

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-P receptor (GUINEA PIG) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards NK1 receptor in the striatal membranes of guinea pig | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50000041 ((+) (2-Methoxy-benzyl)-(2-phenyl-piperidin-3-yl)-a...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity towards NK1 receptor in the striatal membranes of guinea pig | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014156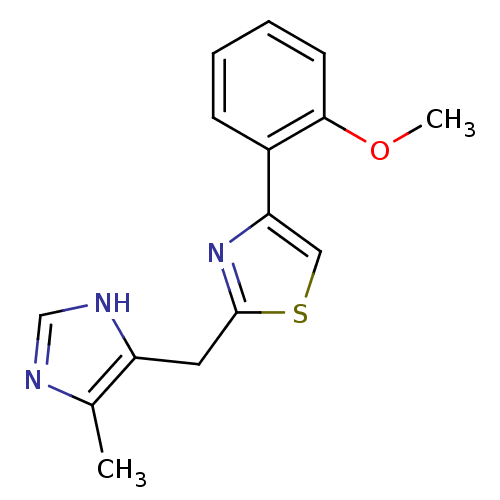 (4-(2-Methoxy-phenyl)-2-(5-methyl-1H-imidazol-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014174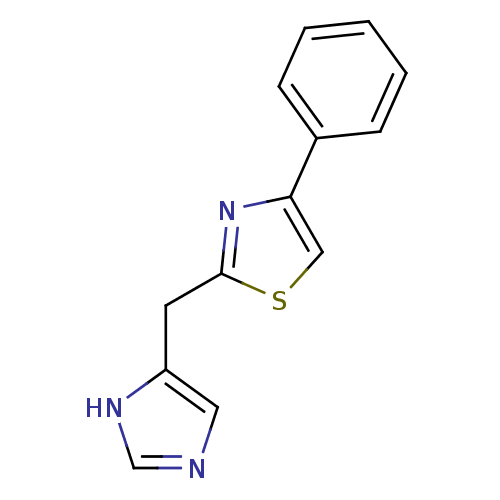 (2-(1H-Imidazol-4-ylmethyl)-4-phenyl-thiazole | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014164 (8-[2-(5-Methyl-1H-imidazol-4-ylmethyl)-thiazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Antibacterial activity against Escherichia coli DHFR | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014159 (4-(2-Fluoro-phenyl)-2-(5-methyl-1H-imidazol-4-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50013039 (CHEMBL40260 | N-[4-(1H-Indol-3-yl)-thiazol-2-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of dihydrofolate reductase of Escherichia coli | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50013043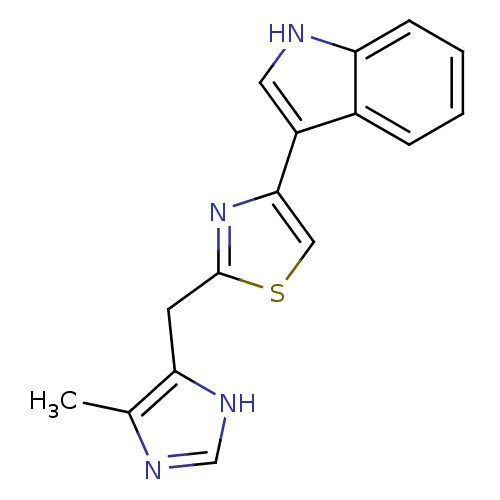 (3-[2-(5-Methyl-1H-imidazol-4-ylmethyl)-thiazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50013043 (3-[2-(5-Methyl-1H-imidazol-4-ylmethyl)-thiazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014600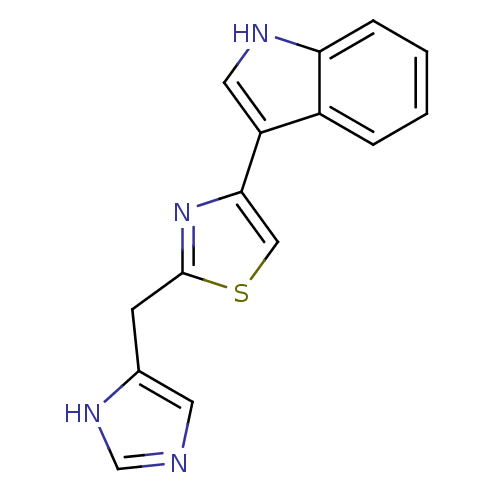 (3-[2-(1H-Imidazol-4-ylmethyl)-thiazol-4-yl]-1H-ind...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM85330 (CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM85330 (CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50033658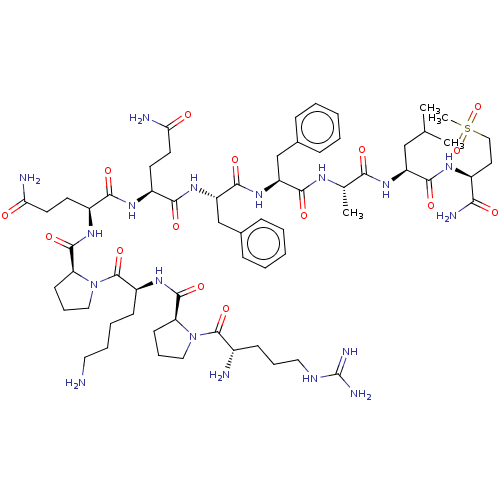 (Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Sarcosine-Leu-Met(...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity against NK1 receptor | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (GUINEA PIG) | BDBM50001450 ((SP)Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity against NK1 receptor | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50106301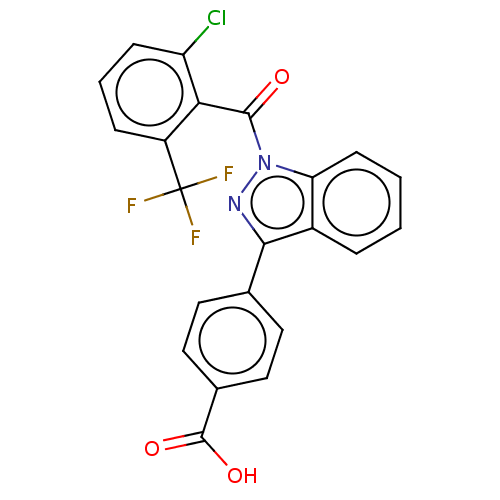 (CHEMBL3598140) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116877 BindingDB Entry DOI: 10.7270/Q2GB282C | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50106301 (CHEMBL3598140) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116877 BindingDB Entry DOI: 10.7270/Q2GB282C | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014601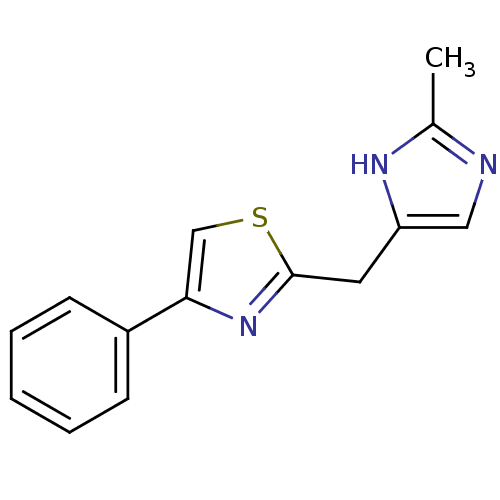 (2-(2-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Escherichia coli dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50106301 (CHEMBL3598140) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116877 BindingDB Entry DOI: 10.7270/Q2GB282C | ||||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50143282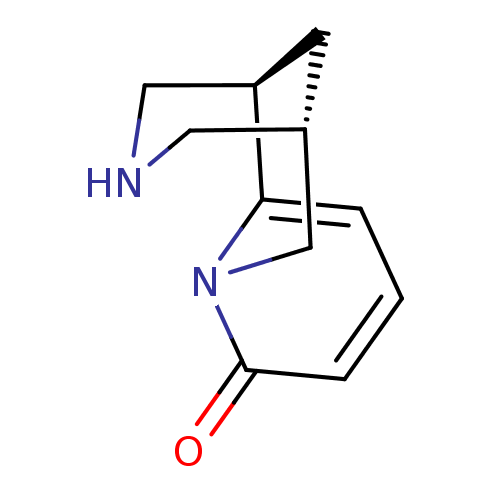 ((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50166908 (5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 322 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha-7 subunit in rat GH4C1 cells | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50166909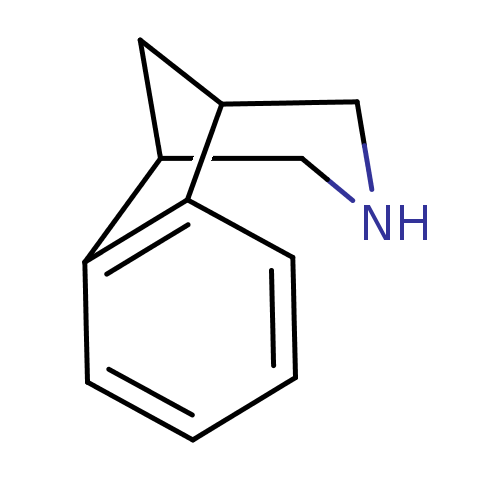 (10-Aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),3,5-trien...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50166907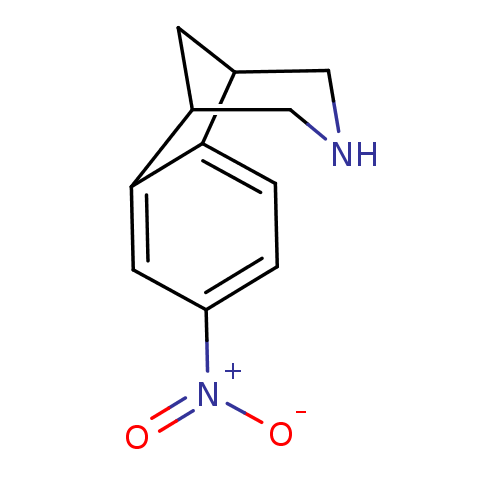 (4-nitro-10-azatricyclo[6.3.1.02,7]dodeca-2(7),3,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1C receptor was determined by using [3H]- mesulergine as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Mus musculus (Mouse)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Dopamine receptor D1 was determined by using [3H]SCH-23390 as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Alpha-1 adrenergic receptor was determined by using [3H]prazosin as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Dopamine receptor D2 was determined by using [3H]spiperone as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor mu 1 was determined by using [3H]naloxone as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1D receptor was determined by using [3H]5-HT as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (BOVINE) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 2 receptor was determined by using [3H]ketanserin as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1B receptor was determined by using [3H]5-HT as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Muscarinic acetylcholine receptor was determined by using [3H]QNB as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Sigma opioid receptor was determined by using [3H](+)-3-(3-hydroxyphenyl)N-1-propyl-piperdine((+)-3-PPP) as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Beta adrenergic receptor was determined by using [3H]dihydroalprenolol as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Rattus norvegicus) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Alpha-2 adrenergic receptor was determined by using [3H]p-aminoclonidine as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1A receptor was determined by using [3H]8-OH-DPAT as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50013045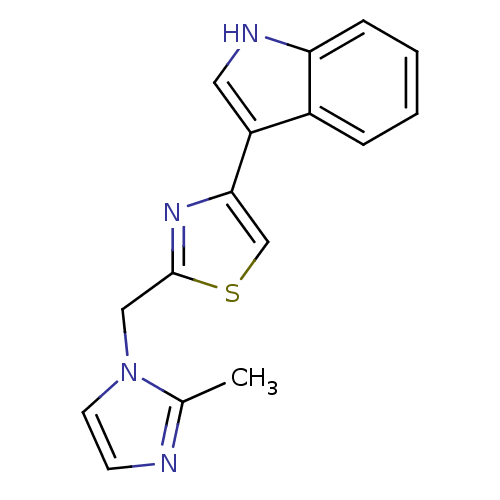 (3-[2-(2-Methyl-imidazol-1-ylmethyl)-thiazol-4-yl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014406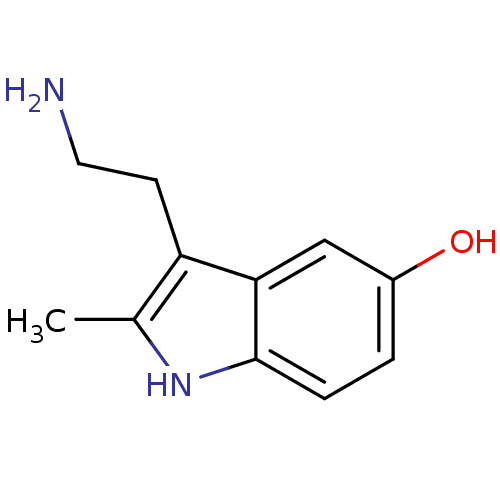 (2-Me 5-HT | 2-Methyl-5-hydroxytryptamine | 2-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Escherichia coli dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM50166908 (5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 3.