/h4-9,12,15,20,26,30H,10-11,13-14,16H2,1-3H3,(H,27,28)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405402
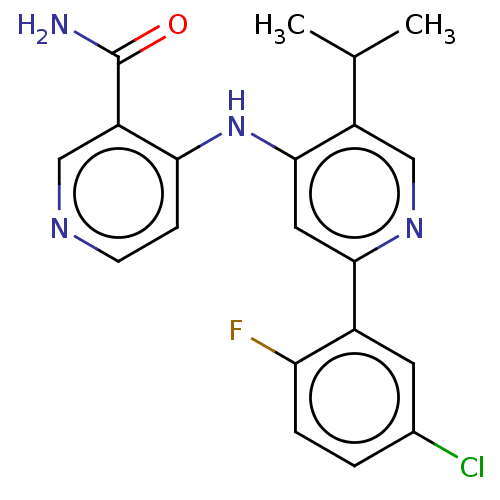
(CHEMBL5267945)Show SMILES COc1ccc(CCNCC(O)COc2ccc(COCc3ncc[nH]3)cc2)cc1OC Show InChI InChI=1S/C24H31N3O5/c1-29-22-8-5-18(13-23(22)30-2)9-10-25-14-20(28)16-32-21-6-3-19(4-7-21)15-31-17-24-26-11-12-27-24/h3-8,11-13,20,25,28H,9-10,14-17H2,1-2H3,(H,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240218
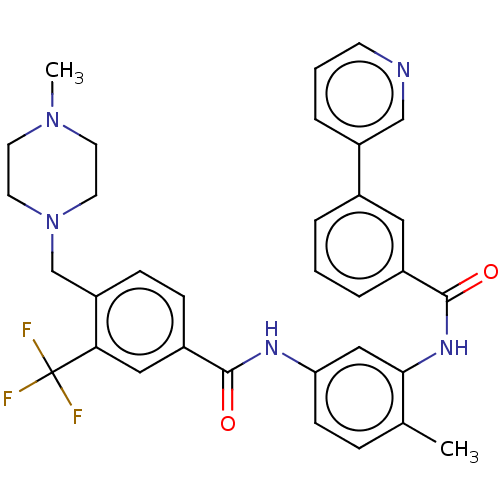
(CHEMBL4059858)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(NC(=O)c3cccc(c3)-c3cccnc3)c2)CC1 Show InChI InChI=1S/C33H32F3N5O2/c1-22-8-11-28(19-30(22)39-32(43)24-6-3-5-23(17-24)26-7-4-12-37-20-26)38-31(42)25-9-10-27(29(18-25)33(34,35)36)21-41-15-13-40(2)14-16-41/h3-12,17-20H,13-16,21H2,1-2H3,(H,38,42)(H,39,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405385
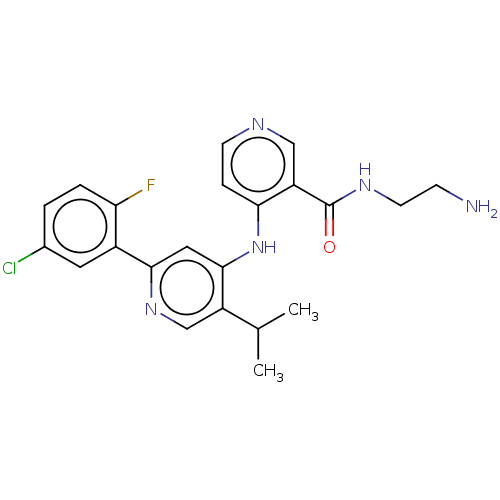
(CHEMBL5285746)Show SMILES CC(CCc1ccccc1)NCC(O)c1cc(C(N)=O)c2[nH]ccc2c1 Show InChI InChI=1S/C21H25N3O2/c1-14(7-8-15-5-3-2-4-6-15)24-13-19(25)17-11-16-9-10-23-20(16)18(12-17)21(22)26/h2-6,9-12,14,19,23-25H,7-8,13H2,1H3,(H2,22,26) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405403

(CHEMBL5274165)Show InChI InChI=1S/C16H22BrN3O2/c1-11(2)18-8-13(21)10-22-14-5-3-12(4-6-14)7-16-19-9-15(17)20-16/h3-6,9,11,13,18,21H,7-8,10H2,1-2H3,(H,19,20) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM514529
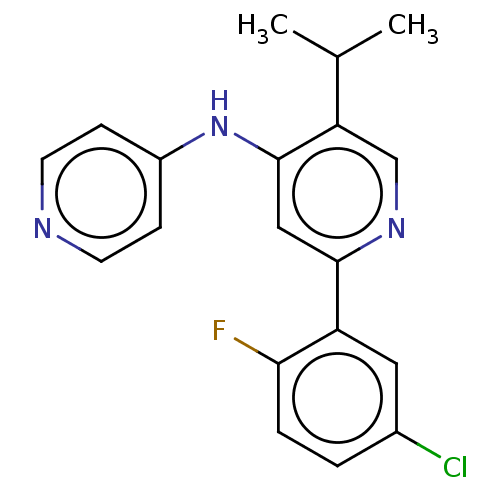
(N-(2-aminoethyl)-4-[[2-(5-chloro-2-fluoro-phenyl)-...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCCN)-c1cc(Cl)ccc1F | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240219
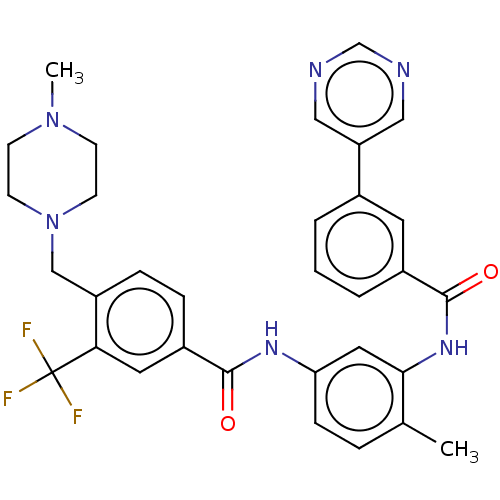
(CHEMBL4100838)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(NC(=O)c3cccc(c3)-c3cncnc3)c2)CC1 Show InChI InChI=1S/C32H31F3N6O2/c1-21-6-9-27(16-29(21)39-31(43)23-5-3-4-22(14-23)26-17-36-20-37-18-26)38-30(42)24-7-8-25(28(15-24)32(33,34)35)19-41-12-10-40(2)11-13-41/h3-9,14-18,20H,10-13,19H2,1-2H3,(H,38,42)(H,39,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240227