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (GUINEA PIG) | BDBM50001447 (CHEMBL217406 | His-Lys-Thr-Asp-Ser-Phe-Val-Gly-Leu...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Evaluated for the binding affinity against NK2 receptor | J Med Chem 36: 3197-201 (1993) BindingDB Entry DOI: 10.7270/Q29887NM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50143282 ((-)-cytisine | (1R,5S)-1,2,3,4,5,6-Hexahydro-1,5-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha-7 subunit in rat GH4C1 cells | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50166909 (10-Aza-tricyclo[6.3.1.0*2,7*]dodeca-2(7),3,5-trien...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha-7 subunit in rat GH4C1 cells | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholine receptor subunit alpha/beta/delta/gamma (Torpedo californica) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha1 beta gamma delta of electroplax | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM82070 (CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase KEGG PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 6.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha-7 subunit in rat GH4C1 cells | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuronal acetylcholine receptor subunit alpha-7 (Rattus norvegicus (Rat)) | BDBM50166907 (4-nitro-10-azatricyclo[6.3.1.02,7]dodeca-2(7),3,5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of [125I]alpha-bungarotoxin binding to nicotinic acetylcholine receptor alpha-7 subunit in rat GH4C1 cells | J Med Chem 48: 3474-7 (2005) Article DOI: 10.1021/jm050069n BindingDB Entry DOI: 10.7270/Q2N87BJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [S768I] (Homo sapiens (Human)) | BDBM557850 (6-((1-acryloylpiperidin-4-yl)oxy)-4-((3,4-dichloro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Cell viability was determined using the Cell Titer Glo assay (Promega) as previously described. Briefly, 2000-3000 cells per well were plated in 384-... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z322V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor [S768I] (Homo sapiens (Human)) | BDBM557852 (1-(3-((4-((3,4-dichloro-2-fluorophenyl)amino)-7-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Cell viability was determined using the Cell Titer Glo assay (Promega) as previously described. Briefly, 2000-3000 cells per well were plated in 384-... | Citation and Details BindingDB Entry DOI: 10.7270/Q2Z322V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 580 total ) | Next | Last >> |