(CHEMBL4084391)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1NC(=O)c1cncc(c1)-c1cnn(C)c1 Show InChI InChI=1S/C25H20F3N5O2/c1-15-6-7-21(31-23(34)16-4-3-5-20(9-16)25(26,27)28)10-22(15)32-24(35)18-8-17(11-29-12-18)19-13-30-33(2)14-19/h3-14H,1-2H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HSV-1 Ribonucleoside diphosphate reductase |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240217
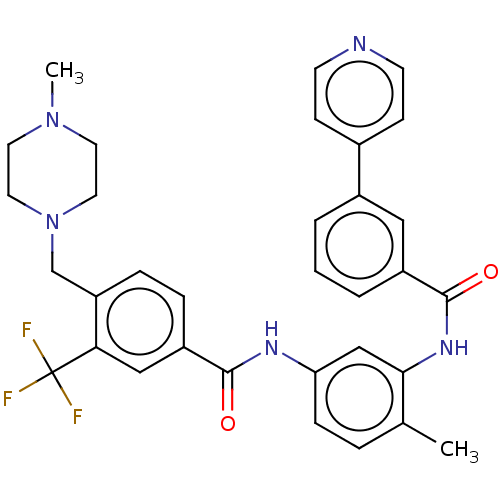
(CHEMBL4063659)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(NC(=O)c3cccc(c3)-c3ccncc3)c2)CC1 Show InChI InChI=1S/C33H32F3N5O2/c1-22-6-9-28(20-30(22)39-32(43)25-5-3-4-24(18-25)23-10-12-37-13-11-23)38-31(42)26-7-8-27(29(19-26)33(34,35)36)21-41-16-14-40(2)15-17-41/h3-13,18-20H,14-17,21H2,1-2H3,(H,38,42)(H,39,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240204
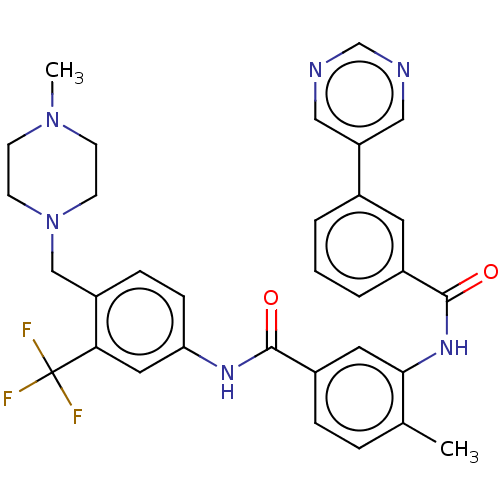
(CHEMBL4081527)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(NC(=O)c4cccc(c4)-c4cncnc4)c3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C32H31F3N6O2/c1-21-6-7-24(15-29(21)39-31(43)23-5-3-4-22(14-23)26-17-36-20-37-18-26)30(42)38-27-9-8-25(28(16-27)32(33,34)35)19-41-12-10-40(2)11-13-41/h3-9,14-18,20H,10-13,19H2,1-2H3,(H,38,42)(H,39,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against HSV-1 Ribonucleoside diphosphate reductase was determined |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405404
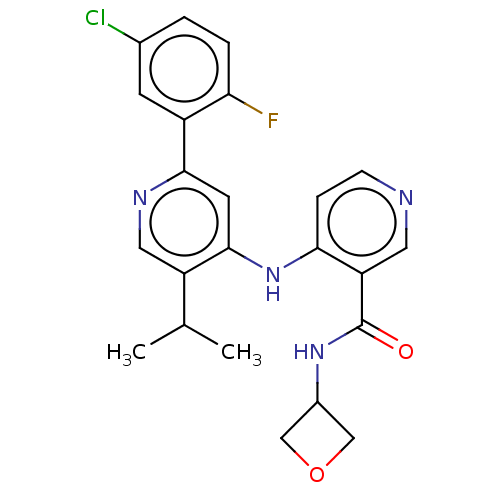
(CHEMBL5268400)Show InChI InChI=1S/C15H21N3O2/c1-11(2)18-9-13(19)10-20-14-5-3-12(4-6-14)15-16-7-8-17-15/h3-8,11,13,18-19H,9-10H2,1-2H3,(H,16,17) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240211
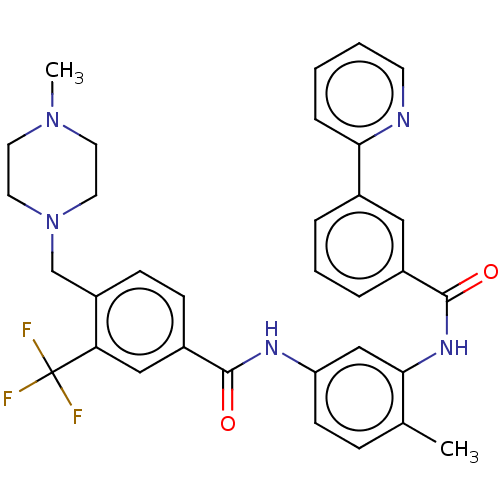
(CHEMBL4104586)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(NC(=O)c3cccc(c3)-c3ccccn3)c2)CC1 Show InChI InChI=1S/C33H32F3N5O2/c1-22-9-12-27(20-30(22)39-32(43)24-7-5-6-23(18-24)29-8-3-4-13-37-29)38-31(42)25-10-11-26(28(19-25)33(34,35)36)21-41-16-14-40(2)15-17-41/h3-13,18-20H,14-17,21H2,1-2H3,(H,38,42)(H,39,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405409
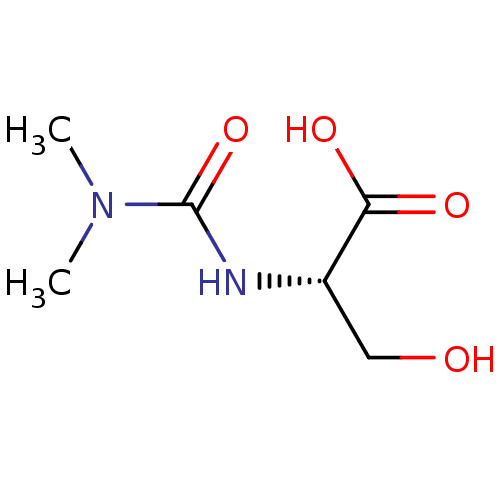
(CHEMBL5267264)Show SMILES COc1ccc(CCNCC(O)COc2ccc(cc2Cl)-c2ncc(Br)[nH]2)cc1OC Show InChI InChI=1S/C22H25BrClN3O4/c1-29-19-5-3-14(9-20(19)30-2)7-8-25-11-16(28)13-31-18-6-4-15(10-17(18)24)22-26-12-21(23)27-22/h3-6,9-10,12,16,25,28H,7-8,11,13H2,1-2H3,(H,26,27) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280348
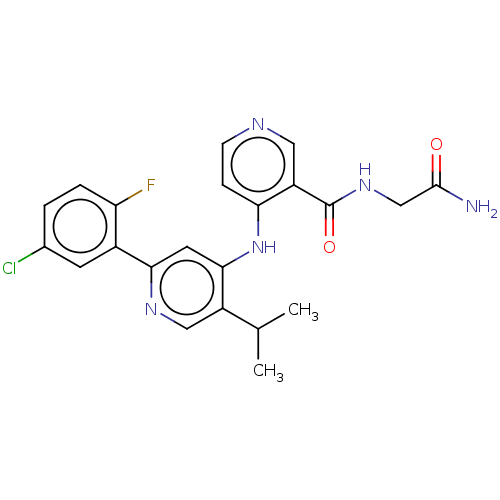
(N-(2-amino-2-oxo-ethyl)-4-[[2-(5-chloro-2-fluoro-p...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCC(N)=O)-c1cc(Cl)ccc1F Show InChI InChI=1S/C22H21ClFN5O2/c1-12(2)15-10-27-19(14-7-13(23)3-4-17(14)24)8-20(15)29-18-5-6-26-9-16(18)22(31)28-11-21(25)30/h3-10,12H,11H2,1-2H3,(H2,25,30)(H,28,31)(H,26,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240227

(CHEMBL4084391)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1NC(=O)c1cncc(c1)-c1cnn(C)c1 Show InChI InChI=1S/C25H20F3N5O2/c1-15-6-7-21(31-23(34)16-4-3-5-20(9-16)25(26,27)28)10-22(15)32-24(35)18-8-17(11-29-12-18)19-13-30-33(2)14-19/h3-14H,1-2H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus expression system using fluorescen... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240210
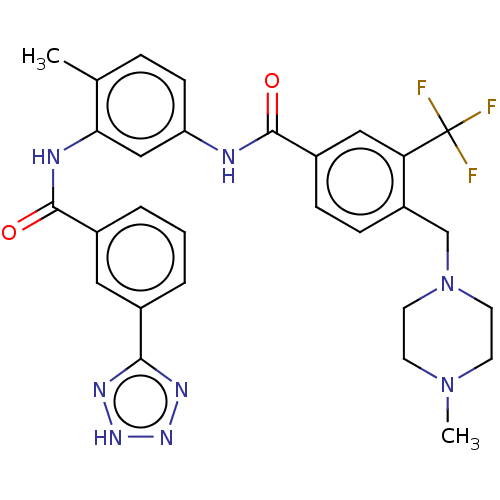
(CHEMBL4084299)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(NC(=O)c3cccc(c3)-c3nn[nH]n3)c2)CC1 Show InChI InChI=1S/C29H29F3N8O2/c1-18-6-9-23(16-25(18)34-28(42)20-5-3-4-19(14-20)26-35-37-38-36-26)33-27(41)21-7-8-22(24(15-21)29(30,31)32)17-40-12-10-39(2)11-13-40/h3-9,14-16H,10-13,17H2,1-2H3,(H,33,41)(H,34,42)(H,35,36,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against HSV-1 Ribonucleoside diphosphate reductase was determined |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50240209

(CHEMBL4068526)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(NC(=O)c3cccc(c3)-c3cn[nH]c3)c2)CC1 Show InChI InChI=1S/C31H31F3N6O2/c1-20-6-9-26(16-28(20)38-30(42)22-5-3-4-21(14-22)25-17-35-36-18-25)37-29(41)23-7-8-24(27(15-23)31(32,33)34)19-40-12-10-39(2)11-13-40/h3-9,14-18H,10-13,19H2,1-2H3,(H,35,36)(H,37,41)(H,38,42) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human c-Kit using poly[Glu:Tyr] (4:1) as substrate measured after 2 hrs in presence of [33gammaP]ATP by P18 ion exchange chromatographi... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240228

(CHEMBL4060882)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(NC(=O)c4cccc(c4)-c4cccnc4)c3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C33H32F3N5O2/c1-22-8-9-25(18-30(22)39-32(43)24-6-3-5-23(17-24)26-7-4-12-37-20-26)31(42)38-28-11-10-27(29(19-28)33(34,35)36)21-41-15-13-40(2)14-16-41/h3-12,17-20H,13-16,21H2,1-2H3,(H,38,42)(H,39,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280340

((S)-4-(2-(5-chloro-2-fluorophenyl)-5-isopropylpyri...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)N[C@@H](C)CO)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H24ClFN4O2/c1-13(2)17-11-27-21(16-8-15(24)4-5-19(16)25)9-22(17)29-20-6-7-26-10-18(20)23(31)28-14(3)12-30/h4-11,13-14,30H,12H2,1-3H3,(H,28,31)(H,26,27,29)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405408

(CHEMBL5290233)Show SMILES COCc1cnc([nH]1)-c1ccc(OC[C@@H](O)CNCCc2ccc(OC)c(OC)c2)cc1 |r| Show InChI InChI=1S/C24H31N3O5/c1-29-15-19-13-26-24(27-19)18-5-7-21(8-6-18)32-16-20(28)14-25-11-10-17-4-9-22(30-2)23(12-17)31-3/h4-9,12-13,20,25,28H,10-11,14-16H2,1-3H3,(H,26,27)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240227

(CHEMBL4084391)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1NC(=O)c1cncc(c1)-c1cnn(C)c1 Show InChI InChI=1S/C25H20F3N5O2/c1-15-6-7-21(31-23(34)16-4-3-5-20(9-16)25(26,27)28)10-22(15)32-24(35)18-8-17(11-29-12-18)19-13-30-33(2)14-19/h3-14H,1-2H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus expression system using fluorescen... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280355
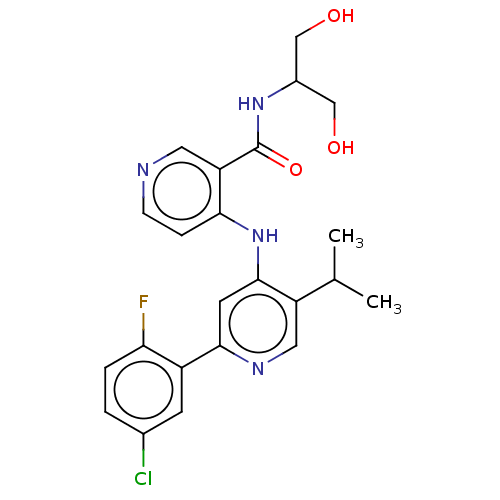
(4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropyl-4-pyr...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NC(CO)CO)-c1cc(Cl)ccc1F Show InChI InChI=1S/C23H24ClFN4O3/c1-13(2)17-10-27-21(16-7-14(24)3-4-19(16)25)8-22(17)29-20-5-6-26-9-18(20)23(32)28-15(11-30)12-31/h3-10,13,15,30-31H,11-12H2,1-2H3,(H,28,32)(H,26,27,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280337
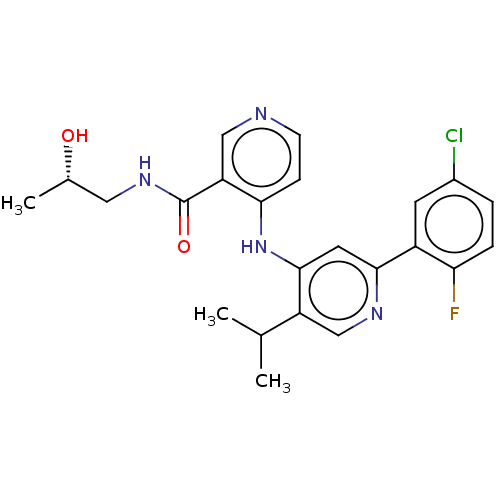
((S)-4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropyl-4...)Show SMILES C[C@H](O)CNC(=O)c1cnccc1Nc1cc(ncc1C(C)C)-c1cc(Cl)ccc1F |r| Show InChI InChI=1S/C23H24ClFN4O2/c1-13(2)17-12-27-21(16-8-15(24)4-5-19(16)25)9-22(17)29-20-6-7-26-11-18(20)23(31)28-10-14(3)30/h4-9,11-14,30H,10H2,1-3H3,(H,28,31)(H,26,27,29)/t14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50205634
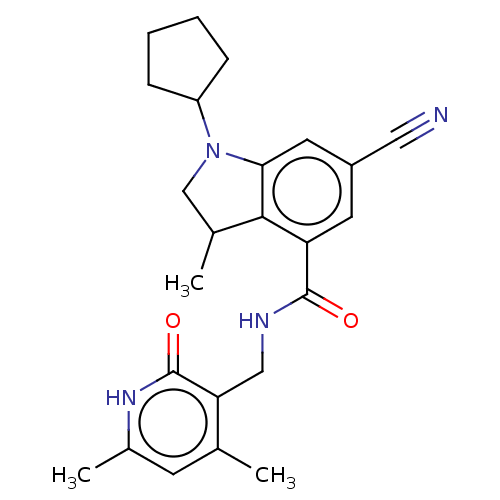
(CHEMBL3933203)Show SMILES CC1CN(C2CCCC2)c2cc(cc(C(=O)NCc3c(C)cc(C)[nH]c3=O)c12)C#N Show InChI InChI=1S/C24H28N4O2/c1-14-8-16(3)27-24(30)20(14)12-26-23(29)19-9-17(11-25)10-21-22(19)15(2)13-28(21)18-6-4-5-7-18/h8-10,15,18H,4-7,12-13H2,1-3H3,(H,26,29)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis |
Bioorg Med Chem Lett 27: 217-222 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.080
BindingDB Entry DOI: 10.7270/Q2S46TZW |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240226

(CHEMBL4065459)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(N[C@H]4CCCC[C@@H]4O)sc3c2)ccn1 |r| Show InChI InChI=1S/C20H22N4O3S/c1-21-19(26)16-10-13(8-9-22-16)27-12-6-7-15-18(11-12)28-20(24-15)23-14-4-2-3-5-17(14)25/h6-11,14,17,25H,2-5H2,1H3,(H,21,26)(H,23,24)/t14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus expression system using fluorescen... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240206
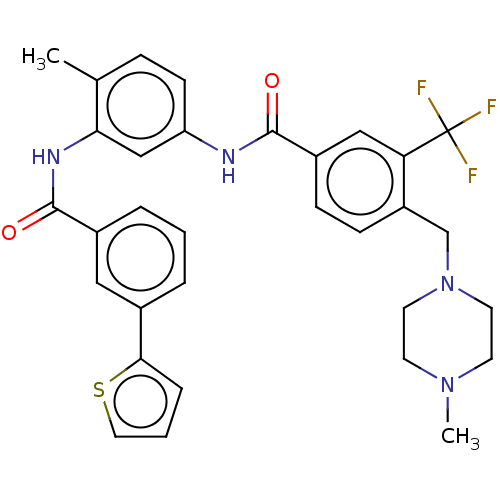
(CHEMBL4064012)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(NC(=O)c3cccc(c3)-c3cccs3)c2)CC1 Show InChI InChI=1S/C32H31F3N4O2S/c1-21-8-11-26(19-28(21)37-31(41)23-6-3-5-22(17-23)29-7-4-16-42-29)36-30(40)24-9-10-25(27(18-24)32(33,34)35)20-39-14-12-38(2)13-15-39/h3-11,16-19H,12-15,20H2,1-2H3,(H,36,40)(H,37,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240212
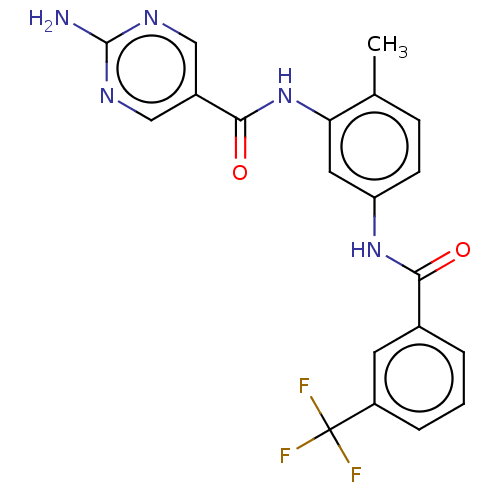
(CHEMBL4103299)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1NC(=O)c1cnc(N)nc1 Show InChI InChI=1S/C20H16F3N5O2/c1-11-5-6-15(8-16(11)28-18(30)13-9-25-19(24)26-10-13)27-17(29)12-3-2-4-14(7-12)20(21,22)23/h2-10H,1H3,(H,27,29)(H,28,30)(H2,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280347
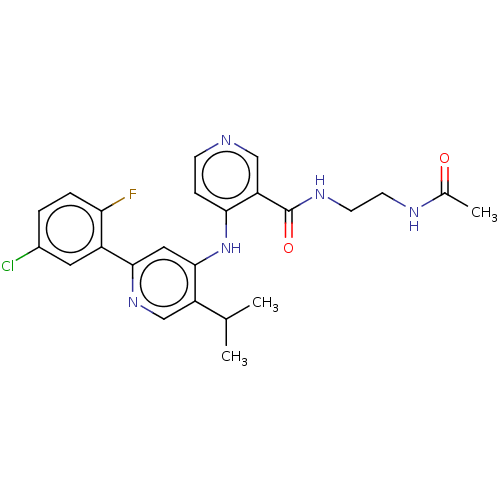
(N-(2-acetamidoethyl)-4-[[2-(5-chloro-2-fluoro-phen...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCCNC(C)=O)-c1cc(Cl)ccc1F Show InChI InChI=1S/C24H25ClFN5O2/c1-14(2)18-13-30-22(17-10-16(25)4-5-20(17)26)11-23(18)31-21-6-7-27-12-19(21)24(33)29-9-8-28-15(3)32/h4-7,10-14H,8-9H2,1-3H3,(H,28,32)(H,29,33)(H,27,30,31) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405410

(CHEMBL5266024)Show SMILES COc1ccc(CCNCC(O)COc2ccc(cn2)-c2nc(c[nH]2)-c2cccs2)cc1OC Show InChI InChI=1S/C25H28N4O4S/c1-31-21-7-5-17(12-22(21)32-2)9-10-26-14-19(30)16-33-24-8-6-18(13-27-24)25-28-15-20(29-25)23-4-3-11-34-23/h3-8,11-13,15,19,26,30H,9-10,14,16H2,1-2H3,(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM280351

(4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropyl-4-pyr...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NCCNS(C)(=O)=O)-c1cc(Cl)ccc1F Show InChI InChI=1S/C23H25ClFN5O3S/c1-14(2)17-13-28-21(16-10-15(24)4-5-19(16)25)11-22(17)30-20-6-7-26-12-18(20)23(31)27-8-9-29-34(3,32)33/h4-7,10-14,29H,8-9H2,1-3H3,(H,27,31)(H,26,28,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240222

(CHEMBL4082748)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(Cl)c(NC(=O)c4cccc(c4)-c4cccs4)c3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C31H28ClF3N4O2S/c1-38-11-13-39(14-12-38)19-23-7-9-24(18-25(23)31(33,34)35)36-29(40)22-8-10-26(32)27(17-22)37-30(41)21-5-2-4-20(16-21)28-6-3-15-42-28/h2-10,15-18H,11-14,19H2,1H3,(H,36,40)(H,37,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human CSF1R using poly[Glu:Tyr] (4:1) as substrate incubated for 2 hrs in presence of [33gammaP]ATP by P81 ion exchange chromatographic... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405405
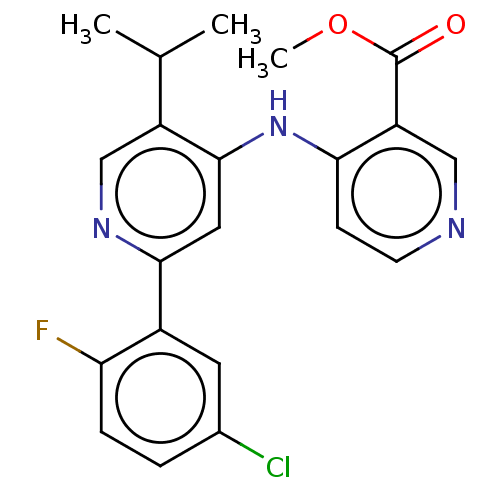
(CHEMBL5287924)Show SMILES COc1ccc(CCNCC(O)COc2ccc(cc2)-c2nc(c[nH]2)C(C)(C)C)cc1OC Show InChI InChI=1S/C26H35N3O4/c1-26(2,3)24-16-28-25(29-24)19-7-9-21(10-8-19)33-17-20(30)15-27-13-12-18-6-11-22(31-4)23(14-18)32-5/h6-11,14,16,20,27,30H,12-13,15,17H2,1-5H3,(H,28,29) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
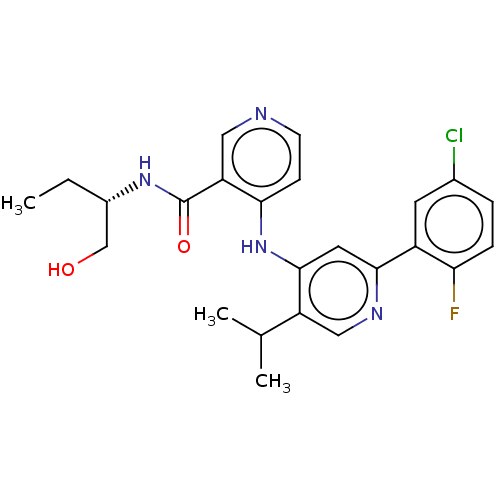
(Homo sapiens (Human)) | BDBM50405406
(CHEMBL5285227)Show InChI InChI=1S/C19H23N3O2S/c1-2-9-20-11-15(23)13-24-16-7-5-14(6-8-16)19-21-12-17(22-19)18-4-3-10-25-18/h3-8,10,12,15,20,23H,2,9,11,13H2,1H3,(H,21,22) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Activin receptor type-1B
(Homo sapiens (Human)) | BDBM280355

(4-[[2-(5-chloro-2-fluoro-phenyl)-5-isopropyl-4-pyr...)Show SMILES CC(C)c1cnc(cc1Nc1ccncc1C(=O)NC(CO)CO)-c1cc(Cl)ccc1F Show InChI InChI=1S/C23H24ClFN4O3/c1-13(2)17-10-27-21(16-7-14(24)3-4-19(16)25)8-22(17)29-20-5-6-26-9-18(20)23(32)28-15(11-30)12-31/h3-10,13,15,30-31H,11-12H2,1-2H3,(H,28,32)(H,26,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240226

(CHEMBL4065459)Show SMILES CNC(=O)c1cc(Oc2ccc3nc(N[C@H]4CCCC[C@@H]4O)sc3c2)ccn1 |r| Show InChI InChI=1S/C20H22N4O3S/c1-21-19(26)16-10-13(8-9-22-16)27-12-6-7-15-18(11-12)28-20(24-15)23-14-4-2-3-5-17(14)25/h6-11,14,17,25H,2-5H2,1H3,(H,21,26)(H,23,24)/t14-,17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity against HSV-1 Ribonucleoside diphosphate reductase was determined |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50240227

(CHEMBL4084391)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1NC(=O)c1cncc(c1)-c1cnn(C)c1 Show InChI InChI=1S/C25H20F3N5O2/c1-15-6-7-21(31-23(34)16-4-3-5-20(9-16)25(26,27)28)10-22(15)32-24(35)18-8-17(11-29-12-18)19-13-30-33(2)14-19/h3-14H,1-2H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human c-Kit using poly[Glu:Tyr] (4:1) as substrate measured after 2 hrs in presence of [33gammaP]ATP by P18 ion exchange chromatographi... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240224

(CHEMBL4095856)Show SMILES CN1CCN(Cc2ccc(cc2C(F)(F)F)C(=O)Nc2ccc(C)c(NC(=O)c3cccc(c3)-c3cnn(C)c3)c2)CC1 Show InChI InChI=1S/C32H33F3N6O2/c1-21-7-10-27(17-29(21)38-31(43)23-6-4-5-22(15-23)26-18-36-40(3)19-26)37-30(42)24-8-9-25(28(16-24)32(33,34)35)20-41-13-11-39(2)12-14-41/h4-10,15-19H,11-14,20H2,1-3H3,(H,37,42)(H,38,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human His-tagged CSF1R cytoplasmic domain (538 to 910 residues) expressed in baculovirus expression system using fluorescen... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405413

(CHEMBL5273556)Show SMILES COc1ccc(CCNCC(O)COc2ccc(CCc3ncc(Br)[nH]3)cc2)cc1OC Show InChI InChI=1S/C24H30BrN3O4/c1-30-21-9-5-18(13-22(21)31-2)11-12-26-14-19(29)16-32-20-7-3-17(4-8-20)6-10-24-27-15-23(25)28-24/h3-5,7-9,13,15,19,26,29H,6,10-12,14,16H2,1-2H3,(H,27,28) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against angiotensin converting enzyme (ACE) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405412

(CHEMBL5276114)Show InChI InChI=1S/C22H26N2O5/c1-26-20-8-3-16(13-21(20)27-2)9-10-23-14-18(25)15-29-19-6-4-17(5-7-19)22-24-11-12-28-22/h3-8,11-13,18,23,25H,9-10,14-15H2,1-2H3 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
In vitro inhibitory activity against neutral endopeptidase (NEP) |
Citation and Details
|
More data for this
Ligand-Target Pair | |
TGF-beta receptor type-1
(Homo sapiens (Human)) | BDBM50405411
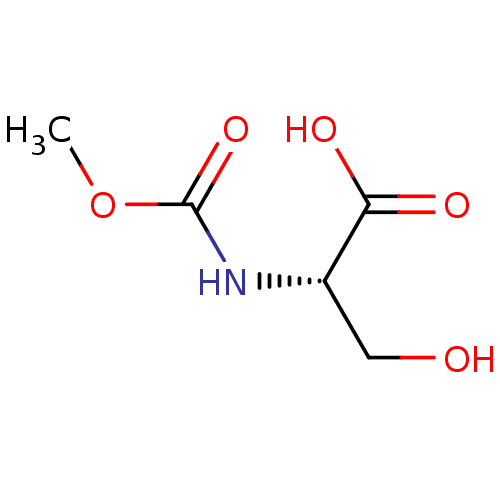
(CHEMBL5277114)Show SMILES COc1ccc(CCNCC(O)COc2ccc(cc2Cl)-c2nc(c[nH]2)-c2cccs2)cc1OC Show InChI InChI=1S/C26H28ClN3O4S/c1-32-23-7-5-17(12-24(23)33-2)9-10-28-14-19(31)16-34-22-8-6-18(13-20(22)27)26-29-15-21(30-26)25-4-3-11-35-25/h3-8,11-13,15,19,28,31H,9-10,14,16H2,1-2H3,(H,29,30) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibitory activity against Escherichia coli leader peptidase using substrate A |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Macrophage colony-stimulating factor 1 receptor
(Homo sapiens (Human)) | BDBM50240220
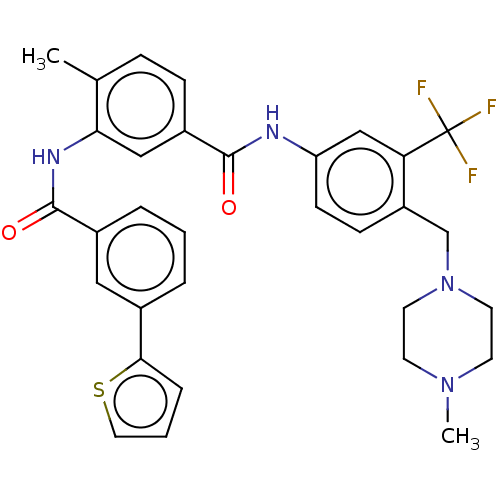
(CHEMBL4079932)Show SMILES CN1CCN(Cc2ccc(NC(=O)c3ccc(C)c(NC(=O)c4cccc(c4)-c4cccs4)c3)cc2C(F)(F)F)CC1 Show InChI InChI=1S/C32H31F3N4O2S/c1-21-8-9-24(18-28(21)37-31(41)23-6-3-5-22(17-23)29-7-4-16-42-29)30(40)36-26-11-10-25(27(19-26)32(33,34)35)20-39-14-12-38(2)13-15-39/h3-11,16-19H,12-15,20H2,1-2H3,(H,36,40)(H,37,41) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HSV-1 Ribonucleoside diphosphate reductase |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50205628
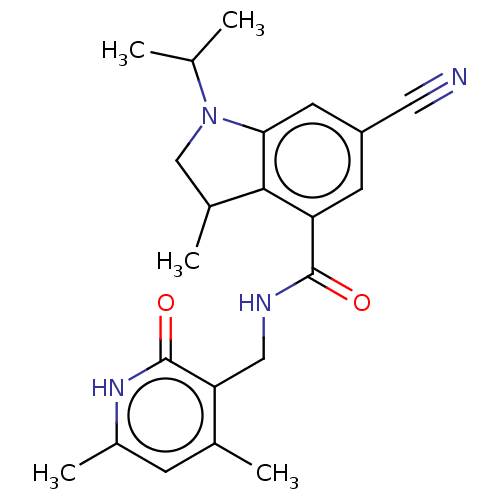
(CHEMBL3906288)Show SMILES CC(C)N1CC(C)c2c1cc(cc2C(=O)NCc1c(C)cc(C)[nH]c1=O)C#N Show InChI InChI=1S/C22H26N4O2/c1-12(2)26-11-14(4)20-17(7-16(9-23)8-19(20)26)21(27)24-10-18-13(3)6-15(5)25-22(18)28/h6-8,12,14H,10-11H2,1-5H3,(H,24,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human wild type EZH2 using histone H3 as substrate after 1 hr in presence of 3H-SAM by filter paper detection analysis |
Bioorg Med Chem Lett 27: 217-222 (2017)
Article DOI: 10.1016/j.bmcl.2016.11.080
BindingDB Entry DOI: 10.7270/Q2S46TZW |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50240215

(CHEMBL4062707)Show SMILES Cc1ccc(NC(=O)c2cccc(c2)C(F)(F)F)cc1NC(=O)c1cccc(c1)-c1cnn(C)c1 Show InChI InChI=1S/C26H21F3N4O2/c1-16-9-10-22(31-24(34)19-7-4-8-21(12-19)26(27,28)29)13-23(16)32-25(35)18-6-3-5-17(11-18)20-14-30-33(2)15-20/h3-15H,1-2H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibitory activity was determined against HSV-1 Ribonucleoside diphosphate reductase; value ranges from 36-60 uM |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |
Mast/stem cell growth factor receptor Kit
(Homo sapiens (Human)) | BDBM50240213

(CHEMBL4085366)Show SMILES Cc1ncc(NC(=O)c2cccc(c2)C(F)(F)F)cc1NC(=O)c1cncc(c1)-c1cnn(C)c1 Show InChI InChI=1S/C24H19F3N6O2/c1-14-21(32-23(35)17-6-16(9-28-10-17)18-11-30-33(2)13-18)8-20(12-29-14)31-22(34)15-4-3-5-19(7-15)24(25,26)27/h3-13H,1-2H3,(H,31,34)(H,32,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Integral BioSciences Pvt. Ltd
Curated by ChEMBL
| Assay Description
Inhibition of human c-Kit using poly[Glu:Tyr] (4:1) as substrate measured after 2 hrs in presence of [33gammaP]ATP by P18 ion exchange chromatographi... |
Bioorg Med Chem Lett 27: 2153-2160 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.064
BindingDB Entry DOI: 10.7270/Q2SX6GC2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data